Polymethylolacrylamide/AuNPs Nanocomposites: Electrochemical Synthesis and Functional Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solutions for the Formation of Composites
2.1.1. Chemicals
2.1.2. Electrolytes
2.2. Electrochemical Synthesis of the Polymethylolacrylamide/AuNPs Composites
2.3. Swelling Capacity of the Composite Films
2.4. Coefficients of Linear Extension/Compression of the Films
2.5. Research Methods
3. Results and Discussion
3.1. Mechanism of Reduction of [AuCl4]− and Formation of the PMAA/AuNPs Composite
3.1.1. Possibility of Chemical Reduction of a Tetrachloroaurate Ion [AuCl4]−
3.1.2. Electrochemical Reduction of a Tetrachloraurate Ion [AuCl4]−
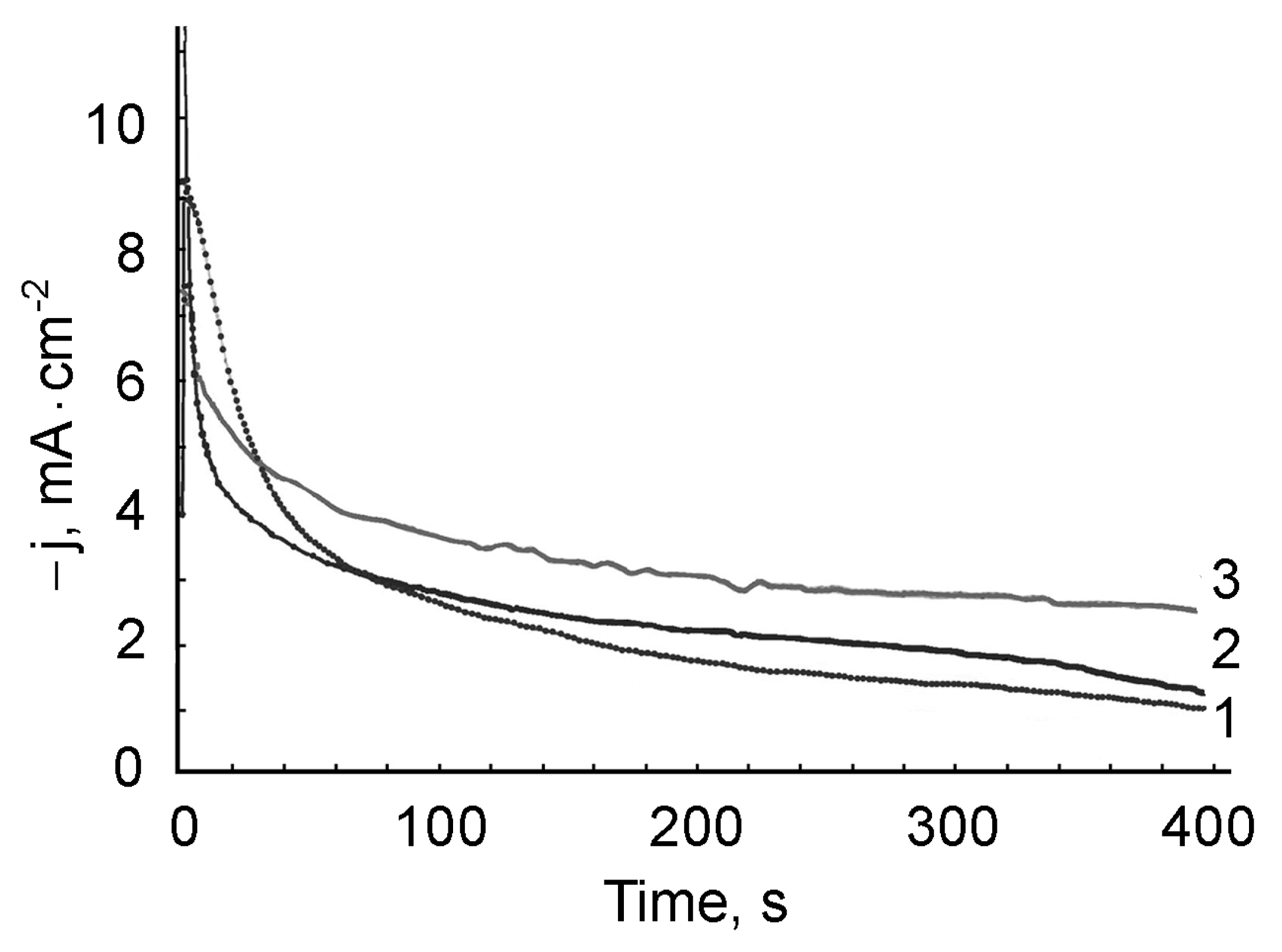
3.2. Dynamics of the Formation of PMAA Films and PMAA/AuNPs Composite
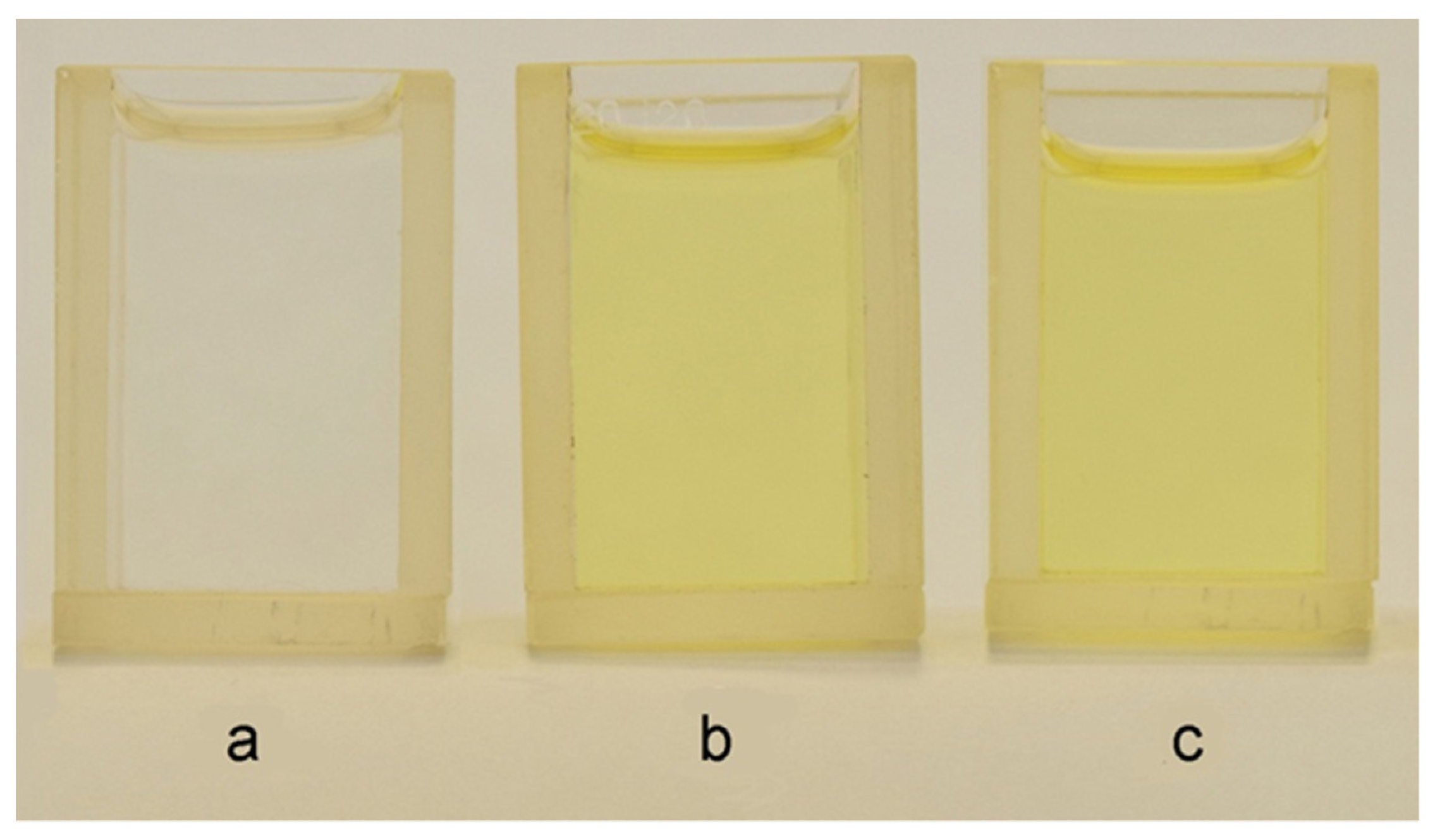
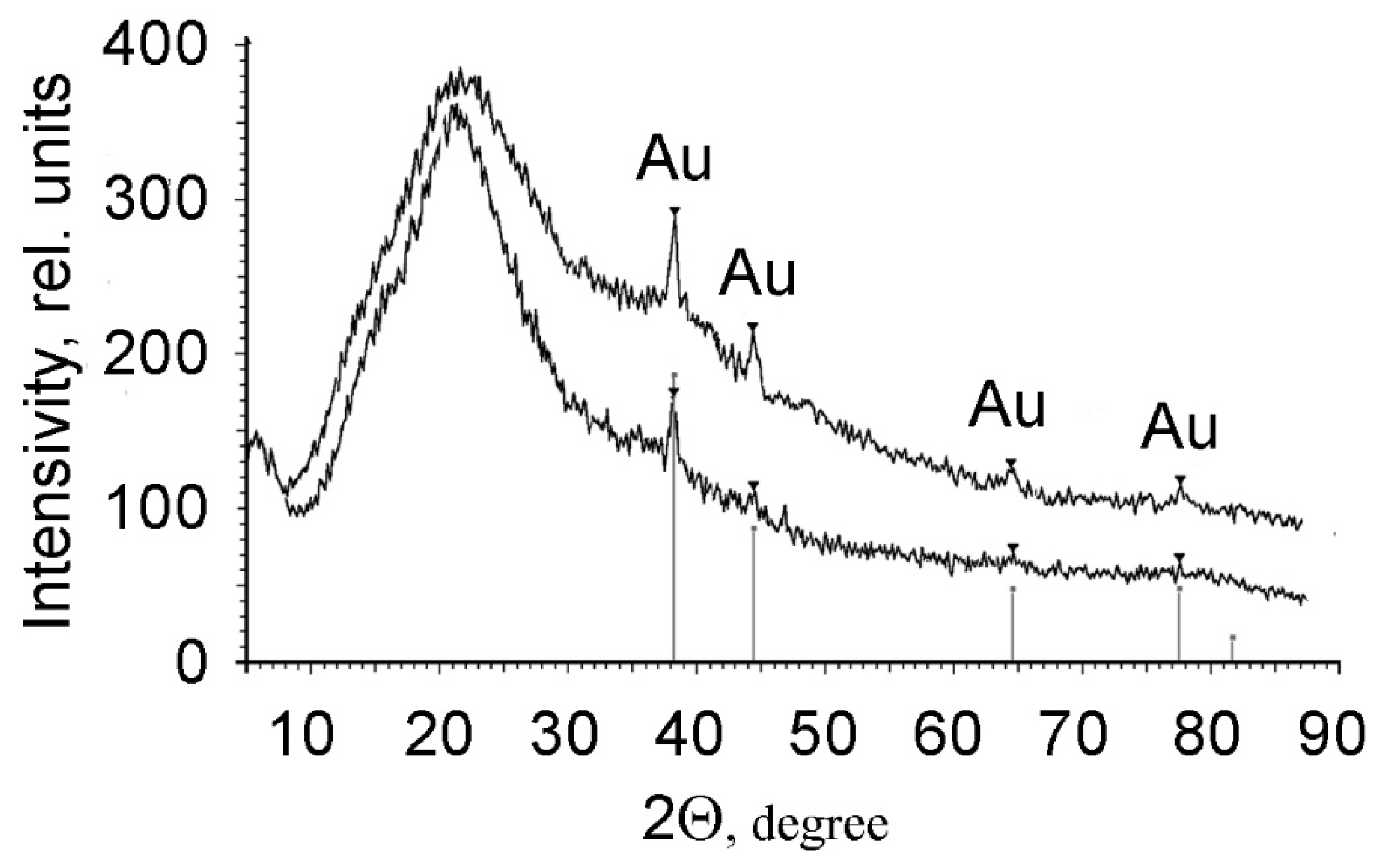
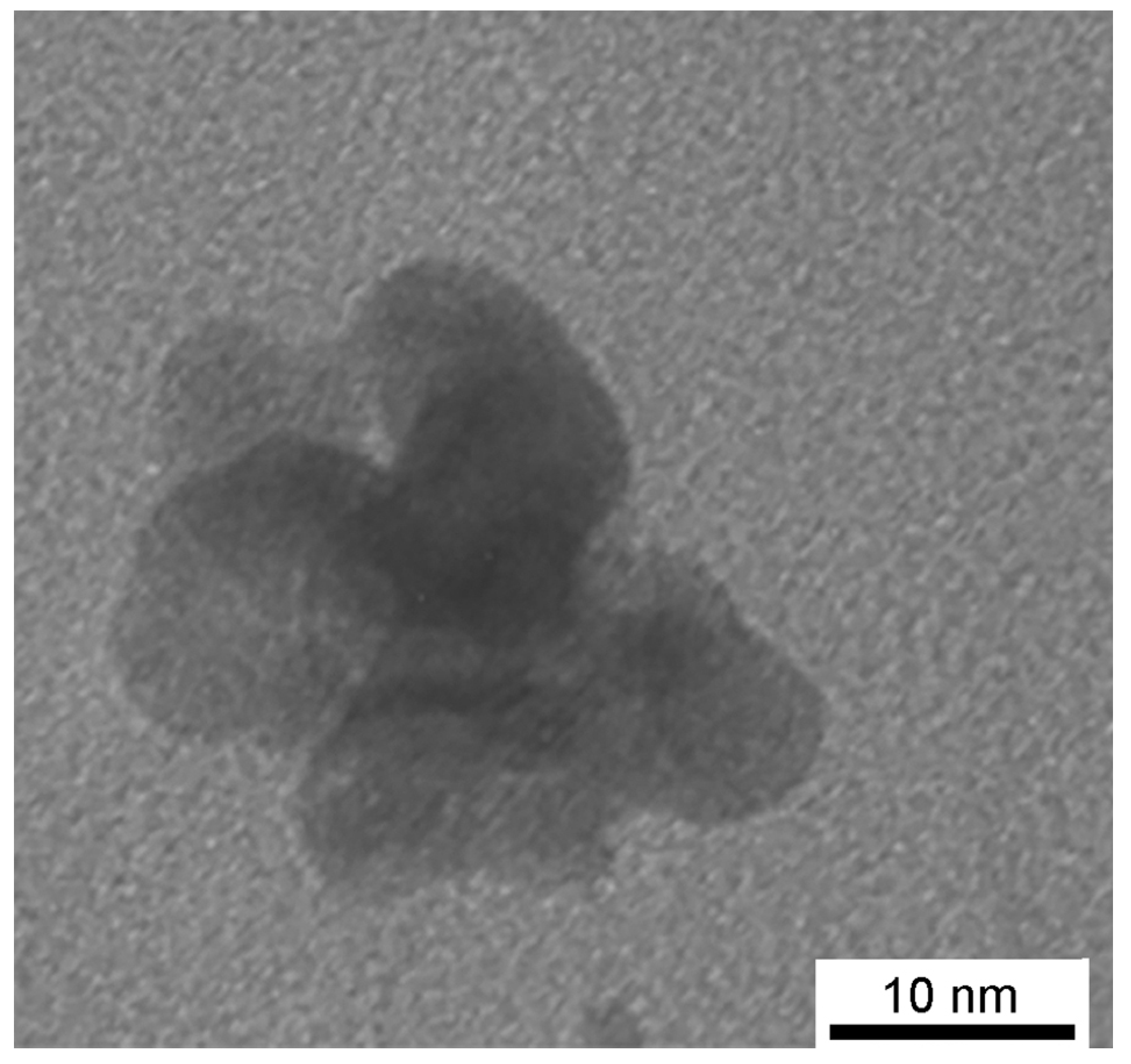
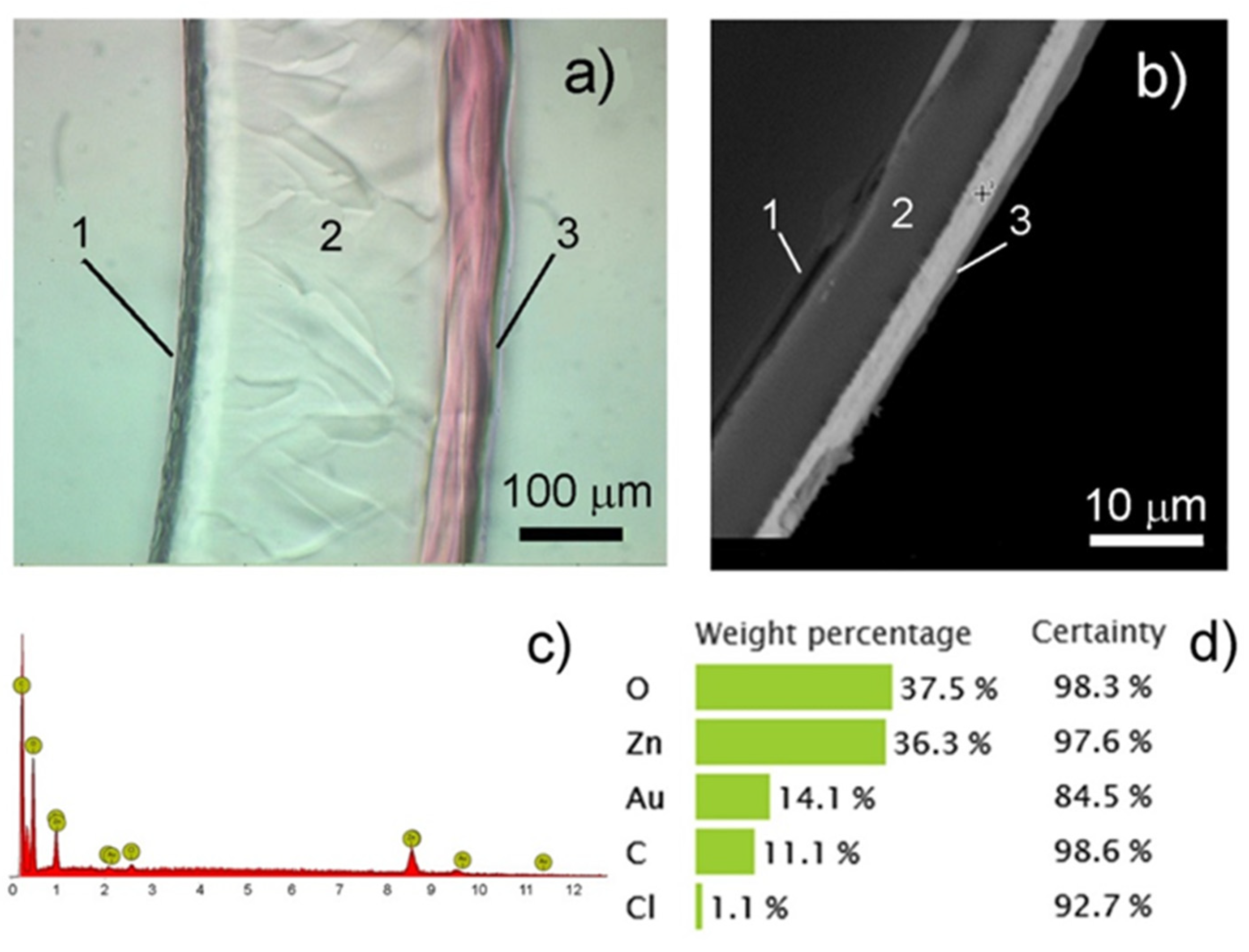
3.3. Structure, Morphology and Properties of PMAA/AuNPs Composites
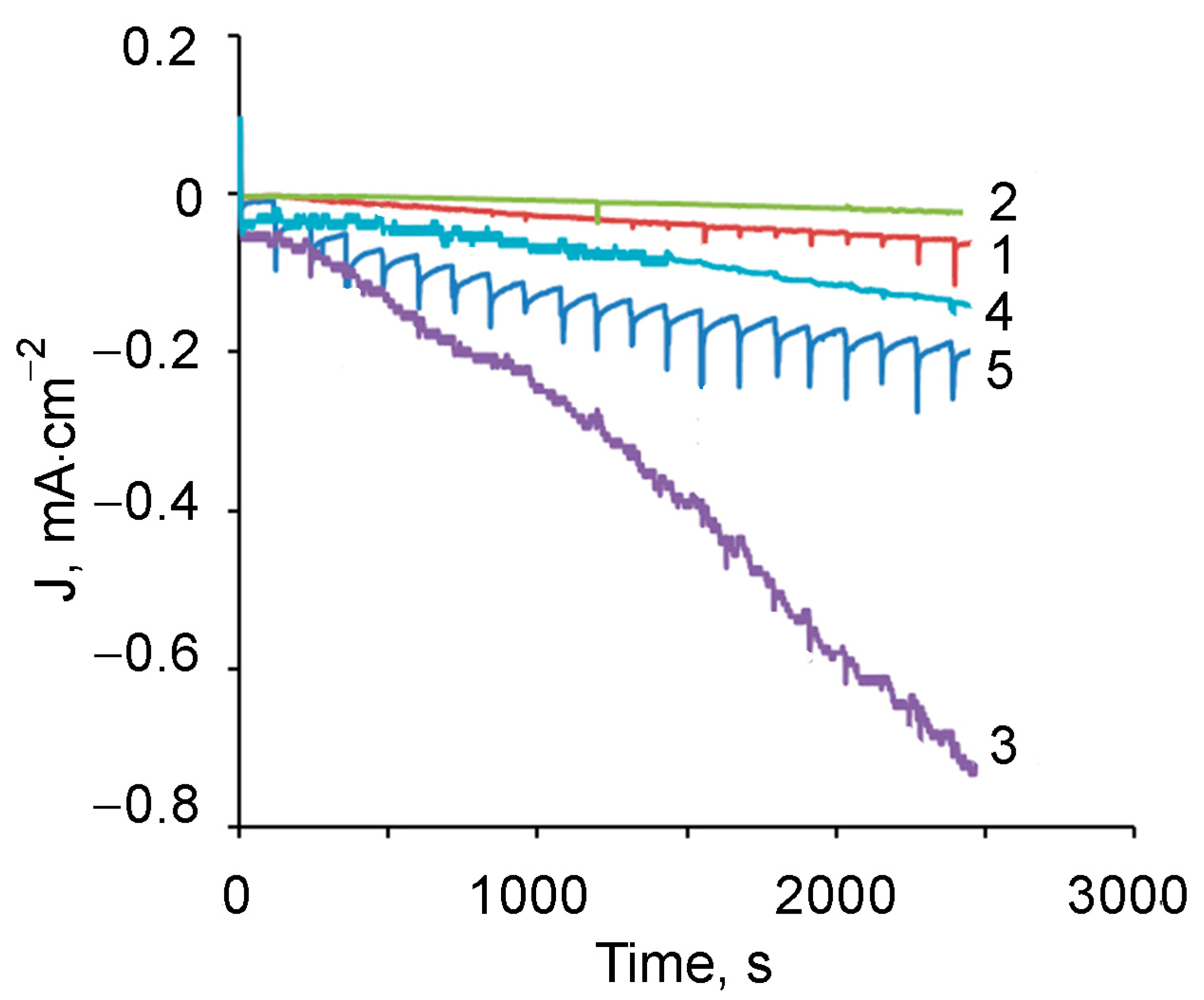
3.4. Sensor Properties of the Composite PMAA/AuNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Z.; Feng, J. Highly thermally conductive composite films based on nanofibrillated cellulose in-situ coated with a small amount of silver nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 24193–24200. [Google Scholar] [CrossRef]
- Ullman, A.M.; Jones, C.G.; Doty, F.P.; Stavila, V.; Talin, A.A.; Allendorf, M.D. Hybrid polymer/MOF films for colorimetric water sensing over a wide concentration range. ACS Appl. Mater. Interfaces 2018, 10, 24201–24208. [Google Scholar] [CrossRef]
