Environmentally Friendly, High-Performance Fire Retardant Made from Cellulose and Graphite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Characterization
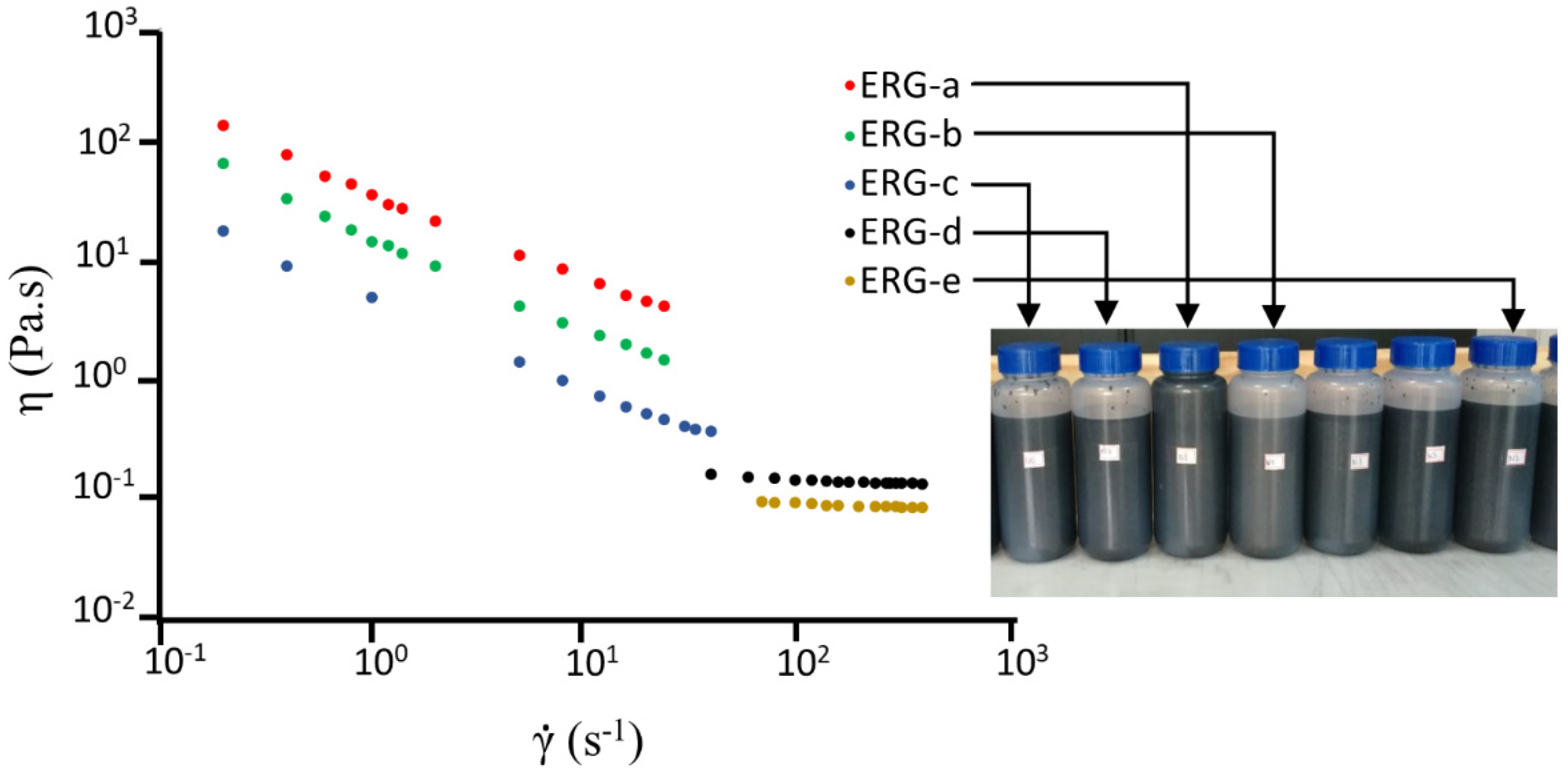
2.2.1. Graphite Dispersion and Rheology Characterization
2.2.2. Exfoliated and Reassembled Graphite (ERG)-Coated Samples Preparation and Characterization
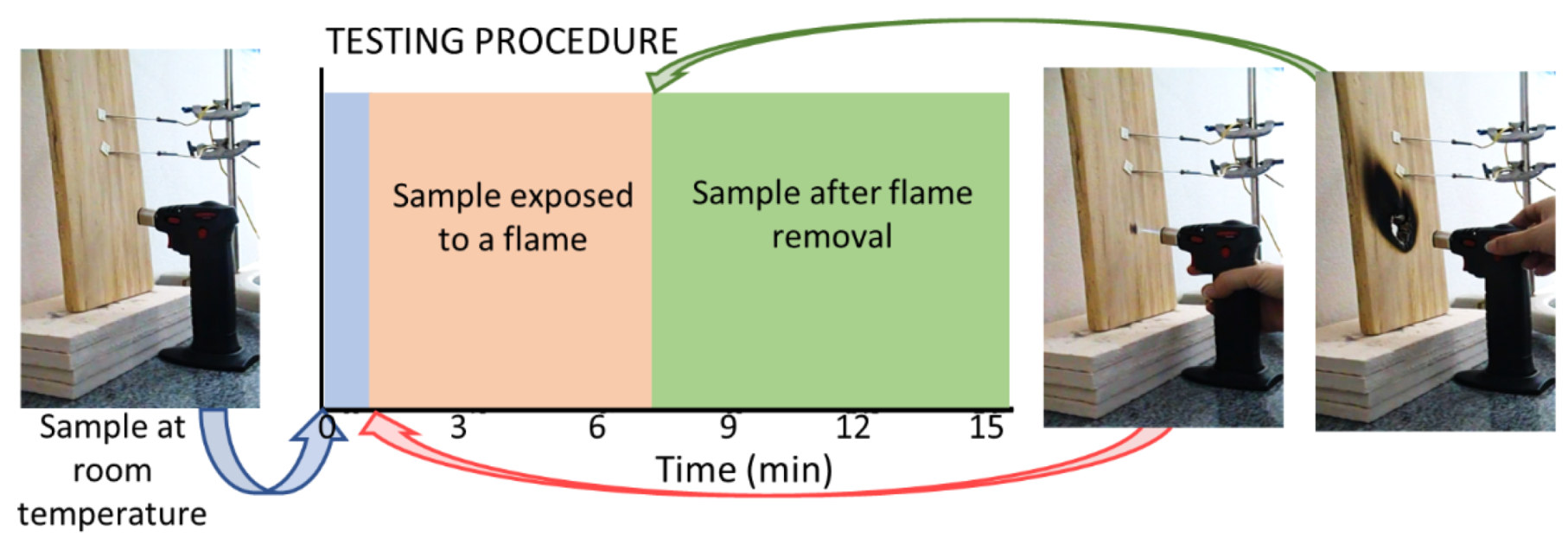
2.2.3. Flame-Resistance Tests
3. Results
3.1. Preparation and Properties of Graphite Dispersions
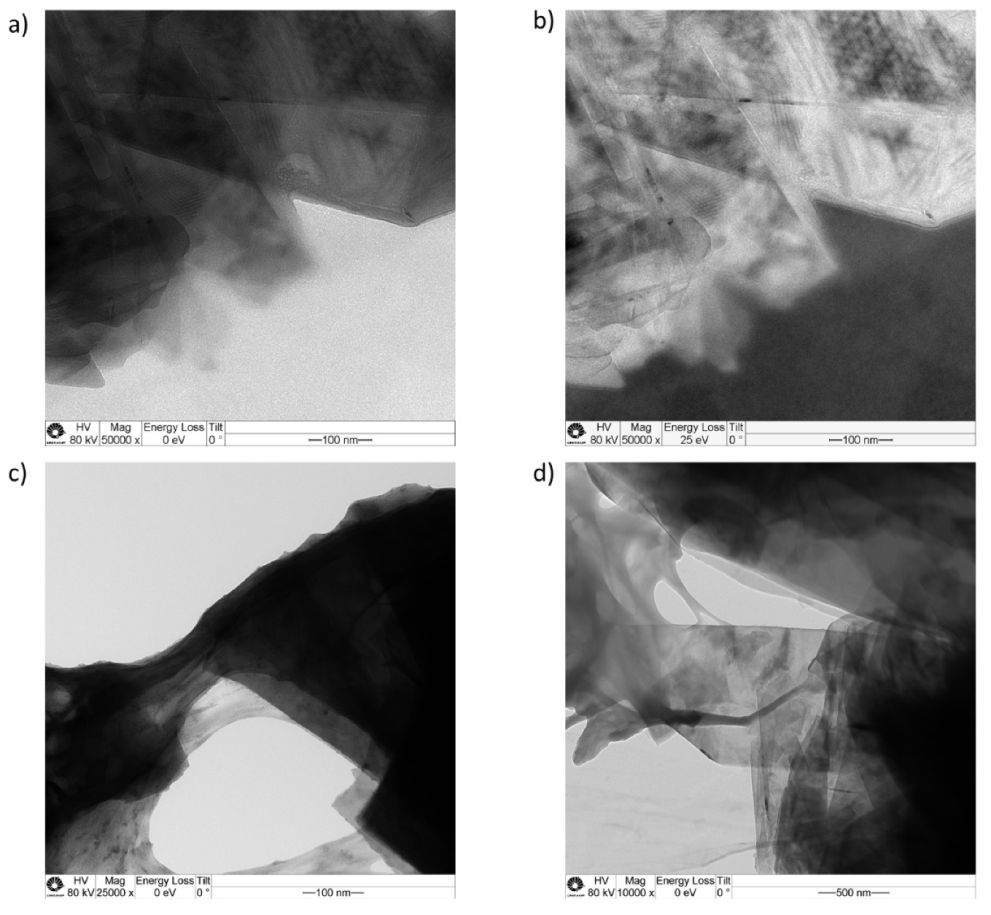
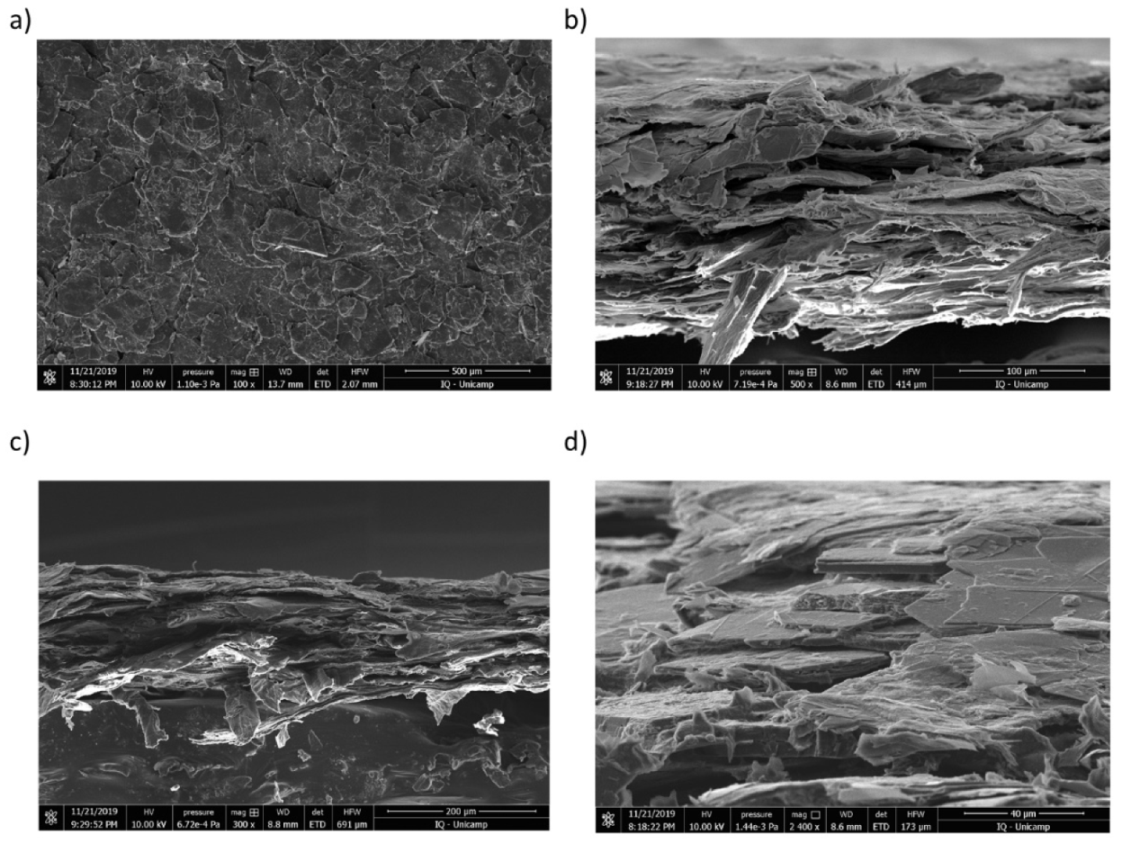
3.2. Coating Characteristics
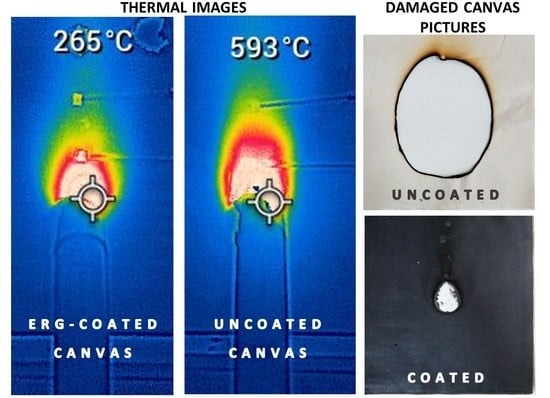
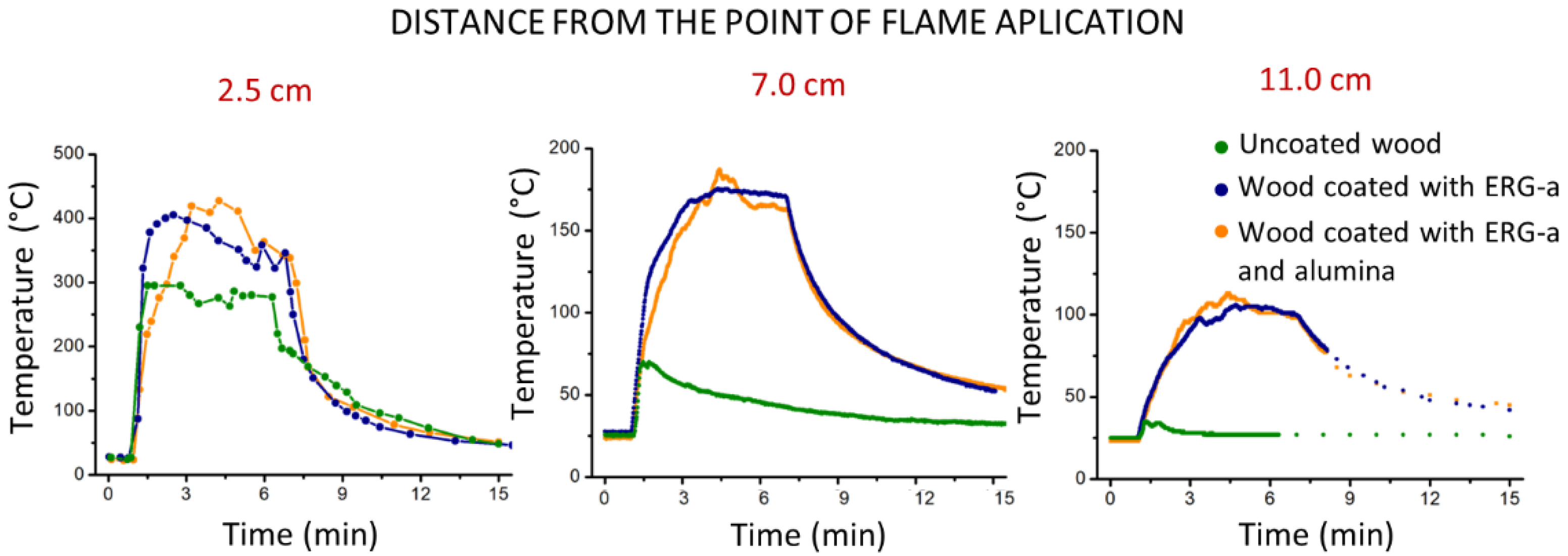
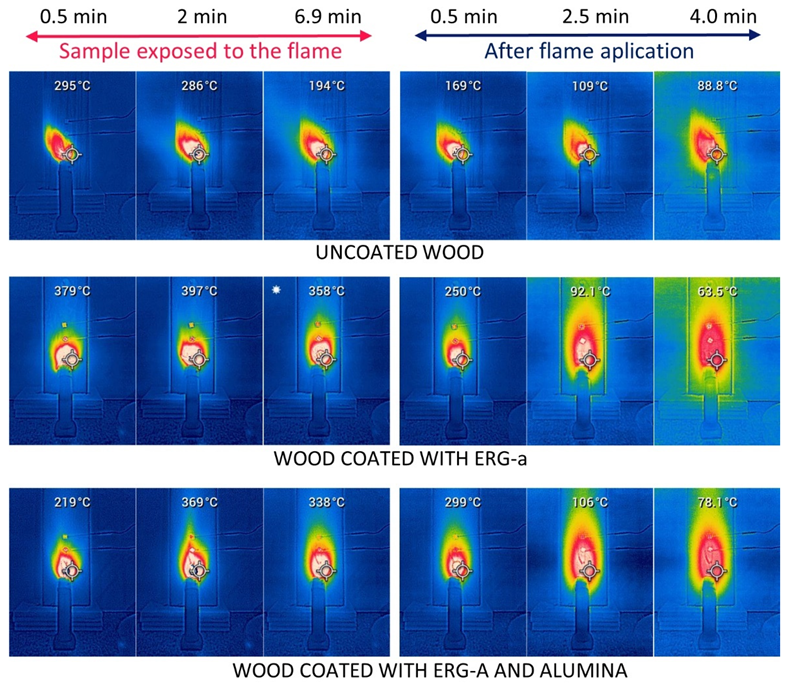
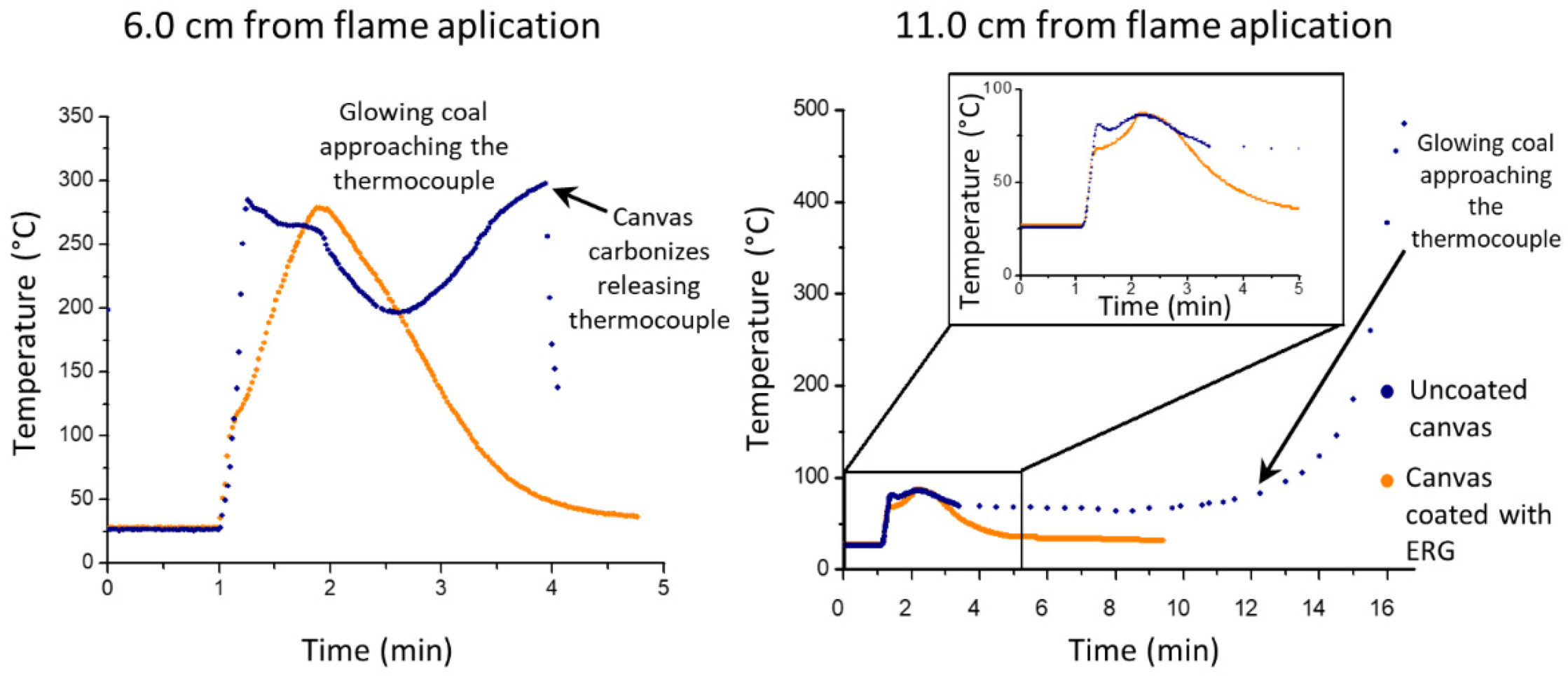
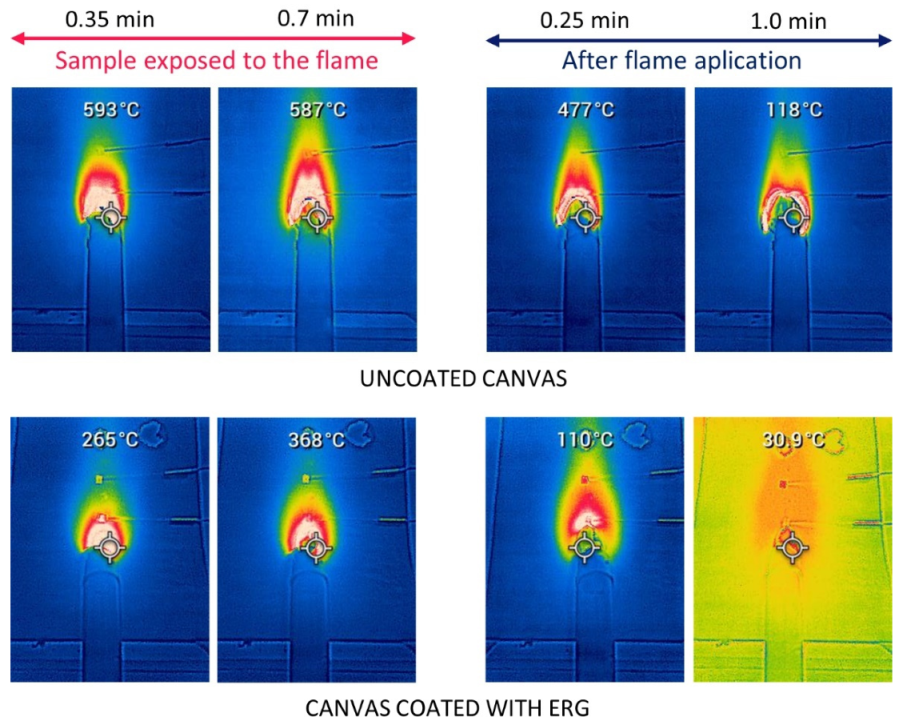
3.3. Exposure to Flame: Laboratory Tests
3.4. Exposure to Flame: Standard Tests
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habibi, Y.; Lucia, L.; Rojas, O. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Linares, M.; Zozoulenko, I. Theoretical Rationalization of Self-Assembly of Cellulose Nanocrystals: Effect of Surface Modifications and Counterions. Biomacromolecules 2020, 21, 3069–3080. [Google Scholar] [CrossRef]
- Zhu, B.; Johansen, V.E.; Kamita, G.; Guidetti, G.; Bay, M.M.; Parton, T.G.; Frka-Petesic, B.; Vignolini, S. Hyperspectral Imaging of Photonic Cellulose Nanocrystal Films: Structure of Local Defects and Implications for Self-Assembly Pathways. ACS Nano 2020, 14, 15361–15373. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Pham, C.; Bilatto, S.; Azeredo, H.M.; Cranston, E.D.; Moreira, F.K.; Mattoso, L.H.C.; Bras, J. Effect of Tannic Acid and Cellulose Nanocrystals on Antioxidant and Antimicrobial Properties of Gelatin Films. ACS Sustain. Chem. Eng. 2021, 9, 8539–8549. [Google Scholar] [CrossRef]
