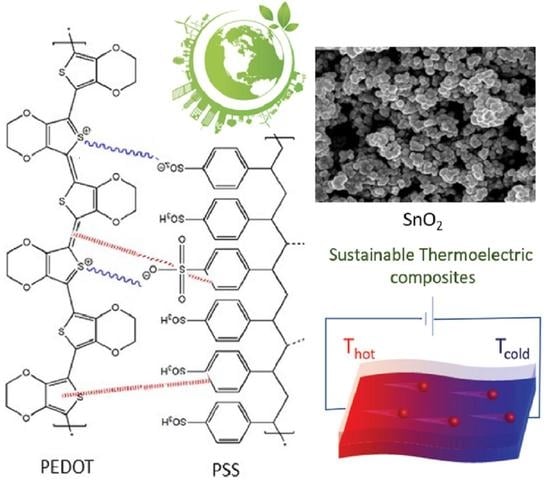
Environmentally Friendly Synthesis of Poly(3,4-Ethylenedioxythiophene): Poly(Styrene Sulfonate)/SnO2 Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of PEDOT:PSS/SnO2 Nanocomposites
2.3. Characterization Techniques
3. Results and Discussion
3.1. Characterization of SnO2 Nanoparticles
3.2. Morphology of the Nanocomposites
3.3. Raman Spectra of the Nanocomposites
3.4. Thermoelectric Performance of PEDOT:PSS/SnO2 Nanocomposites
3.5. Thermal Stability of PEDOT:PSS/SnO2
3.6. Tensile Properties of PEDOT:PSS/SnO2 Nanocomposites
3.7. Optical Properties of PEDOT:PSS/SnO2 Nanocomposites
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [Green Version]
- Díez-Pascual, A.M.; Luceño Sánchez, J.A.; Peña Capilla, R.; García Díaz, P. Recent Developments in Graphene/Polymer Nanocomposites for Application in Polymer Solar Cells. Polymers 2018, 10, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.-M.; Yeo, J.-S.; Kim, J.; Jeong, H.-G.; Kim, D.-Y.; Noh, Y.-J.; Kim, S.-S.; Ku, B.-C.; Na, S.-I. Solution-processable reduced graphene oxide as a novel alternative to PEDOT:PSS hole transport layers for highly efficient and stable polymer solar cells. Adv. Mater. 2011, 23, 4923–4928. [Google Scholar] [CrossRef]
- Naffakh, M.; Diez-Pascual, A.M. Thermoplastic Polymer Nanocomposites Based on Inorganic Fullerene-like Nanoparticles and Inorganic Nanotubes. Inorganics 2014, 2, 291–312. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Liu, C.; Jiang, F.; Xu, J.; Liu, E. Effective treatment methods on PEDOT: PSS to enhance its thermoelectric performance. Synth. Met. 2017, 225, 31–40. [Google Scholar] [CrossRef]
- Culebras, M.; Gomez, C.M.; Cantarero, A. Review on polymers for thermoelectric applications. Materials 2014, 7, 6701–6732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luceño-Sánchez, J.A.; Charas, A.; Díez-Pascual, A.M. Effect of HDI-Modified GO on the Thermoelectric Performance of Poly(3,4-ethylenedioxythiophene): Poly(Styrenesulfonate) Nanocomposite Films. Polymers 2021, 13, 1503. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, G. Conducting polymer/carbon particle thermoelectric composites: Emerging green energy materials. Compos. Sci. Technol. 2016, 124, 52–70. [Google Scholar] [CrossRef]
- Lian, H.T.; Zhenyu, T.; Hongen, G.; Zheng, Z.; Jian, W.; Qingchen, D.; Furong, Z.; Bin, W.; Wong, W.-Y. Magnetic nanoparticles/PEDOT:PSS composite hole-injection layer for efficient organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 4903–4911. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, H.; Fu, Q. Recent progress on PEDOT:PSS based polymer blends and composites for flexible electronics and thermoelectric devices. Mater. Chem. Front. 2020, 4, 3130. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Katz, H.; Fang, F.; Opila, R. Promising thermoelectric properties of commercial PEDOT: PSS materials and their Bi2Te3 powder composites. ACS Appl. Mater. Interfaces 2010, 2, 3170–3178. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, L.; Xu, J.; Liu, C.; Zhou, W.; Shi, H.; Jiang, Q.; Jiang, F. Thermoelectric performance of PEDOT: PSS/ Bi2 Te3-nanowires: A comparison of hybrid types. J. Mater. Sci. Mater. Electron. 2016, 27, 1769–1776. [Google Scholar] [CrossRef]
- Yee, S.K.; Coates, N.E.; Majumdar, A.; Urban, J.J.; Segalman, R.A. Thermoelectric power factor optimization in PEDOT: PSS tellurium nanowire hybrid composites. Phys. Chem. Chem. Phys. 2013, 15, 4024–4032. [Google Scholar] [CrossRef]
- Yu, C.; Choi, K.; Yin, L.; Grunlan, J.C. Light-Weight Flexible Carbon Nanotube Based Organic Composites with Large Thermoelectric Power Factors. ACS Nano 2011, 5, 7885–7892. [Google Scholar] [CrossRef]
- Li, F.; Cai, K.; Shen, S.; Chen, S. Preparation and thermoelectric properties of reduced graphene oxide/PEDOT: PSS composite films. Synth. Met. 2014, 197, 58–61. [Google Scholar] [CrossRef]
