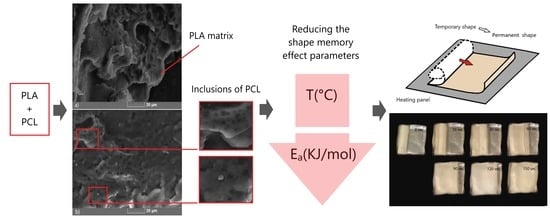
Polymer Composite Materials Based on Polylactide with a Shape Memory Effect for “Self-Fitting” Bone Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Test Specimens
2.2. Characterization
2.2.1. Structure
2.2.2. Mechanical Properties
2.2.3. Shape Memory Properties
2.2.4. Cytotoxicity Assay
3. Results and Discussion
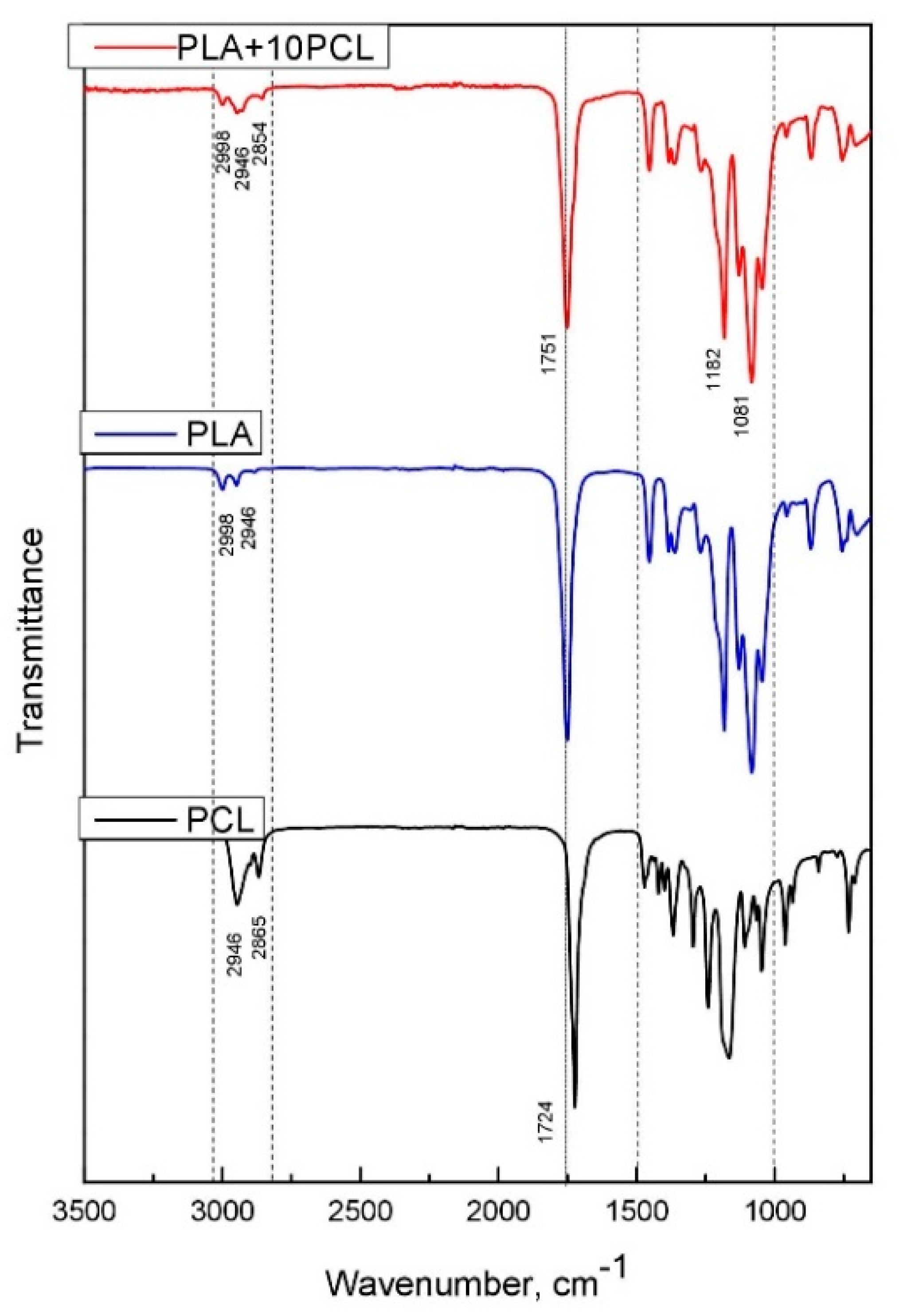
3.1. Analysis of Material Composition and Structure
3.2. Mechanical Properties
3.3. Shape Memory Effect
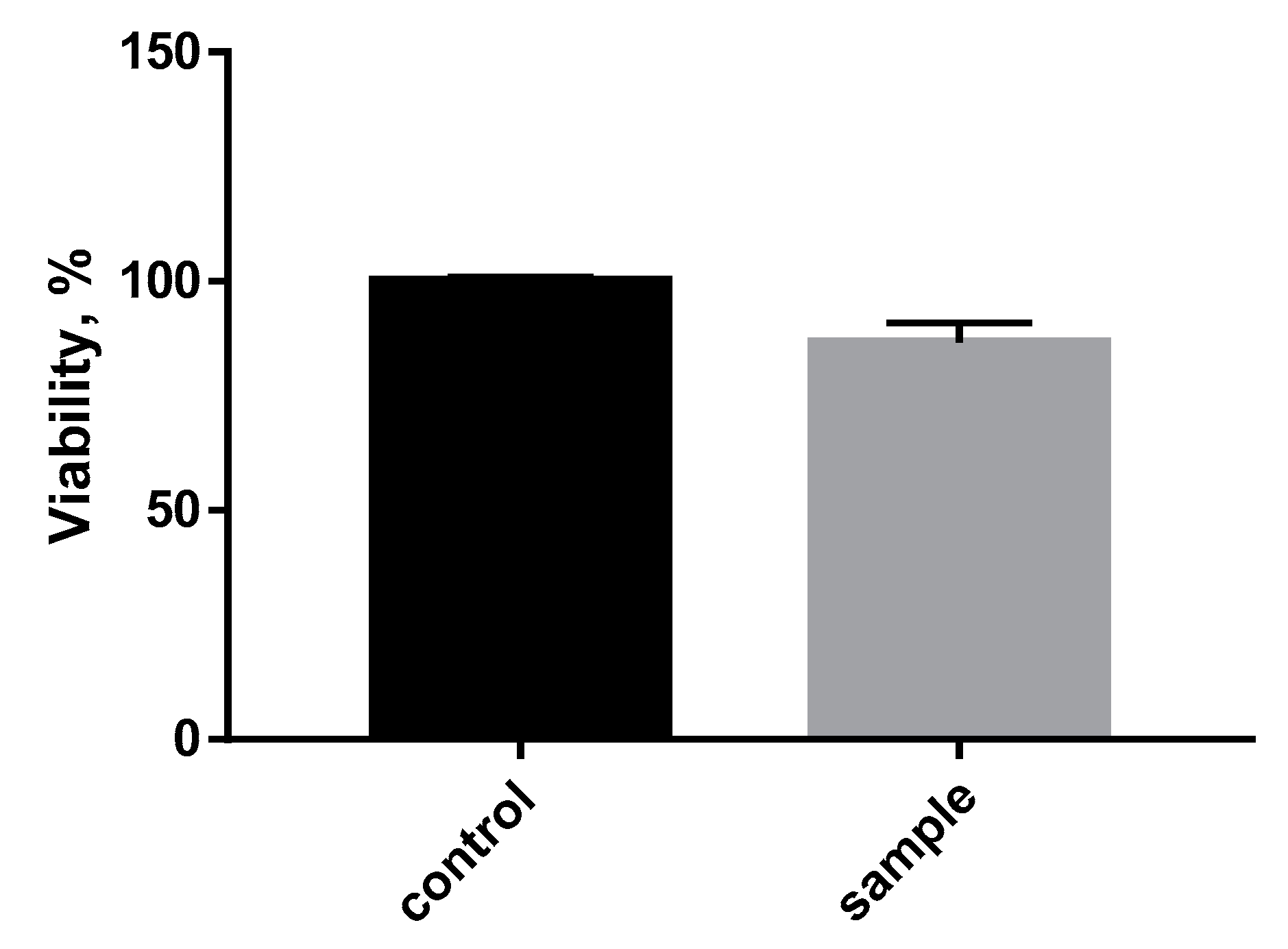
3.4. Biological Compatibility Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hornat, C.C.; Urban, M.W. Shape Memory Effects in Self-Healing Polymers. Prog. Polym. Sci. 2020, 102, 101208. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, G.; Singh, S.; Jha, K.; Prakash, C. 3D printed biodegradable functional temperature-stimuli shape memory polymer for customized scaffoldings. JMBBM 2020, 108, 103781. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Hu, J.; Hoffmann, O.; Zhang, Y.; Ng, F.; Qin, T.; Guo, X. Self-fitting shape memory polymer foam inducing bone regeneration: A rabbit femoral defect study. BBA-General Subj. 2018, 1862, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Kolesov, I.; Dolynchuk, O.; Radusch, H.J. Additives and surface properties of implants and delivery tools in biomedical applications. Express Polym. Lett. 2015, 9, 255–276. [Google Scholar] [CrossRef]
- Maksimkin, A.; Kaloshkin, S.; Zadorozhnyy, M.; Tcherdyntsev, V. Comparison of shape memory effect in UHMWPE for bulk and fiber state. JAllC 2014, 586, S214–S217. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, S.; Xiao, Y.; Yu, X.; Li, X.; Wu, P. Shape memory effect of poly(d,l-lactide)/Fe3O4 nanocomposites by inductive heating of magnetite particles. Colloids Surf. B 2009, 71, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Wu, J.M.; Song, F.; Li, D.D.; Wang, X.L.; Chen, L.; Wang, Y.Z. Flexible and electro-induced shape memory Poly(Lactic Acid)-based material constructed by inserting a main-chain liquid crystalline and selective localization of carbon nanotubes. Compos. Sci. Technol. 2019, 173, 1–6. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, X. A fast water-induced shape memory polymer based on hydroxyethyl cellulose/graphene oxide composites. Compos Part A Appl. Sci. Manuf. 2017, 103, 9–16. [Google Scholar] [CrossRef]