- Gu, Z.; Lu, M.; Feng, K.; Jin, Z. The different composites of cellulose nanocrystals with d- or l-histidine. Nanoscale 2021, 13, 8174–8180. [Google Scholar] [CrossRef]
- Pracella, M.; Mura, C.; Galli, G. Polyhydroxyalkanoate Nanocomposites with Cellulose Nanocrystals as Biodegradable Coating and Packaging Materials. ACS Appl. Nano Mater. 2021, 4, 260–270. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Rezende, C.A.; Cranston, E.D. Fundamentals of cellulose lightweight materials: Bio-based assemblies with tailored properties. Green Chem. 2021, 23, 3542–3568. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Norgren, M.; Nordenskiöld, L. Hydrophobic interactions control the self-assembly of DNA and cellulose. Q. Rev. Biophys. 2021, 54, e3. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Bellesia, G.; Chundawat, S.P.S.; Dale, B.E.; Langan, P.; Gnanakaran, S. Insights into Hydrogen Bonding and Stacking Interactions in Cellulose. J. Phys. Chem. A 2011, 115, 14191–14202. [Google Scholar] [CrossRef]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Brown, R.M., Jr.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the structure of cellulose: Debating the Lindman hypothesis. Cellulose. 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Lanzoni, E.M.; Costa, C.A.R.; Deneke, C.; Bernardes, J.S.; Galembeck, F. Adhesive and Reinforcing Properties of Soluble Cellulose: A Repulpable Adhesive for Wet and Dry Cellulosic Substrates. ACS Appl. Mater. Interfaces 2015, 7, 18750–18758. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.S.; Da Silva, D.S.; Burgo, T.A.L.; Batista, B.C.; Galembeck, F. Graphite exfoliation in cellulose solutions. Nanoscale 2017, 9, 10219–10226. [Google Scholar] [CrossRef]