- Pananon, P.; Sriprachuabwong, C.; Wisitsoraat, A.; Chuysinuan, P.; Tuantranont, A.; Saparpakornd, P.; Dechtrirat, D. A facile one-pot green synthesis of gold nanoparticle-graphene-PEDOT:PSS nanocomposite for selective electrochemical detection of dopamine. RSC Adv. 2018, 8, 12724. [Google Scholar] [CrossRef] [Green Version]
- Di, L.B.; Zhang, X.L.; Xu, Z.J.; Wang, K. Atmospheric-Pressure Cold Plasma for Preparation of High Performance Pt/TiO2 Photocatalyst and Its Mechanism. Plasma Chem. Plasma Process. 2014, 34, 301. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Loyer, F.; Frache, G.; Choquet, P.; Boscher, N.D. Atmospheric Pressure Plasma-Initiated Chemical Vapor Deposition (AP-PiCVD) of Poly(alkyl acrylates): An Experimental Study. Macromolecules 2017, 50, 4351. [Google Scholar] [CrossRef]
- Molina, R.; Ligero, C.; Jovančić, P.; Bertran, E. In Situ Polymerization of Aqueous Solutions of NIPAAm Initiated by Atmospheric Plasma Treatment. Plasma Process. Polym. 2013, 10, 506–516. [Google Scholar] [CrossRef]
- Nguyen, L.N. In situ plasma-assisted synthesis of polydopamine-functionalized gold nanoparticles for biomedical applications. Green Chem. 2020, 22, 6588–6599. [Google Scholar] [CrossRef]
- Sanon, G.; Rup, R.; Mansingh, A. Band-gap narrowing and band structure in degenerate tin oxide (SnO2) films. Phys. Rev. B Condens. Matter Mater. Phys. 1991, 44, 5672–5680. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Venugopal, N.; Raja, K.P.; Rao, M.M. Nano SnO2–Al2O3 mixed oxide and SnO2–Al2O3–carbon composite oxides as new and novel electrodes for supercapacitor applications. J. Power Sour. 2006, 158, 1538–1543. [Google Scholar] [CrossRef]
- Pandimurugan, A.R.; Sankaranarayanan, K. Antibacterial and photocatalytic activity of ZnO, SnO2 and Zn2SnO4 nanoparticles prepared by Microwave assisted method. Mater. Technol. 2021, 1, 11. [Google Scholar] [CrossRef]
- Soumen, D.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Leung, T.L.; Liu, F. Doped SnO2 nanoparticles for solar-cell application. Proc. SPIE 2019, 10919, 109192K. [Google Scholar] [CrossRef]
- Chiappim, W.; Sampaio, A.d.G.; Miranda, F.; Fraga, M.; Petraconi, G.; da Silva Sobrinho, A.; Kostov, K.; Koga-Ito, C.; Pessoa, R. Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus aureus, Escherichia coli, and Candida albicans. Water 2021, 13, 1480. [Google Scholar] [CrossRef]
- Pawłat, J.; Terebun, P.; Kwiatkowski, M.; Tarabová, B.; Kovaľová, Z.; Kučerová, K.; Machala, Z.; Janda, M.; Hensel, K. Evaluation of Oxidative Species in Gaseous and Liquid Phase Generated by Mini-Gliding Arc Discharge. Plasma Chem. Plasma Process. 2019, 39, 627–642. [Google Scholar] [CrossRef] [Green Version]
- Van Alphen, S.; Jardali, F.; Creel, J.; Trenchev, G.; Snyders, R.; Bogaerts, A. Sustainable gas conversion by gliding arc plasmas: A new modelling approach for reactor design improvement. Sustain. Energy Fuels 2021, 5, 1786–1800. [Google Scholar] [CrossRef]
- Debataraja, A.; Zulhendri, D.; Yuliarto, B.; Tapran, N.; Purwasasmita, B. Investigation of Nanostructured SnO2 Synthesized with Polyol Technique for CO Gas Sensor Applications. Procedia Eng. 2017, 170, 60–64. [Google Scholar] [CrossRef]
- Patterson, A. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Dieguez, A.; Romano-Rodriguez, A.; Vila, A.; Morante, J.R. The Complete Raman Spectrum of Nanometric SnO2 Particles. J. Appl. Phys. 2001, 90, 1550–1557. [Google Scholar] [CrossRef] [Green Version]
- Luceño Sánchez, J.A.; Peña Capilla, R.; Díez-Pascual, A.M. High-Performance PEDOT:PSS/Hexamethylene Diisocyanate-Functionalized Graphene Oxide Nanocomposites: Preparation and Properties. Polymers 2018, 10, 1169. [Google Scholar] [CrossRef] [Green Version]