- Li, N.; Nan, C.; Mei, X.; Sun, Y.; Feng, H.; Li, Y. Electrochemical sensor based on dual-template molecularly imprinted polymer and nanoporous gold leaf modified electrode for simultaneous determination of dopamine and uric acid. Microchim. Acta 2020, 187, 496. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, X.-L.; Zeng, Q.; Wang, L.-S. A glassy carbon electrode modified with graphene nanoplatelets, gold nanoparticles and chitosan, and coated with a molecularly imprinted polymer for highly sensitive determination of prostate specific antigen. Microchim. Acta 2017, 184, 4469–4476. [Google Scholar] [CrossRef]
- Bonakdar, M.; Yu, J.; Mottola, H.A. Continuous-flow performance of carbon electrodes modified with immobilized Fe(II)/Fe(III) centers. Amperometric response to N2O, NO and NO2. Talanta 1989, 36, 219–225. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Gold nanoparticles assisted laser desorption/ionization mass spectrometry and applications: From simple molecules to intact cells. Anal. Bioanal. Chem. 2016, 408, 4485–4502. [Google Scholar] [CrossRef] [PubMed]
- Ulvestad, A.; Sasikumar, K.; Kim, J.W.; Harder, R.; Maxey, E.R.; Clark, J.N.; Narayanan, B.; Deshmukh, S.A.; Ferrier, N.J.; Mulvaney, P.; et al. In Situ 3D imaging of catalysis induced strain in gold nanoparticles. J. Phys. Chem. Lett. 2016, 7, 3008–3013. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yun, W.; Chen, Y.; Wu, H.; Liu, X.; Fu, M.; Huang, Y. Ultrasensitive colorimetric and fluorometric detection of Hg(II) based on the use of gold nanoparticles and a catalytic hairpin assembly. Microchim. Acta 2017, 184, 4741–4747. [Google Scholar] [CrossRef]