- Molavi, F.K.; Ghasemi, I.; Messori, M.; Esfandeh, M. Nanocomposites based on poly(L-lactide)/poly(ε-caprolactone) blends with triple-shape memory behavior: Effect of the incorporation of graphene nanoplatelets (GNps). Compos. Sci. Technol. 2017, 151, 219–227. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-Memory Polymers. Angew. Chem. Int. Edit. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sandstroröm, R.; Miyazaki, S. Shape-memory materials and hybrid composites for smart systems: Part I Shape-memory materials. J. Mater. Sci. 1998, 33, 3743–3762. [Google Scholar] [CrossRef]
- Liu, Y.; Gall, K.; Dunn, M.L.; McCluskey, P. Thermomechanics of shape memory polymer nanocomposites. Mech. Mater. 2004, 36, 929–940. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Zhang, F.; Leng, J.; Liu, Y. Shape memory polymers and their composites in biomedical applications. Mater. Sci. Eng. C 2019, 97, 864–883. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Rigogliuso, S.; Ghersi, G. Integration of PCL and PLA in a monolithic porous scaffold for interface tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 63, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Pengyan, W. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Senatov, F.S.; Zadorozhnyy, M.Y.; Niaza, K.V.; Medvedev, V.V.; Kaloshkin, S.D.; Anisimova, N.Y.; Kiselevskiy, M.V.; Yang, K.C. Shape memory effect in 3D-printed scaffolds for self-fitting implants. Eur. Polym. J. 2017, 93C, 222–231. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Xu, J.; Song, J. Thermal Responsive Shape Memory Polymers for Biomedical Applications. Orthop. Phys. Rehabil. Publ. Present. 2011, 125–142. [Google Scholar]
- Leonés, A.; Sonseca, A.; López, D.; Fiori, S.; Peponi, L. Shape memory effect on electrospun PLA-based fibers tailoring their thermal response. Eur. Polym. J. 2019, 117, 217–226. [Google Scholar] [CrossRef]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.S. Incorporation of poly (ethylene glycol) grafted cellulose nanocrystals in poly (lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef]