- Santos, L.P.; Da Silva, D.S.; Bertacchi, J.P.F.; Moreira, K.S.; Burgo, T.A.D.L.; Batista, B.C.; Dos Santos, J.; De Paula, P.A.; Galembeck, F. Multifunctional coatings of exfoliated and reassembled graphite on cellulosic substrates. Faraday Discuss. 2020, 227, 105–124. [Google Scholar] [CrossRef] [Green Version]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef] [Green Version]
- Bar, M.; Alagirusamy, R.; Das, A. Flame retardant polymer composites. Fibers Polym. 2015, 16, 705–717. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; John, M.J.; Jacobs, V.; Luyt, A.S. Review on flammability of biofibres and biocomposites. Carbohydr. Polym. 2014, 111, 149–182. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Sun, P.; Hai, Y.; Jiang, S. A self-healing, recyclable, and degradable fire-retardant gelatin-based biogel coating for green buildings. Soft Matter 2021, 17, 5231–5239. [Google Scholar] [CrossRef]
- Malucelli, G. Biomacromolecules and Bio-Sourced Products for the Design of Flame Retarded Fabrics: Current State of the Art and Future Perspectives. Molecules 2019, 24, 3774. [Google Scholar] [CrossRef] [Green Version]
- Elvira-León, J.; Chimenos, J.M.; Isábal, C.; Monton, J.; Formosa, J.; Haurie, L. Epsomite as flame retardant treatment for wood: Preliminary study. Constr. Build. Mater. 2016, 126, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Kwon, D.-J.; Shin, P.-S.; Baek, Y.-M.; Park, H.-S.; DeVries, K.L.; Park, J.-M. The evaluation of the interfacial and flame retardant properties of glass fiber/unsaturated polyester composites with ammonium dihydrogen phosphate. Compos. Part B: Eng. 2019, 167, 221–230. [Google Scholar] [CrossRef]
