Metal-Based Nanocomposite Materials for Efficient Photocatalytic Degradation of Phenanthrene from Aqueous Solutions
Abstract
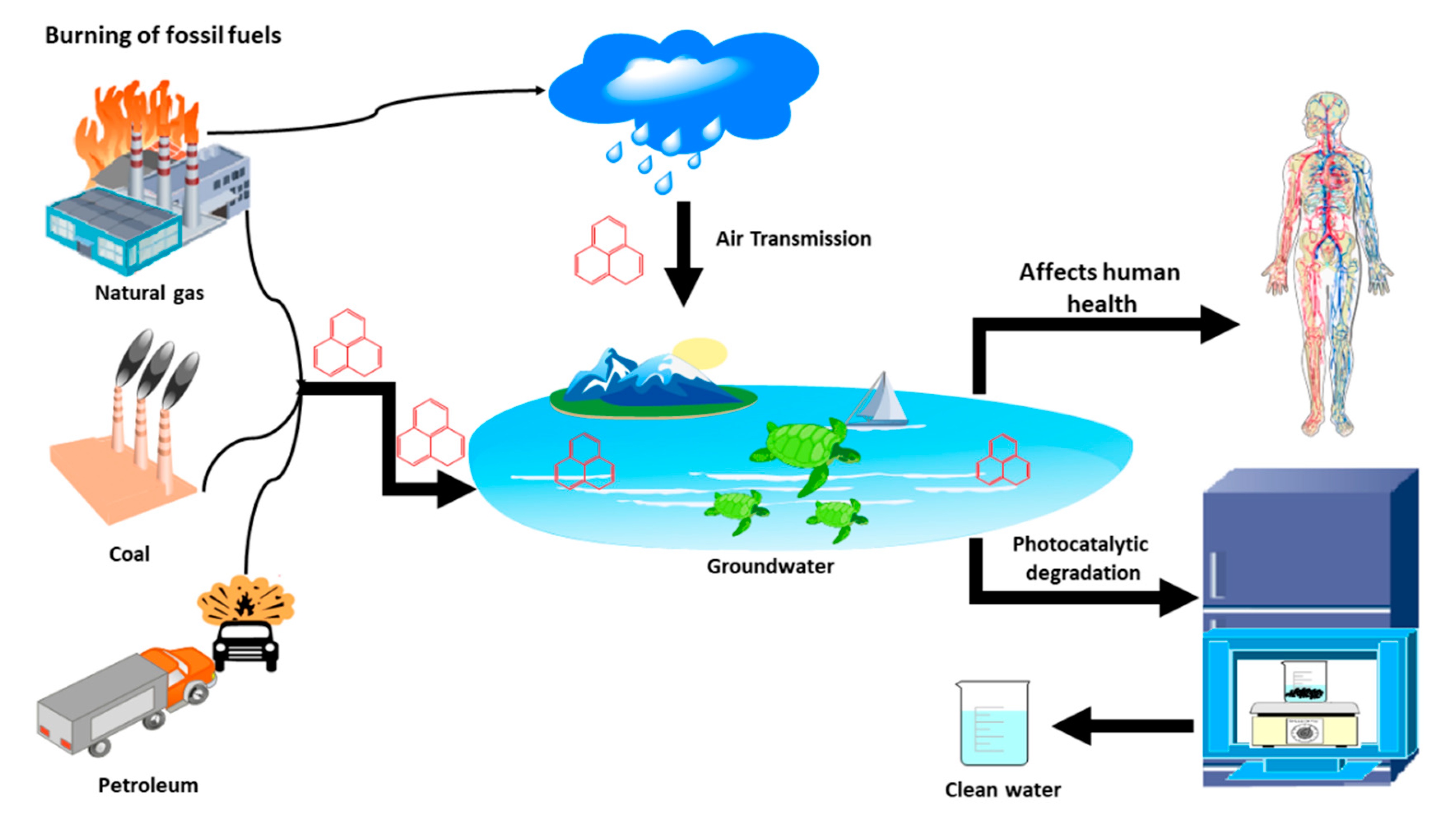
:1. Introduction
2. Photocatalytic Degradation of Phenanthrene
2.1. Titanium Dioxide-Based Photocatalysts
2.2. Iron-Based Photocatalysts
2.3. Silver-Based Photocatalysts
2.4. Carbon-Based Photocatalysts
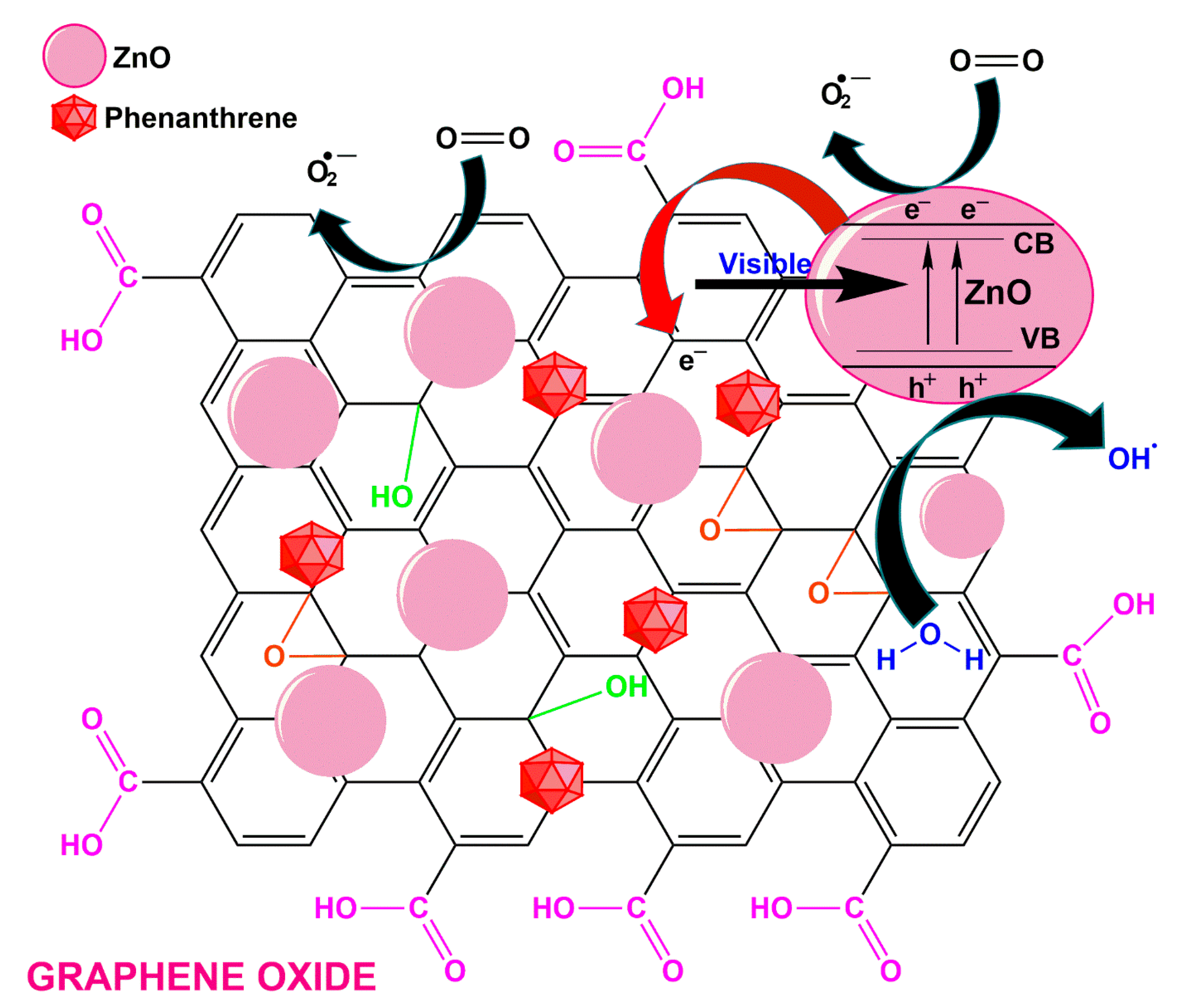
2.5. Zinc-Based Photocatalysts
3. Mechanism and Degradation Pathways of Phenanthrene
4. Knowledge Gaps and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerniglia, C.E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 1992, 3, 351–368. [Google Scholar] [CrossRef]
- Wilson, S.C.; Jones, K.C. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): A review. Environ. Pollut. 1993, 81, 229–249. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Naidu, R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo[a]pyrene. Int. Biodeterior. Biodegrad. 2000, 45, 57–88. [Google Scholar] [CrossRef]
- Beltran, F.J.; González, M.; Ribas, F.J.; Alvarez, P.; Beltran, F.J.; González, M.; Ribas, F.J.; Alvarez, P. Fenton Reagent Advanced Oxidation of Polynuclear Aromatic Hydrocarbons in Water. Water Air Soil Pollut. 1998, 105, 685–700. [Google Scholar] [CrossRef]
- Beltran, F.J.; Ovejero, G.; Rivas, J. Oxidation of Polynuclear Aromatic Hydrocarbons in Water. Ozone Combined with Hydrogen Peroxide. Ind. Eng. Chem. Res. 1996, 35, 891–898. [Google Scholar] [CrossRef]
- Menzie, C.A.; Potocki, B.B.; Santodonato, J. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 2002, 26, 1278–1284. [Google Scholar] [CrossRef]
- Busetti, F.; Heitz, A.; Cuomo, M.; Badoer, S.; Traverso, P. Determination of sixteen polycyclic aromatic hydrocarbons in aqueous and solid samples from an Italian wastewater treatment plant. J. Chromatogr. A 2006, 1102, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoli, E.; Samara, C.; Manoli, E.; Samara, C. The removal of Polycyclic Aromatic Hydrocarbons in the wastewater treatment process: Experimental calculations and model predictions. Environ. Pollut. 2008, 151, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, S.; Deschaux, P. The effects of polycyclic aromatic hydrocarbons on the immune system of fish: A review. Aquat. Toxicol. 2006, 77, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.F.; Haus, N.W.; Sukkariyah, B.F.; Haering, K.C.; Daniels, W.L. Remediation of PAH-contaminated soils and sediments: A literature rview. In Environmental Soil Science, Wetland Restoration and Mined Land Reclamation; CSES: Sacramento, CA, USA, 2011; pp. 1–102. [Google Scholar]
- Zhang, Y.; Zhang, L.; Huang, Z.; Li, Y.; Li, J.; Wu, N.; He, J.; Zhang, Z.; Liu, Y.; Niu, Z.; et al. Pollution of polycyclic aromatic hydrocarbons (PAHs) in drinking water of China: Composition, distribution and influencing factors. Ecotoxicol. Environ. Saf. 2019, 177, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nykamp, J.; Huang, X.-D.; Gerhardt, K.; Dixon, D.G.; Greenberg, B.M. Examination of The Mechanism of Phenanthrenequinone Toxicity to Vibrio Fischeri: Evidence for A Reactive Oxygen Species—Mediated Toxicity Mechanism. Environ. Toxicol. Chem. 2009, 28, 1655–1662. [Google Scholar] [CrossRef]