- Boyacioglu, S.; Kodal, M.; Ozkoc, G. A comprehensive study on shape memory behavior of PEG plasticized PLA/TPU bio-blends. Eur. Polym. J. 2020, 122, 109372. [Google Scholar] [CrossRef]
- Dogan, S.K.; Boyacioglu, S.; Kodal, M.; Gokce, O.; Ozkoc, G. Thermally induced shape memory behavior, enzymatic degradation and biocompatibility of PLA/TPU blends: “Effects of compatibilization”. J. Mech. Behav. Biomed. Mater. 2017, 71, 349–361. [Google Scholar] [CrossRef]
- Zhang, C.; Xiong, X. Miscibility, crystallization and morphology of poly(β-hydroxybutyrate)/poly(d,l-lactide) blends. Polymer 1996, 37, 235–241. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Duan, Y.X.; Noda, I. Structure, Dispersibility, and Crystallinity of Poly(hydroxybutyrate)/Poly(l-lactic acid) Blends Studied by FT-IR Microspectroscopy and Differential Scanning Calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Peponi, L.; Sessini, V.; Arrieta, M. Thermally-activated shape memory effect on biodegradable nanocomposites based on PLA/PCL blend reinforced with hydroxyapatite. Polym. Degrad. Stab. 2018, 151, 36–51. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Marcos-Fernandez, A.; Kenny, J.M.; Peponi, L. Crystallization behavior of diblock copolymers based on PCL and PLLA biopolymers. J. Appl. Crystallogr. 2014, 47, 1948–1957. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Giacobazzi, G.; Rizzuto, M.; Zubitur, M.; Mugica, A.; Caretti, D.; Miller, A.J. Crystallization kinetics as a sensitive tool to detect degradation in poly(lactide)/poly(ε-caprolactone)/ PCL-co-PC copolymers blends. Polym. Degrad. Stab. 2019, 168, 108939. [Google Scholar] [CrossRef]
- Sartore, L.; Inverardi, N.; Pandini, S.; Bignotti, F.; Chiellini, F. PLA/PCL-based foams as scaffolds for tissue engineering applications. Mater. Today Proc. 2019, 7, 410–417. [Google Scholar] [CrossRef]
- Huang, M.H.; Li, S.; Vert, M. Synthesis and degradation of PLA–PCL–PLA triblock copolymer prepared by successive polymerization of ε-caprolactone and dl-lactide. Polymer 2004, 45, 8675–8681. [Google Scholar] [CrossRef]