- Oikawa, K.; Toyota, K.; Sakatani, S.; Hayashi, Y.; Takizawa, H. Facile synthesis and thermal properties of waterglass-based silica xerogel nanocomposites containing reduced graphene oxide. Ceram. Int. 2019, 45, 4201–4207. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Comparative study on flame retardancy of silica fume-based geopolymer activated by different activators. J. Alloys Compd. 2018, 743, 108–114. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Benign design and the evaluation of pyrolysis kinetics of polyester resin based intumescent system comprising of alkali-activated silica fume. Prog. Org. Coatings 2018, 122, 30–37. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Preliminary study on decanoic/palmitic eutectic mixture modified silica fume geopolymer-based coating for flame retardant plywood. Constr. Build. Mater. 2018, 189, 1–7. [Google Scholar] [CrossRef]
- Vahabi, H.; Saeb, M.R.; Formela, K.; Cuesta, J.-M.L. Flame retardant epoxy/halloysite nanotubes nanocomposite coatings: Exploring low-concentration threshold for flammability compared to expandable graphite as superior fire retardant. Prog. Org. Coatings 2018, 119, 8–14. [Google Scholar] [CrossRef]
- Tan, Y.; He, Z.; Li, X.; Jiang, B.; Li, J.; Zhang, Y. Research on the Flame Retardancy Properties and Mechanism of Modified Asphalt with Halloysite Nanotubes and Conventional Flame Retardant. Materials. 2020, 13, 4509. [Google Scholar] [CrossRef]
- Wang, H.; Du, X.; Wang, S.; Du, Z.; Wang, H.; Cheng, X. Improving the flame retardancy of waterborne polyurethanes based on the synergistic effect of P–N flame retardants and a Schiff base. RSC Adv. 2020, 10, 12078–12088. [Google Scholar] [CrossRef]
- Qin, S.; Yang, Z.; Zhang, S.; Zhang, Z.; Wang, X.; Yang, X.; Luo, T.; Yang, L. Comparison of Flame-retardancy Property and Mechanism between a Phosphate Ester and a Phosphoramine Flame-retardants. J. Wuhan Univ. Technol. Sci. Ed. 2021, 36, 148–156. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, W. Synthesis and properties of a novel hyperbranched polyphosphate acrylate applied to UV curable flame retardant coatings. Eur. Polym. J. 2007, 43, 1302–1312. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, G.; Zhao, J.; Han, Y. Effect of flaky graphite with different particle sizes on flame resistance of intumescent flame retardant coating. Results Mater. 2020, 5, 100061. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, S.; Zhang, Z.; Mai, C.; Xie, Y.; Wang, Q. Fire retardancy of an aqueous, intumescent, and translucent wood varnish based on guanylurea phosphate and melamine-urea-formaldehyde resin. Prog. Org. Coatings 2018, 121, 64–72. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Effect of Graphene on Flame Retardancy of Graphite Doped Intumescent Flame Retardant (IFR) Coatings: Synergy or Antagonism. Coatings 2019, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Liu, Y.; Zhang, L.; Wang, H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Effect of graphite on the flame resistance of silica fume-based geopolymeric coatings. Mater. Chem. Phys. 2020, 239, 122088. [Google Scholar] [CrossRef]
- Khalili, P.; Tshai, K.Y.; Kong, I. Natural fiber reinforced expandable graphite filled composites: Evaluation of the flame retardancy, thermal and mechanical performances. Compos. Part A: Appl. Sci. Manuf. 2017, 100, 194–205. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.M.; Seong, D.G.; Lee, D. Synergistic improvement of flame retardant properties of expandable graphite and multi-walled carbon nanotube reinforced intumescent polyketone nanocomposites. Carbon 2019, 143, 650–659. [Google Scholar] [CrossRef]