- Kasumba, J.; Holmén, B.A. Heterogeneous ozonation reactions of PAHs and fatty acid methyl esters in biodiesel particulate matter. Atmos. Environ. 2018, 175, 15–24. [Google Scholar] [CrossRef]
- Bhateria, R.; Jain, D. Water quality assessment of lake water: A review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Hu, W.; Xu, J.; Ma, R. Development of a visualization platform oriented to Lake water quality targets management—A case study of Lake Taihu. Ecol. Inform. 2017, 41, 40–53. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Fu, X.; Gu, X.; Lu, S.; Xu, M.; Miao, Z.; Zhang, X.; Zhang, Y.; Xue, Y.; Qiu, Z.; Sui, Q. Enhanced degradation of benzene in aqueous solution by sodium percarbonate activated with chelated-Fe(II). Chem. Eng. J. 2016, 285, 180–188. [Google Scholar] [CrossRef]
- Saleh, T. The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl. Surf. Sci. 2011, 257, 7746–7751. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Singh, J.; Choi, J.-S.; Chang, Y.-Y.; Yang, J.-K.; Karri, R.R.; Koduru, J.R. Multivariate modeling via artificial neural network applied to enhance methylene blue sorption using graphene-like carbon material prepared from edible sugar. J. Mol. Liq. 2018, 265, 416–427. [Google Scholar] [CrossRef]
- Akinpelu, A.A.; Ali, E.; Johan, M.R.; Saidur, R.; Qurban, M.A.; Saleh, T.A. Polycyclic aromatic hydrocarbons extraction and removal from wastewater by carbon nanotubes: A review of the current technologies, challenges and prospects. Process. Saf. Environ. Prot. 2019, 122, 68–82. [Google Scholar] [CrossRef]
- Liang, S.; Li, G.; Tian, R. Multi-walled carbon nanotubes functionalized with a ultrahigh fraction of carboxyl and hydroxyl groups by ultrasound-assisted oxidation. J. Mater. Sci. 2016, 51, 3513–3524. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Sharma, M.; Nandy, A.; Taylor, N.; Venkatesan, S.V.; Kollath, V.O.; Karan, K.; Thangadurai, V.; Tsesmetzis, N.; Gieg, L.M.; Sharma, M.; et al. Bioelectrochemical remediation of phenanthrene in a microbial fuel cell using an anaerobic consortium enriched from a hydrocarbon-contaminated site. J. Hazard. Mater. 2020, 389, 121845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fang, Z.; Liu, Y.; Liu, Y.; Xu, Y.; Ruan, X.; Zhang, X.; Cao, W. Phosphorus speciation and bioavailability of sewage sludge derived biochar amended with CaO. Waste Manag. 2019, 87, 71–77. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Zhao, W.; Wang, Y.; Li, Z.; Lin, Z. A Simple Route to Reduced Graphene Oxide-Draped Nanocomposites with Markedly Enhanced Visible-Light Photocatalytic Performance. Small 2016, 12, 4077–4085. [Google Scholar] [CrossRef]
- Rachna; Rani, M.; Shanker, U. Degradation of tricyclic polyaromatic hydrocarbons in water, soil and river sediment with a novel TiO2 based heterogeneous nanocomposite. J. Environ. Manag. 2019, 248, 109340. [Google Scholar] [CrossRef]
- Cheng, K.; Cai, Z.; Fu, J.; Sun, X.; Sun, W.; Chen, L.; Zhang, D.; Liu, W. Synergistic adsorption of Cu(II) and photocatalytic degradation of phenanthrene by a jaboticaba-like TiO2/titanate nanotube composite: An experimental and theoretical study. Chem. Eng. J. 2019, 358, 1155–1165. [Google Scholar] [CrossRef]