- Jin, S.-A.; Heo, Y.; Lin, L.-K.; Deering, A.J.; Chiu, G.T.-C.; Allebach, J.P.; Stanciu, L.A. Gold decorated polystyrene particles for lateral flow immunodetection of Escherichia coli O157:H7. Microchim. Acta 2017, 184, 4879–4886. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, J.; Mu, Z.; Huang, Y.; Lu, M.; Gu, Z. Gold nanoparticle incorporated inverse opal photonic crystal capillaries for optofluidic surface enhanced Raman spectroscopy. Biosens. Bioelectron. 2015, 72, 268–274. [Google Scholar] [CrossRef]
- Etorki, A.M.; Awin, L.A.; El-Rais, M.; Elhabbat, M.S.; Shaban, I.S. Application of gold nanoparticles with 1,6-Hexanedithiol modified screen-printed carbon electrode as a sensor for determination of arsenic in environmental samples. Sens. Lett. 2019, 17, 762–768. [Google Scholar] [CrossRef]
- Zong, J.; Jin, Q.; Huang, C. Effect of wetted graphene on the performance of Pt/PPy-graphene electrocatalyst for methanol electrooxidation in acid medium. J. Solid State Electrochem. 2013, 17, 1339–1348. [Google Scholar] [CrossRef]
- Ponnaiah, S.K.; Periakaruppan, P.; Ponnaiah, S.K.; Periakaruppan, P. A glassy carbon electrode modified with a copper tungstate and polyaniline nanocomposite for voltammetric determination of quercetin. Microchim. Acta 2018, 185, 524. [Google Scholar] [CrossRef]
- Vdovina, S.N.; Ferapontov, N.B.; Zolotukhina, E.V.; Nesterova, E.A. Chemical deposition of copper in crosslinked polyvinyl alcohol and polyacrylamide gels. Condens. Media Interphase Boundaries 2010, 12, 93–100. [Google Scholar]
- Vdovina, S.N.; Ferapontov, N.B. The role of the properties of the polymer matrix in the chemical deposition of a metal in a polymer gel. Sorpt. Chromatogr. Process 2001, 11, 132–138. [Google Scholar]