- Ho, Q.B.; Osazuwa, O.; Modler, R.; Daymond, M.; Gallerneault, M.T.; Kontopoulou, M. Exfoliation of graphite and expanded graphite by melt compounding to prepare reinforced, thermally and electrically conducting polyamide composites. Compos. Sci. Technol. 2019, 176, 111–120. [Google Scholar] [CrossRef]
- Sever, K.; Tavman, I.H.; Seki, Y.; Turgut, A.; Omastova, M.; Ozdemir, I. Electrical and mechanical properties of expanded graphite/high density polyethylene nanocomposites. Compos. Part B: Eng. 2013, 53, 226–233. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motaung, T.E.; Motloung, S.V. Morphology, flammability, and properties of graphite reinforced polymer composites. Systematic review. Polym. Compos. 2018, 39, E1487–E1499. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, Y.; Xu, Y.; Chen, X.; Chen, M.; Yu, J.; Hu, S.; Zhang, Z. Effect of the particle size of expandable graphite on the thermal stability, flammability, and mechanical properties of high-density polyethylene/ethylene vinyl-acetate/expandable graphite composites. Polym. Eng. Sci. 2014, 54, 1162–1169. [Google Scholar] [CrossRef]
- Li, Y.; Zou, J.; Zhou, S.; Chen, Y.; Zou, H.; Liang, M.; Luo, W. Effect of expandable graphite particle size on the flame retardant, mechanical, and thermal properties of water-blown semi-rigid polyurethane foam. J. Appl. Polym. Sci. 2013, 131, 1082–1090. [Google Scholar] [CrossRef]
- Cheng, J.-J.; Zhou, F.-B. Influence of expandable graphite on flame retardancy and mechanical properties of organic–inorganic hybrid material based on sodium silicate and polyisocyanate. J. Therm. Anal. Calorim. 2016, 126, 1417–1426. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Z.; Chen, T.; Zhu, Y.; Lv, Z.; Gong, X.; Niu, Y.; Ma, B. Preparation of highly dispersed expandable graphite/polystyrene composite foam via suspension polymerization with enhanced fire retardation. Carbon 2019, 146, 503–512. [Google Scholar] [CrossRef]