- Rani, C.N.; Karthikeyan, S. Performance of an indigenous integrated slurry photocatalytic membrane reactor (PMR) on the removal of aqueous phenanthrene (PHE). Water Sci. Technol. 2018, 77, 2642–2656. [Google Scholar] [CrossRef]
- Bai, H.; Zhou, J.; Zhang, H.; Tang, G. Enhanced adsorbability and photocatalytic activity of TiO2 -graphene composite for polycyclic aromatic hydrocarbons removal in aqueous phase. Colloids Surf. B Biointerfaces 2017, 150, 68–77. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, Z.; Wang, T.; O’Reilly, S.; Liu, W.; Zhao, D. A new type of cobalt-deposited titanate nanotubes for enhanced photocatalytic degradation of phenanthrene. Appl. Catal. B Environ. 2016, 187, 134–143. [Google Scholar] [CrossRef]
- Luo, Z.-H.; Wei, C.-L.; He, N.-N.; Sun, Z.-G.; Li, H.-X.; Chen, D. Correlation between the Photocatalytic Degradability of PAHs over Pt/TiO2-SiO2 in Water and Their Quantitative Molecular Structure. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wong, J.; Liu, P.; Yuan, M. Heterogeneous photocatalytic degradation of phenanthrene in surfactant solution containing TiO2 particles. J. Hazard. Mater. 2011, 191, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhao, J.; Sheng, G.; Fu, J.; Peng, P. Photocatalytic reactions of phenanthrene at TiO2/water interfaces. Chemosphere 2002, 46, 871–877. [Google Scholar] [CrossRef]
- Cai, Z.; Hao, X.; Sun, X.; Du, P.; Liu, W.; Fu, J. Highly active WO3@anatase-SiO2 aerogel for solar-light-driven phenanthrene degradation: Mechanism insight and toxicity assessment. Water Res. 2019, 162, 369–382. [Google Scholar] [CrossRef]
- Fu, J.; Kyzas, G.; Cai, Z.; Deliyanni, E.A.; Liu, W.; Zhao, D. Photocatalytic degradation of phenanthrene by graphite oxide-TiO2-Sr(OH)2/SrCO3 nanocomposite under solar irradiation: Effects of water quality parameters and predictive modeling. Chem. Eng. J. 2018, 335, 290–300. [Google Scholar] [CrossRef]
- Rani, C.N.; Karthikeyan, S. Photocatalytic Degradation of Aqueous Phenanthrene in a Slurry Photocatalytic Reactor: Optimization and Modelling. Curr. Sci. 2018, 115, 1732–1740. [Google Scholar] [CrossRef]
- Lin, H.F.; Valsaraj, K.T. A titania thin film annular photocatalytic reactor for the degradation of polycyclic aromatic hydro-carbons in dilute water streams. J. Hazard. Mater. 2003, 99, 203–219. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Zhang, J.; Du, J.; Xu, S.; Wang, C.; Zhang, D.; Tang, J.; Zhao, H.; Zhou, J. Degradation of phenanthrene by peroxymonosulfate activated with bimetallic metal-organic frameworks: Kinetics, mechanisms, and degradation products. Chem. Eng. J. 2020, 397, 125401. [Google Scholar] [CrossRef]
- Rani, M.; Rachna; Shanker, U. Rachna; Shanker, U. Metal oxide-chitosan based nanocomposites for efficient degradation of carcinogenic PAHs. J. Environ. Chem. Eng. 2020, 8, 103810. [Google Scholar] [CrossRef]
- Li, S.-X.; Sun, S.; Wu, H.; Wei, C.; Hu, Y. Effects of electron-donating groups on the photocatalytic reaction of MOFs. Catal. Sci. Technol. 2018, 8, 1696–1703. [Google Scholar] [CrossRef]