- Kafi, A.K.M.; Wali, Q.; Jose, R.; Biswas, T.K.; Yusoff, M.M. A glassy carbon electrode modified with SnO2 nanofibers, polyaniline and hemoglobin for improved amperometric sensing of hydrogen peroxide. Microchim. Acta 2017, 184, 4443–4450. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahmad, A.; Islam, S.S. Magnetic Fe3O4@poly(methacrylic acid) particles for selective preconcentration of trace arsenic species. Microchim. Acta 2017, 184, 2007–2014. [Google Scholar] [CrossRef]
- Gubin, S.P.; Yurkov, G.Y.; Kataeva, N.A. Nanochastitsy Blagorodnykh Metallov i Materialy na ikh Osnove (Nanoparticles of Noble Metals and Materials on Their Basis); Azbuka: Moscow, Russia, 2006; p. 156. [Google Scholar]
- Han, J.; Wang, M.; Hu, Y.; Zhou, C.; Guo, R. Conducting polymer-noble metal nanoparticle hybrids: Synthesis mechanism application. Prog. Polym. Sci. 2017, 70, 52–91. [Google Scholar] [CrossRef]
- Shchitovskaya, E.V.; Kolzunova, L.G.; Karpenko, M.A. Electrochemical immobilization of silver nanoparticles in a polymethylolacryalmide matrix. Russ. J. Coord. Chem. 2020, 56, 379–387. [Google Scholar] [CrossRef]
- Smirnova, L.A.; Aleksandrov, A.P.; Yakimovich, N.O.; Sapogova, N.V.; Kirsanov, A.V.; Soustov, L.V.; Bityurin, N.M. UV-induced formation of gold nanoparticles in a poly(methyl methacrylate) matrix. Dokl. Phys. Chem. 2005, 400, 19–21. [Google Scholar] [CrossRef]