- Seidi, F.; Movahedifar, E.; Naderi, G.; Akbari, V.; Ducos, F.; Shamsi, R.; Vahabi, H.; Saeb, M.R. Flame Retardant Polypropylenes: A Review. Polymers. 2020, 12, 1701. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Tran, P.; Nguyen, Q.T.; Lau, K. Fire performance of polymer-based composites for maritime infrastructure. Compos. Part B: Eng. 2018, 155, 31–48. [Google Scholar] [CrossRef]
- Oliwa, R.; Heneczkowski, M.; Oleksy, M.; Galina, H. Epoxy composites of reduced flammability. Compos. Part B: Eng. 2016, 95, 1–8. [Google Scholar] [CrossRef]
- Jung, E.; Lee, Y. Development of a heat dissipating LED headlamp with silicone lens to replace halogen bulbs in used cars. Appl. Therm. Eng. 2015, 86, 143–150. [Google Scholar] [CrossRef]
- Yew, M.C.; Sulong, N.H.R. Fire-resistive performance of intumescent flame-retardant coatings for steel. Mater. Des. 2012, 34, 719–724. [Google Scholar] [CrossRef]
- Vahidi, G.; Bajwa, D.S.; Shojaeiarani, J.; Stark, N.; Darabi, A. Advancements in traditional and nanosized flame retardants for polymers—A review. J. Appl. Polym. Sci. 2021, 138, 50050. [Google Scholar] [CrossRef]
- Araby, S.; Philips, B.; Meng, Q.; Ma, J.; Laoui, T.; Wang, C.H. Recent advances in carbon-based nanomaterials for flame retardant polymers and composites. Compos. Part B: Eng. 2021, 212, 108675. [Google Scholar] [CrossRef]
- Fugallo, G.; Cepellotti, A.; Paulatto, L.; Lazzeri, M.; Marzari, N.; Mauri, F. Thermal Conductivity of Graphene and Graphite: Collective Excitations and Mean Free Paths. Nano Lett. 2014, 14, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liang, F.; Gou, J. Nanopaper enabled shape-memory nanocomposite with vertically aligned nickel nanostrand: Controlled synthesis and electrical actuation. Soft Matter 2011, 7, 7416–7423. [Google Scholar] [CrossRef]