- Kou, J.; Zhang, H.; Yuan, Y.; Li, Z.; Wang, Y.; Yu, T.; Zou, Z. Efficient Photodegradation of Phenanthrene under Visible Light Irradiation via Photosensitized Electron Transfer. J. Phys. Chem. C 2008, 112, 4291–4296. [Google Scholar] [CrossRef]
- Yu, S.; Gu, X.; Lu, S.; Xue, Y.; Zhang, X.; Xu, M.; Qiu, Z.; Sui, Q. Degradation of phenanthrene in aqueous solution by a persulfate/percarbonate system activated with CA chelated-Fe(II). Chem. Eng. J. 2018, 333, 122–131. [Google Scholar] [CrossRef]
- Shanker, U.; Jassal, V.; Rani, M. Green synthesis of iron hexacyanoferrate nanoparticles: Potential candidate for the degradation of toxic PAHs. J. Environ. Chem. Eng. 2017, 5, 4108–4120. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Pu, S.-Y.; Qian, W.; Liang, S.; Kong, L.-J.; Xia, D.-H.; Lei, Z.-X.; Du, J.-J.; Liu, H.; Yang, J.-W. Photocatalytic removal of phenanthrene and algae by a novel Ca-Ag3PO4 composite under visible light: Reactivity and coexisting effect. Chemosphere 2019, 221, 511–518. [Google Scholar] [CrossRef]
- Cai, H.; Sun, L.; Wang, Y.; Song, T.; Bao, M.; Yang, X. Unprecedented efficient degradation of phenanthrene in water by intimately coupling novel ternary composite Mn3O4/MnO2-Ag3PO4 and functional bacteria under visible light irradiation. Chem. Eng. J. 2019, 369, 1078–1092. [Google Scholar] [CrossRef]
- Yang, X.; Cai, H.; Bao, M.; Yu, J.; Lu, J.; Li, Y. Insight into the highly efficient degradation of PAHs in water over graphene oxide/Ag3PO4 composites under visible light irradiation. Chem. Eng. J. 2018, 334, 355–376. [Google Scholar] [CrossRef]
- Yang, X.; Cai, H.; Bao, M.; Yu, J.; Lu, J.; Li, Y. Highly Efficient Photocatalytic Remediation of Simulated Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Wastewater under Visible Light Irradiation by Graphene Oxide Enwrapped Ag3PO4 Composite. Chin. J. Chem. 2017, 35, 1549–1558. [Google Scholar] [CrossRef]
- Lee, H.; Anwer, H.; Park, J.-W. Graphene quantum dots on stainless-steel nanotubes for enhanced photocatalytic degradation of phenanthrene under visible light. Chemosphere 2020, 246, 125761. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmed, B.; Singh, A.; Ojha, A.K. Photodegradation of phenanthrene catalyzed by rGO sheets and disk like structures synthesized using sugar cane juice as a reducing agent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, R.; Li, C.; Guo, W.; Han, X.; He, F.; Ma, Y.; Xing, B. Selective removal of polycyclic aromatic hydrocarbons (PAHs) from soil washing effluents using biochars produced at different pyrolytic temperatures. Bioresour. Technol. 2014, 163, 193–198. [Google Scholar] [CrossRef]
- Rani, M.; Rachna; Shanker, U. Mineralization of carcinogenic anthracene and phenanthrene by sunlight active bimetallic oxides nanocomposites. J. Colloid Interface Sci. 2019, 555, 676–688. [Google Scholar] [CrossRef]