- Ruggeri, G.; Covolan, V.L.; Bernabò, M.; Li, L.M.; Valadares, L.F.; Leite, C.A.P.; Galembeck, F. Metal nanostructures with magnetic and biodegradable properties for medical applications. J. Braz. Chem. Soc. 2013, 24, 191–200. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, E.; Don, S. Incorporation of surface-derivatized gold nanoparticles into electrochemically generated polymer films. Electrochem. Commun. 2002, 4, 210–213. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Zhang, J.; Dai, W.; Lin, X.; Ye, J.; Ye, J. A screen-printed carbon electrode modified with a bismuth film and gold nanoparticles for simultaneous stripping voltammetric determination of Zn(II), Pb(II) and Cu(II). Microchim. Acta 2017, 184, 4731–4740. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, Y.; Liu, J.; Wang, H.; Hou, S. Non-enzymatic amperometric hydrogen peroxide sensor using a glassy carbon electrode modified with gold nanoparticles deposited on CVD-grown grapheme. Microchim. Acta 2017, 184, 4723–4729. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kamada, K.; Enomoto, N. Morphology-selective synthesis of polyhedral gold nanoparticles: What factors control the size and morphology of gold nanoparticles in a wet-chemical process. J. Colloid Interface Sci. 2008, 316, 887–892. [Google Scholar] [CrossRef]
- Schreiber, R.; Santiago, I.; Ardavan, A.; Turberfield, A.J. Ordering gold nanoparticles with DNA origami nanoflowers. ACS Nano. 2016. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Shinde, S.; Saratale, R.; Syed, A.; Ameen, F.; Ghodake, G. Spectrophotometric determination of Fe(III) by uing casein-functionalized gold nanoparticles. Microchim. Acta 2017, 184, 4695–4704. [Google Scholar] [CrossRef]