- Yu, P.; Wang, X.; Zhang, K.; Zhou, D.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Aqueous cellulose solution assisted direct exfoliation of graphite to high concentration graphene dispersion. Mater. Lett. 2021, 285, 129081. [Google Scholar] [CrossRef]
- Kampioti, K.; Matos, C.F.; Galembeck, F.; Jaillet, C.; Derré, A.; Zarbin, A.J.; Pénicaud, A. Highly Conducting, Sustainable, Nanographitic Rubber Composites. ACS Omega 2018, 3, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Run/Sample Designation | Weight Percentage (% m/m) | ||
|---|---|---|---|
| Cellulose | Graphite | Activated Carbon | |
| N11 | 3 | 12.5 | 3.5 |
| N1 | 2 | 10 | 2 |
| N5 | 2 | 10 | 5 |
| N3/ERG-e | 2 | 15 | 2 |
| N4/ERG-b | 4 | 15 | 2 |
| N8/ERG-a | 4 | 15 | 5 |
| N7/ERG-d | 2 | 15 | 5 |
| N6/ERG-c | 4 | 10 | 5 |
| N10 | 3 | 12.5 | 3.5 |
| N2 | 4 | 10 | 2 |
| N9 | 3 | 12.5 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.P.; da Silva, D.S.; Morari, T.H.; Galembeck, F. Environmentally Friendly, High-Performance Fire Retardant Made from Cellulose and Graphite. Polymers 2021, 13, 2400. https://doi.org/10.3390/polym13152400
Santos LP, da Silva DS, Morari TH, Galembeck F. Environmentally Friendly, High-Performance Fire Retardant Made from Cellulose and Graphite. Polymers. 2021; 13(15):2400. https://doi.org/10.3390/polym13152400
Chicago/Turabian StyleSantos, Leandra P., Douglas S. da Silva, Thais H. Morari, and Fernando Galembeck. 2021. "Environmentally Friendly, High-Performance Fire Retardant Made from Cellulose and Graphite" Polymers 13, no. 15: 2400. https://doi.org/10.3390/polym13152400
APA StyleSantos, L. P., da Silva, D. S., Morari, T. H., & Galembeck, F. (2021). Environmentally Friendly, High-Performance Fire Retardant Made from Cellulose and Graphite. Polymers, 13(15), 2400. https://doi.org/10.3390/polym13152400