- Abbasi, M.; Rafique, U.; Murtaza, G.; Ashraf, M.A. Synthesis, characterisation and photocatalytic performance of ZnS coupled Ag2S nanoparticles: A remediation model for environmental pollutants. Arab. J. Chem. 2018, 11, 827–837. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, L.; Wang, Y.; Ma, C.; Zou, Z. One-pot synthesis of bifunctionalized TiO2 mesoporous photocatalyst with visible light response. J. Porous Mater. 2015, 22, 313–319. [Google Scholar] [CrossRef]
- Bellardita, M.; Loddo, V.; Mele, A.; Panzeri, W.; Parrino, F.; Pibiri, I.; Palmisano, L. Photocatalysis in dimethyl carbonate green solvent: Degradation and partial oxidation of phenanthrene on supported TiO2. RSC Adv. 2014, 4, 40859–40864. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Kou, J.H.; Guo, D.M.; Yang, J.; Liu, H.L.; Yu, H.X.; Chu, S.; Jiang, K.R.; Wang, Y.; Zou, Z.G. Synthesis of thiol-functionalized TiO2 nanocomposite and photocatalytic degradation for PAH under visible light irradiation. Chin. Chem. Lett. 2009, 20, 1366–1370. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Boppella, R.; Jeong, Y.-J.; Park, J.; Kim, Y.-B.; Choi, B.; Park, S.H.; Jung, S.; Lee, D.-W. Facile in-situ formation of rGO/ZnO nanocomposite: Photocatalytic remediation of organic pollutants under solar illumination. Mater. Chem. Phys. 2018, 218, 218–228. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Dantas, R.F.; Bayarri, B.; González, O.; Giménez, J.; Esplugas, S.; Machulek, A. Photocatalytic mechanism of metoprolol oxidation by photocatalysts TiO2 and TiO2 doped with 5% B Primary active species and intermediates. Appl. Catal. B Environ. 2016, 194, 111–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Identification of Bi2WO6 as a highly selective visible-light photocatalyst toward oxidation of glycerol to dihydroxyacetone in water. Chem. Sci. 2013, 4, 1820–1824. [Google Scholar] [CrossRef]
- Teel, A.; Watts, R.J. Degradation of carbon tetrachloride by modified Fenton’s reagent. J. Hazard. Mater. 2002, 94, 179–189. [Google Scholar] [CrossRef]
- Woo, O.; Chung, W.; Wong, K.; Chow, A.T.; Wong, P. Photocatalytic oxidation of polycyclic aromatic hydrocarbons: Intermediates identification and toxicity testing. J. Hazard. Mater. 2009, 168, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Li, Z.; Yuan, Y.; Zhang, H.; Wang, Y.; Zou, Z. Visible-Light-Induced Photocatalytic Oxidation of Polycyclic Aromatic Hydrocarbons over Tantalum Oxynitride Photocatalysts. Environ. Sci. Technol. 2009, 43, 2919–2924. [Google Scholar] [CrossRef] [PubMed]
- Shanker, U.; Jassal, V.; Rani, M. Degradation of toxic PAHs in water and soil using potassium zinc hexacyanoferrate nanocubes. J. Environ. Manag. 2017, 204, 337–348. [Google Scholar] [CrossRef] [PubMed]

| S. N. | Photocatalyst | Experimental Conditions | Detector/Column | Light Intensity | Wavelength λ (nm)/Light Power | Reaction Kinetics | Efficiency (%) | References |
|---|---|---|---|---|---|---|---|---|
| I. | Titanium dioxide-based photocatalysts | |||||||
| 1. | TiO2@ZnHCF | pH: 5–9; temp.: 32.3 ± 3.8 °C; dose: 5–25 mg; time: 24 h; conc.: 2–10 mg/L | UV-spectrophotometer | (463 ± 181) W/m2 series: 150–823) | 350/- | First-order | 95 | [27] |
| 2. | Jaboticaba-like TiO2/titanate nanotube composite | pH: 5.0 ± 0.2; temp.: 25 ± 0.5 °C; dose: 0.5 g/L; time: 4 h; conc.: 200 μg/L | HPLC (Agilent 1260, Buffalo, NY, USA) with UV detector/cuboid quartz reactor (5 cm × 5 cm × 12 cm) | 2.5 mW/cm2 | 365/150 W | Pseudo-second-order | 92.3 | [28] |