- Sessini, V.; Navarro-Baena, I.; Arrieta, M.P.; Dominici, F.; López, D.; Torre, L.; Peponi, L. Effect of the addition of polyester-grafted-cellulose nanocrystals on the shape memory properties of biodegradable PLA/PCL nanocomposites. Polym. Degrad. Stab. 2018, 152, 126–138. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Huang, B. Favorable Thermoresponsive Shape Memory Effects of 3D Printed Poly(Lactic Acid)/Poly(ε-Caprolactone) Blends Fabricated by Fused Deposition Modeling. Macromol. Mater. Eng. 2020, 305, 2000295. [Google Scholar] [CrossRef]
- Naddeo, M.; Sorrentino, A.; Pappalardo, D. Thermo-Rheological and Shape Memory Properties of Block and Random Copolymers of Lactide and ε-Caprolactone. Polymers 2021, 13, 627. [Google Scholar] [CrossRef]







| Young’s Modulus, MPa | Yield Strength, MPa | |
|---|---|---|
| PLA | 3295.3 ± 170.0 | 104.5 ± 2.5 |
| PLA + 10%PCL | 3108.4 ± 225.7 | 85.3 ± 3.9 |
| Material | f, Hz | T, °C | tan δ | Slope | Correlation Coefficient | ΔEa, kJ/mol |
|---|---|---|---|---|---|---|
| PLA | 1 | 65.7 | 2.196 | −32.59 | 0.95 | 271 |
| 3 | 68.2 | 0.637 | ||||
| 5 | 71.8 | 0.705 | ||||
| 7 | 72.4 | 0.701 | ||||
| 10 | 73.0 | 0.692 | ||||
| PLA + 10%PCL | 1 | 61.8 | 1.225 | −22.50 | 0.95 | 186 |
| 3 | 67.1 | 0.637 | ||||
| 5 | 71.4 | 0.705 | ||||
| 7 | 72.0 | 0.701 | ||||
| 10 | 72.3 | 0.692 | ||||
| PCL | 1 | 61.6 | 0.118 | −90.56 | 0.945 | 748 |
| 3 | 63.4 | 0.159 | ||||
| 5 | 63.8 | 0.140 | ||||
| 7 | 64.1 | 0.126 | ||||
| 10 | 64.2 | 0.098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukova, P.A.; Senatov, F.S.; Zadorozhnyy, M.Y.; Chmelyuk, N.S.; Zaharova, V.A. Polymer Composite Materials Based on Polylactide with a Shape Memory Effect for “Self-Fitting” Bone Implants. Polymers 2021, 13, 2367. https://doi.org/10.3390/polym13142367
Zhukova PA, Senatov FS, Zadorozhnyy MY, Chmelyuk NS, Zaharova VA. Polymer Composite Materials Based on Polylactide with a Shape Memory Effect for “Self-Fitting” Bone Implants. Polymers. 2021; 13(14):2367. https://doi.org/10.3390/polym13142367
Chicago/Turabian StyleZhukova, P. A., F. S. Senatov, M. Yu. Zadorozhnyy, N. S. Chmelyuk, and V. A. Zaharova. 2021. "Polymer Composite Materials Based on Polylactide with a Shape Memory Effect for “Self-Fitting” Bone Implants" Polymers 13, no. 14: 2367. https://doi.org/10.3390/polym13142367