- Habibi, B.; Pournaghi-Azar, M.H. Composite electrodes consisting Pt nano-particles and poly(aminophenols) film on pre-treated aluminum substrate as electrocatalysts for methanol oxidation. J. Solid State Electrochem. 2010, 14, 599–613. [Google Scholar] [CrossRef]
- Santhosha, P.; Gopalana, A.; Vasudevana, T.; Lee, K.-P. Platinum particles dispersed poly(diphenylamine) modified electrode for methanol oxidation. Appl. Surf. Sci. 2006, 252, 7964–7969. [Google Scholar] [CrossRef]
- Shchitovskaya, E.V.; Kolzunova, L.G.; Kuryavyi, V.G.; Slobodyuk, A.B. Electrochemical formation and properties of polymethylolacrylamide film with inclusion of platinum particles. Russ. J. Electrochem. 2015, 51, 1097–1107. [Google Scholar] [CrossRef]
- García-Hernández, C.; García-Cabezón, C.; Medina-Plaza1, C.; Martín-Pedrosa, F.; Blanco, Y.; de Saja, J.A.; Rodríguez-Méndez, M.L. Electrochemical behavior of polypyrrol/AuNPs composites deposited by different electrochemical methods: Sensing properties towards catechol. Beilstein J. Nanotechnol. 2015, 6, 2052–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolzunova, L.G. Polymer films. In Properties, Performance and Applications; Romano, S.A., Somners, G.P., Eds.; Nova Sci. Publ., Inc.: Hauppauge, NY, USA, 2012; pp. 1–108. [Google Scholar]
- Kolzunova, L.G.; Barinov, N.N. The supramolecular structure of ultrafiltration membranes synthesized by electropolymerization. Anal. Bioanal. Chem. 2002, 374, 746–748. [Google Scholar] [CrossRef]
- Franz, A.W.; Kronemayer, H.; Pfeiffer, D.; Pilz, R.D.; Reuss, G.; Disteldorf, W.; Gamer, A.O.; Hilt, A. Formaldehyde. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinbeim, Germany, 2016. [Google Scholar] [CrossRef]