| 3. | Nano TiO2 | pH: 7; temp.: 300 °C; dose: 0.5 g/L; time: 6 h; conc.: 1000 μg/L | Photocatalytic membrane reactor (PMR)/column (305 mm × 305 mm) | - | 254/16 W | - | 97 | [29] |
| 4. | TiO2 graphene composite (P25-2.5% GR) | pH: 9.2; temp.: room temp.; dose: 25 mg; time: 2 h; conc.: 2 μg/mL | Pyrex glass reactor with water jacket/ HPLC | 4.8 mW/cm2 | >320/300 W | Pseudo-first-order | 81 | [30] |
| 5. | Co-TNTs-600 | pH: 7.0 ± 0.2; temp.: 25 ± 0.2 °C; dose: 1.0 g/L; time: 12 h; conc.: 200 μg/L | Agilent 1260 Infinity HPLC/Poroshell 120 EC- C18 column (50 mm × 4.6 mm, 2.7 μm) | (85 ± 0.5) mW/cm2 | 254/450 W | First-order | 98.6 | [31] |
| 6. | Pt/TiO2-SiO2 | pH: NA; temp.: NA; dose: NA; time: NA; conc.: 5 × 10−8 M | High performance liquid chromatography; Hitachi L-7300 HPLC with a fluorescence detector Hitachi L-7485 | - | 368/- | Pseudo-first-order | 34.5 | [32] |
| 7. | TiO2 particles | pH: >6.8; temp.: 300 °C; dose: 0.2 g/L; time: 15 min; conc.: 1 mg/L | Agilent 6890 N GC system/capillary column (30 m × 320 μm × 0.25 μm) | 6 × 10−7 Einstein/L/s | 253.7/8 W | Pseudo-second-order | 100 | [33] |
| 8. | Aqueous TiO2 suspensions | pH: 2–10; temp.: NA; dose: 50 mg; time: 90 min; conc.: 5.6 × 10−5 mol/g | GC-MS, Trio-2000/BPX 70 column (30 m × 0.25 mm) | 30 mW/m2 | >320/100 W | - | Over 90 | [34] |
| 9. | WO3@anatase-SiO2 aerogel | pH: 10.7; temp.: 30 ± 5 °C; dose: 1.0 wt.%; time: 3 h; conc.: 500 μg/L | HPLC system with UV detector (1260 series, Agilent, USA) | 100 mW/cm2 | NA/300 W | First-order | 95 | [35] |
| 10. | Graphite oxide- TiO2-Sr(OH)2/SrCO3 | pH: 7.0 ± 0.2; temp.: 22 ± 1 °C; dose: 50 mg/L; time: 6 h; conc.: 1 mg/L | Cylindrical quartz tank reactor with Pyrex pillar/column (80 mm × 75 mm) | 100 mW/cm2 | 250/- | Pseudo-first-order | - | [36] |
| 11. | Nano TiO2 | pH: 3; temp.: 21 ± 1 °C; dose: 0.5 g/L; time: 3 h; conc.: 1000 μg/L | GC-MS Agilent technology system consisting of a 6890 GC equipped with DB-5 MS mid polar/column (30 m × 0.25 mm) | - | 254/16 W | Pseudo-first-order | 84 | [37] |
| 12. | Titania immobilized on a quartz tube | pH: NA; temp.: 325 °C; dose: 2.128 g; time: 3 h; conc.: 9.653 × 10−6 mol | Cylindrical glass reactor (38.2 cm long and 0.6 cm i.d) fabricated and quartz rod (38 cm long and 0.3 cm i.d)/column Phenomenex Envirosep -PP (125 mm × 3.2 mm) | 8.1 mW/cm2 | >290/- | Linear isotherm | 35–67 | [38] |
| II. | Iron-based photocatalysts | |||||||
| 1. | Peroxymonosulfate activated with bimetallic metal-organic frameworks (FeCo-BDC) | pH: 3.5; temp.: 25 °C; dose: 50 mg/L; time: 30 min; conc.: 1.0 mg/L | Cylindrical glass reactor | - | - | Pseudo-first-order | 99 | [39] |
| 2. | Iron-based chitosan nanocomposites | pH: neutral pH; temp.: 42.3 ± 4.2 °C; dose: 20 mg; time: 12 h; conc.: 2.0 mg/L | UV-spectrophotometer | (483 ± 181) W/m2 (range −150–23) | - | - | 92 | [40] |
| 3. | MIL-101(Fe)-X (X= -OH, -NH2, -COOH, -NO2, -H) | pH: 7.08; temp.: room temperature; dose: 0.05 g; time: 150 min; conc.: 10 mg/L | 1260 HPLC | 35 W/m2 | >330/175 W | - | 99.98, 99.6, 90.01, 84.89, 77.01 respectively | [41] |