- Chemistry LibreTexts, Electrochemistry Tables, P1: Standard Reduction Potentials by Element. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Electrochemistry_Tables/P1%3A_Standard_Reduction_Potentials_by_Element (accessed on 27 April 2021).
- Kolzunova, L.G. Formation of supramolecular structure of electropolymerized acryl films. Russ. J. Electrochem. 2004, 40, 337–343. [Google Scholar] [CrossRef]
- Zinchenko, A.; Miwa, Y.; Lopatina, L.I.; Sergeyev, V.G.; Murata, S. DNA hydrogel as a template for synthesis of ultrasmall gold nanoparticles for catalytic applications. Appl. Mater. Interfaces 2014, 6, 3226–3232. [Google Scholar] [CrossRef]
- Che, Y.; Zinchenko, A.; Murata, S. Control of a catalytic activity of gold nanoparticles embedded in DNA hydrogel by swelling/shrinking the hydrogel’s matrix. J. Colloid Interface Sci. 2015, 445, 364–370. [Google Scholar] [CrossRef]
- Chen, S.H.; Yuan, R.; Chai, Y.Q.; Hu, F.X. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Chen, X.M.; Wu, G.H.; Cai, Z.X.; Oyama, M.; Chen, X. Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim. Acta 2014, 181, 689–705. [Google Scholar] [CrossRef]







| Components | Concentration | Electrolyte | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Acrylamide (AA) | 3 mol/L | AA | AA | AA | AA |
| Formaldehyde (F) | 3 mol/L | F | F | F | F |
| Zinc chloride | 0.2 mol/L | ZnCl2 | ZnCl2 | ZnCl2 | ZnCl2 |
| N, N-methylene-bis-acrylamide (MBAA) | 0.05 mol/L | MBAA | MBAA | MBAA | MBAA |
| Chitosan (Chs) | 0.1% | - | Chs | - | Chs |
| Tetrachloroauric acid | 1–4 mmol/L | - | - | HAuCl4 | HAuCl4 |
| Concentranion (HAuCl4), mmmol/L | Average Radius AuNPs, nm |
|---|---|
| 1 | 5.12 ± 0.30 |
| 2 | 5.78 ± 0.15 |