| 4. | Fe3+ ions | pH: NA; temp.: 280 °C; dose: 1.20 × 10−4 mol; time: 3 h; conc.:1.68 × 10−5 mol | Glass reactor with a Xe lamp | - | >420/300 W | - | 100 | [42] |
| 5. | Persulfate/percarbonate system activated with citric acid (CA) chelated Fe (II) | pH: 9; temp.: 20 ± 0.5 °C; dose: 0.5 mM; time: 60 min; conc.: 1.0 mg/L | High performance liquid chromatography (HPLC), LC-20 AT, Shimadzu, Japan with an UV detector/C18 reverse phase column (Inertsil ODS) | - | 254/- | Pseudo-first-order | 92 | [43] |
| 6. | FeHCF nanoparticles | pH: 7; temp.: 31.1 ± 1.7 °C; dose: 25 mg; time: 48 h; conc: 50 mg/L | Gas chromatograph (GC 1300) coupled with mass spectrometer (TSQ8000) | 452 ± 183 W/m2 | 254/- | - | 87 | [44] |
| III. | Silver-based photocatalysts | |||||||
| 1. | Novel Ca-Ag3PO4 composite | pH: 8.01 ± 0.02; temp.: 25 ± 0.1 °C; dose: 0.9 g/L; time: 12 h; conc.: 0.3 mg/L | Photoreactor equipped with a Xenon lamp | - | >420/500 W | - | 96 | [45] |
| 2. | Mn3O4/MnO2-cubic Ag3PO4 composite | pH: NA; temp.: 25 ± 1 °C; dose: 0.4 wt.%; time: 20 min; conc.: 10 mg/L | GC-MS device (Agilent 7890 A GC with 5975C series mass spectrometry)/Agilent DB EUPAH column (122–5532, 30 m × 250 μm × 0.25 μm) | 150 mW/m2 | >420/300 W | First-order | 96.2 | [46] |
| 3. | GO/Ag3PO4 composite | pH: NA; temp.: NA; dose: 1.0 g/L; time: 7 min; conc.: 600 μg/L | Agilent 1260 Infinity high performance liquid chromatography (HPLC) system/Venusil XBP-C18 column (5 μm, 4.6 mm × 250 mm) | 150 mW/m2 | 254/300 W | First-order | 100 | [47] |
| 4. | Ag3PO4 /GO | pH: NA; temp.: 25 ± 2 °C; dose: 3 wt.%; time: 5 min; conc.: 600 μg/L | Agilent 1260 Infinity high performance liquid chromatography (HPLC) system/Venusil XBP-C18 column (5 μm, 4.6 mm × 250 mm) | 150 mW/m2 | >420/300 W | Pseudo-first-order | 100 | [48] |
| IV. | Carbon-based photocatalysts | |||||||
| 1. | SSNT@GQD with persulfate and without persulfate | pH: 7.0; temp.: 300 °C; dose: 0.268 g/L with 1 mM PS; time: 4 h; conc.: 0.1 mM | HPLC, Younglin 9100 equipped with diode array detector (DAD) Younglin 9120/Zorbax SB-C18 column (4.6 mm × 150 mm, 5 μm particle size, Agilent) | 1.65 mW/cm2 | 325/4 W | Pseudo-first-order | 81 and 91 respectively | [49] |
| 2. | rG1 and rG2 Slurry | pH: NA; temp.: 150 °C; dose: 2 mg; time: 280 min; conc.: 50 mL | Photoreactor with a UV lamp | - | 254/16 W | - | 25–30 | [50] |
| 3. | Biochar | pH: NA; temp.: 25 ± 1 °C; dose: 6 g/L; time: 6 h; conc.: 9.07 ± 0.08 mg/L | DIONEX U3000 HPLC (Dionex, USA) using an ultraviolet detector/reversed phase SUPELCOSIL LC-PAH column (150 mm × 4.6 mm, 5 μm) | - | 254/16 W | - | 71.8–98.6 | [51] |
| V. | Zinc-based photocatalysts | |||||||
| 1. | NiO-ZnO | pH: neutral pH ~7; temp.: 33.4 ± 3.7 °C; dose: 80 mg; time: 12 h; conc.: 2 mg/L | Agilent (7890, USA) with HP5-MS capillary column | - | 350/- | First-order | 93 | [52] |
| 2. | ZnS coupled Ag2S nanoparticles | pH: NA; temp.: 598 °C; dose: NA; time: 90 min; conc.: NA | Agilent 1260 HPLC | - | 300–800/- | - | > 80 | [53] |
| S.N. | Photocatalyst | Active Species | Degradation Products | References |
|---|---|---|---|---|
| 1. | TiO2@ZnHCF | •OH | 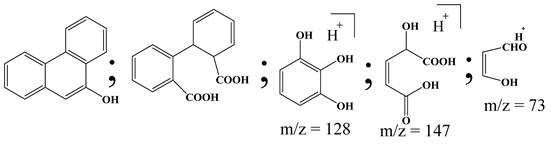 | [27] |
| 2. | Jaboticaba-like TiO2/titanate nanotube composite | •OH | 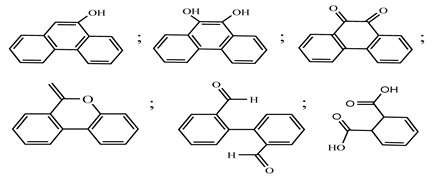 | [28] |
| 3. | TiO2 graphene composite (P25-2.5% GR) | •OH, h+ and •O2− | 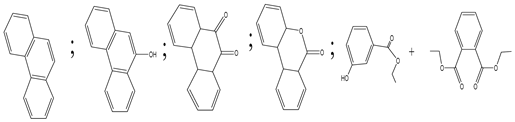 | [30] |
| 4. | Co-TNTs-600 | •OH | 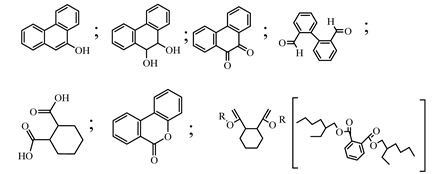 | [31] |
| 5. | TiO2 particles | OH• | 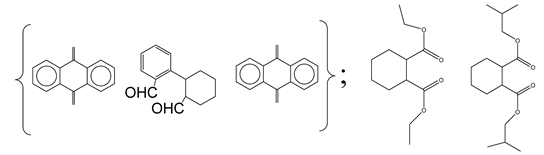 | [33] |
| 6. | WO3@anatase-SiO2 aerogel | •OH and O2•− | 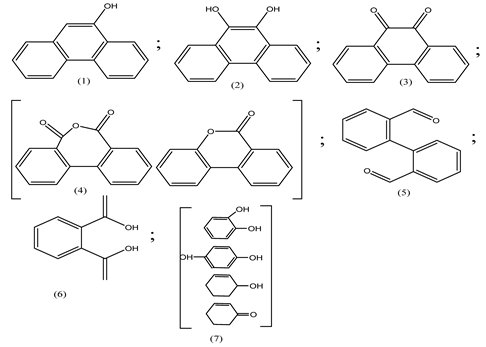 | [35] |
| 7. | Graphite oxide-TiO2-Sr(OH)2/SrCO3 | •OH and O2•− | 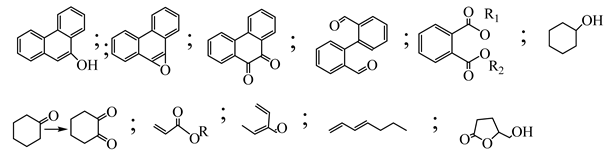 | [36] |
| 8. | Titania immobilized on a quartz tube | OH• and •O2− |  | [38] |
| 9. | Peroxymonosulfate activated with bimetallic metal-organic frameworks (FeCo-BDC) | SO4•−, •OH and O2•− | 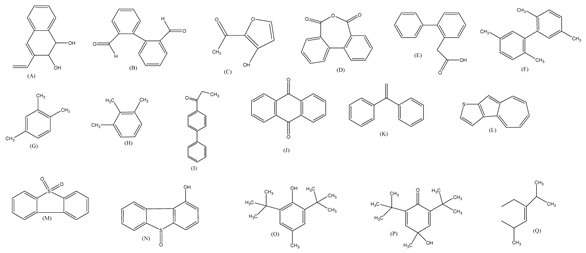 | [39] |
| 10. | Iron-based chitosan nanocomposite | •OH, h+ and •O2− |  | [40] |
| 11. | Fe3+ ions | O2 | 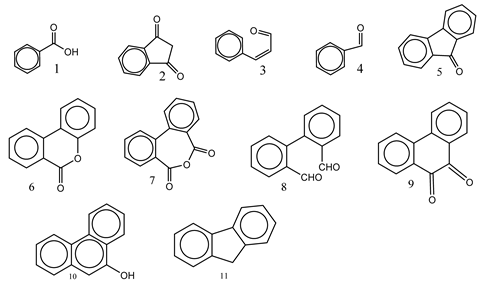 | [42] |
| 12. | Persulfate/percarbonate system activated with citric acid (CA) chelated Fe (II) | •OH and SO4•− | 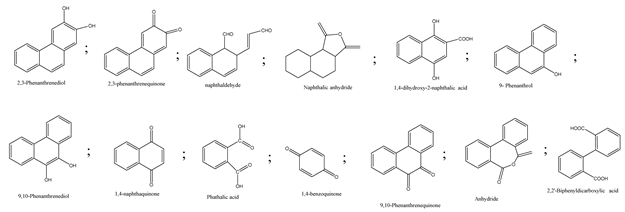 | [43] |
| 13. | Mn3O4/MnO2-cubic Ag3PO4 composite | •OH | 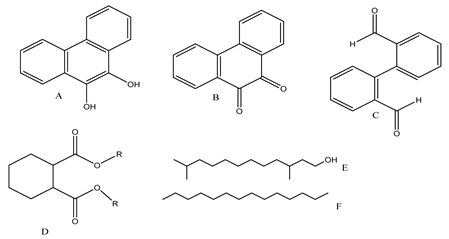 | [46] |
| 14. | GO/Ag3PO4 nanocomposite | •OH, h+ and •O2− | 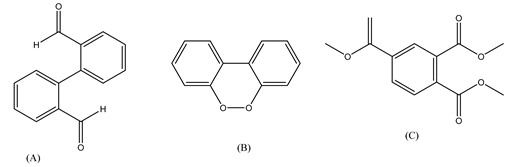 | [47] |
| 15. | SSNT@GQD with persulfate and without persulfate | SO4•−, holes and O2•− | 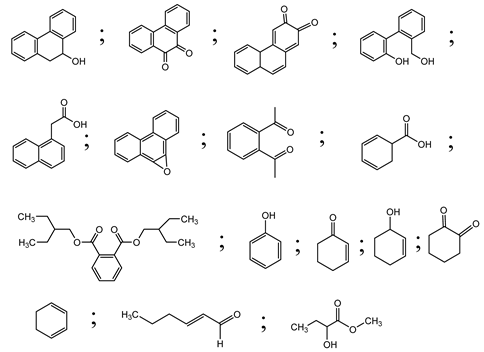 | [49] |
| 16. | NiO-ZnO | •OH | 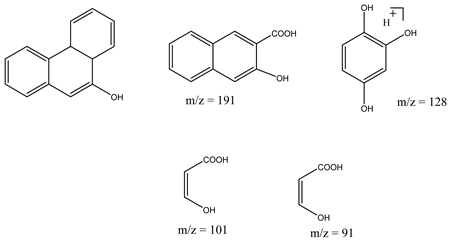 | [52] |
| 17. | TiO2 | •OH and h+ | 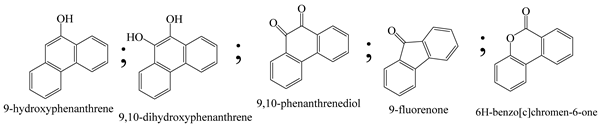 | [55] |
| 18. | Acetone system | OH• | 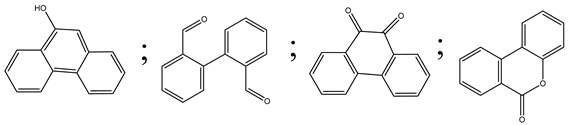 | [62] |
| 19. | Pt-TaON | •O2−, H2O2 and h+ | 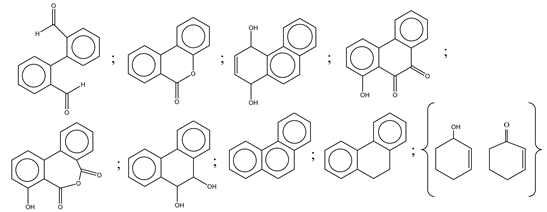 | [63] |
| 20. | KZnHCF nanocubes | •OH |  | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, H.A.; Rafatullah, M.; Ahmed Ali, K.; Siddiqui, M.R.; Khan, M.A.; Alshareef, S.A. Metal-Based Nanocomposite Materials for Efficient Photocatalytic Degradation of Phenanthrene from Aqueous Solutions. Polymers 2021, 13, 2374. https://doi.org/10.3390/polym13142374
Chauhan HA, Rafatullah M, Ahmed Ali K, Siddiqui MR, Khan MA, Alshareef SA. Metal-Based Nanocomposite Materials for Efficient Photocatalytic Degradation of Phenanthrene from Aqueous Solutions. Polymers. 2021; 13(14):2374. https://doi.org/10.3390/polym13142374
Chicago/Turabian StyleChauhan, Husn Ara, Mohd. Rafatullah, Khozema Ahmed Ali, Masoom Raza Siddiqui, Moonis Ali Khan, and Shareefa Ahmed Alshareef. 2021. "Metal-Based Nanocomposite Materials for Efficient Photocatalytic Degradation of Phenanthrene from Aqueous Solutions" Polymers 13, no. 14: 2374. https://doi.org/10.3390/polym13142374
APA StyleChauhan, H. A., Rafatullah, M., Ahmed Ali, K., Siddiqui, M. R., Khan, M. A., & Alshareef, S. A. (2021). Metal-Based Nanocomposite Materials for Efficient Photocatalytic Degradation of Phenanthrene from Aqueous Solutions. Polymers, 13(14), 2374. https://doi.org/10.3390/polym13142374