| 3 | 6.90 ± 0.47 |
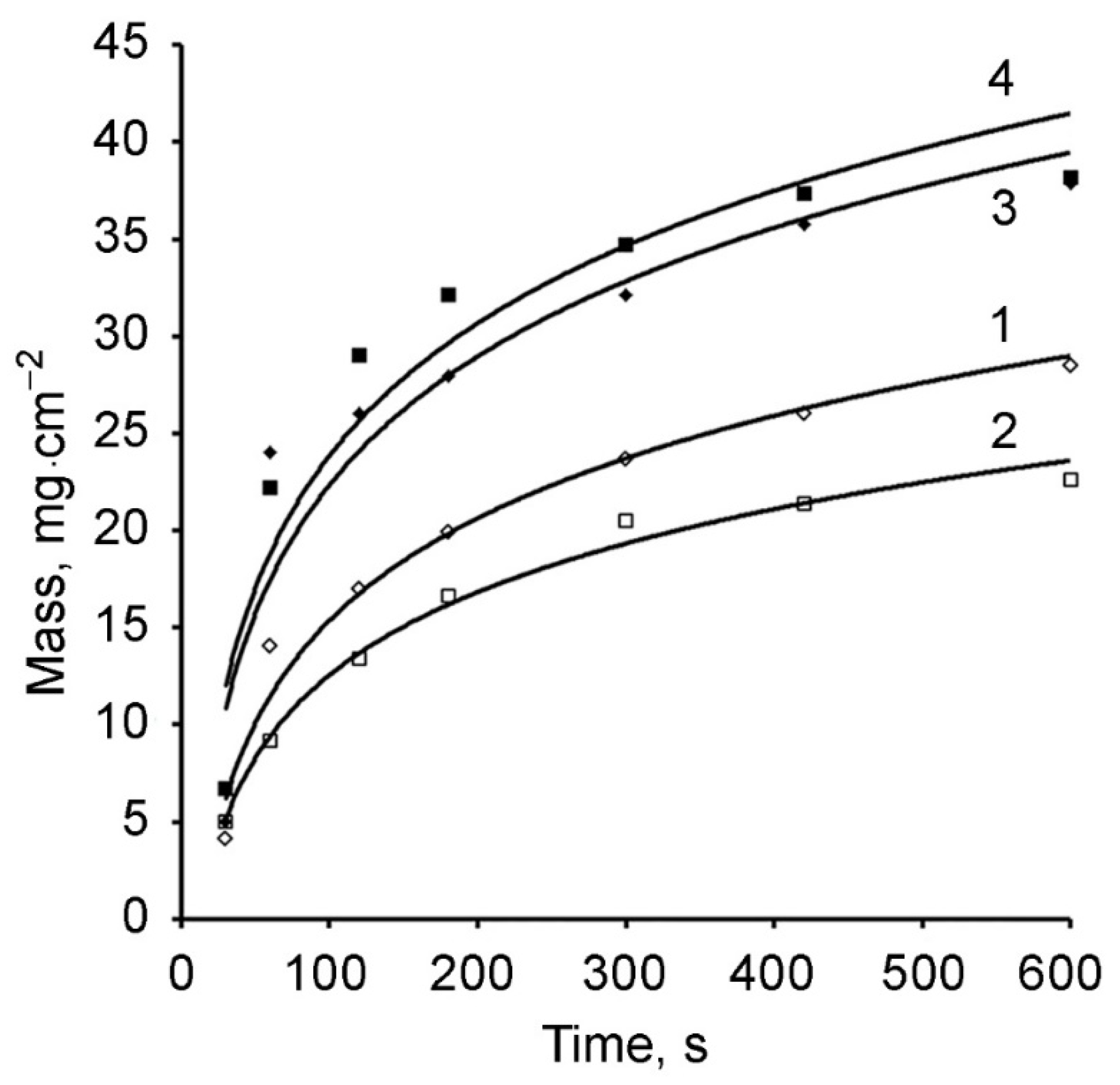
| Time, s | Polymer Mass, mg/cm2 | |||
|---|---|---|---|---|
| Electrolyte 1 | Electrolyte 2 2 | Electrolyte 3 3 | Electrolyte 4 | |
| 30 | 4.10 | - | 4.99 | 6.70 |
| 60 | 14.07 | 9.20 | 24.02 | 22.21 |
| 120 | 17.00 | 13.40 | - | - |
| 180 | 19.90 | 16.65 | 27.97 | 32.15 |
| 300 | 23.67 | 20.50 | 32.12 | 33.67 |
| 600 | 28.48 | 22.60 | 37.88 | 38.20 |
| Composition | msw, gm | mdry, gm | Ssw, % | Øin, mm | Øsw, mm | Ødry, mm | L1 | L2 |
|---|---|---|---|---|---|---|---|---|
| Electrolyte 1 | 0.0217 | 0.0044 | 393 | 8.5 | 12 | 7 | 1.41 | 1.21 |
| Electrolyte 4 | 0.0129 | 0.0027 | 377 | 8.5 | 11 | 6 | 1.29 | 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolzunova, L.; Shchitovskaya, E.; Karpenko, M. Polymethylolacrylamide/AuNPs Nanocomposites: Electrochemical Synthesis and Functional Characteristics. Polymers 2021, 13, 2382. https://doi.org/10.3390/polym13142382
Kolzunova L, Shchitovskaya E, Karpenko M. Polymethylolacrylamide/AuNPs Nanocomposites: Electrochemical Synthesis and Functional Characteristics. Polymers. 2021; 13(14):2382. https://doi.org/10.3390/polym13142382
Chicago/Turabian StyleKolzunova, Lidiia, Elena Shchitovskaya, and Maxim Karpenko. 2021. "Polymethylolacrylamide/AuNPs Nanocomposites: Electrochemical Synthesis and Functional Characteristics" Polymers 13, no. 14: 2382. https://doi.org/10.3390/polym13142382
APA StyleKolzunova, L., Shchitovskaya, E., & Karpenko, M. (2021). Polymethylolacrylamide/AuNPs Nanocomposites: Electrochemical Synthesis and Functional Characteristics. Polymers, 13(14), 2382. https://doi.org/10.3390/polym13142382