- Alonso, E.; Faria, M.; Ferreira, A.; Corderio, N. Influence of the matrix and polymerization methods on the synthesis of BC/PANi nanocomposites: An IGC study. Cellulose 2018, 25, 2343–2354. [Google Scholar] [CrossRef]
- Chiu, W.W.; Travaš-Sejdic, J.; Cooney, R.P.; Bowmaker, G.A. Studies of dopant effects in poly(3,4-ethylenedi-oxythiophene) using Raman spectroscopy. J. Raman Spectrosc. 2006, 37, 1354–1361. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Lee, S.H.; Cho, W.; Choi, H.H.; Kin, F.S.; Kim, J.H. Effects of one- and two-dimensional carbon hybridization of PEDOT:PSS on the power factor of polymer thermoelectric energy conversion devices. J. Mater. Chem. A 2015, 3, 6526–6533. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1–16. [Google Scholar] [CrossRef]
- Rajeeva, M.P. Study on low temperature DC electrical conductivity of SnO2 nanomaterial synthesized by simple gel combustion method. AIP Conf. Proc. 2015, 1665, 050091. [Google Scholar] [CrossRef]
- Diantoro, M.; Kholid, A.A.; Yudiyanto, M. The Influence of SnO2 Nanoparticles on Electrical Conductivity, and Transmittance of PANI-SnO2 Films. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012034. [Google Scholar] [CrossRef]
- Lang, U.; Muller, E.; Naujoks, N.; Dual, J. Microscopical investigations of PEDOT:PSS thin films. Adv. Funct. Mater. 2009, 19, 1215–1220. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; Janssen, R.A.J. Anisotropic hopping conduction in spin-coated PEDOT:PSS thin films. Phys. Rev. B 2007, 76, 085208. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Xu, Q.; Chu, C.-W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Xiong, J.; Jiang, F.; Shi, H.; Xu, J.; Liu, C.; Zhou, W.; Jiang, Q.; Zhu, Z.; Hu, Y. Liquid exfoliated graphene as dopant for improving the thermoelec-tric power factor of conductive PEDOT:PSS nanofilm with hydrazine treatment. ACS Appl. Mater. Interfaces 2015, 7, 14917–14925. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.-C.; Chang, J.; Wu, F.-C.; Cheng, H.-L.; Hsu, S.L.-C.; Chen, J.-S.; Chou, W.-Y. Alignment of poly(3,4-ethylenedioxythiophene) polymer chains in photovoltaic cells by ultraviolet irradiation. J. Mater. Chem. 2012, 22, 22409–22417. [Google Scholar] [CrossRef]
- Jin, Y.; Gerhardt, R.A. Prediction of the Percolation Threshold and Electrical conductivity of Self-Assembled Antimony-Doped Tin Oxide Nanoparticles into Ordered Structures in PMMA/ATO Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 22264–22271. [Google Scholar] [CrossRef]
- Stöcker, T.; Köhler, A.; Moos, R. Why does the electrical conductivity in PEDOT:PSS decrease with PSS content? A study combining thermoelectric measurements with impedance spectroscopy. J. Polym. Sci. B Polym. Phys. 2012, 50, 976–983. [Google Scholar] [CrossRef]
- Boor, J.; Müller, E. Data analysis for Seebeck coefficient measurements. Rev. Sci. Instrum. 2013, 84, 065102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wang, S.; Zhang, X.; Zhang, Y.; Cui, Y.; Qiu, J. Thermoelectric performance of p-type nanohybrids filled polymer composites. Nano Energy 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Du, F.-P.; Cao, N.-N.; Zhang, Y.-F.; Fu, P.; Wu, Y.-G.; Lin, Z.-D.; Shi, R.; Amini, A.; Cheng, C. PEDOT:PSS/graphene quantum dots films with enhanced thermoelectric properties via strong interfacial interaction and phase separation. Sci. Rep. 2018, 8, 6441. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Hwang, D.H.; Woo, S.I. Thermoelectric properties of nanocomposite thin films prepared with poly(3,4- ethylenedioxythiophene) poly(styrenesulfonate) and graphene. Phys. Chem. Chem. Phys. 2012, 14, 3530–3536. [Google Scholar] [CrossRef]
- Du, Y.; Cai, K.; Shen, S.; Yang, W.; Jiayue, X.; Lin, T. ZnO flower/PEDOT:PSS thermoelectric composite films. J. Mater. Sci. Mater. Electr. 2016, 27, 10289–10293. [Google Scholar] [CrossRef]
- Stepien, L.; Roch, A.; Tkachov, R.; Leupolt, B.; Han, L.; van Ngo, N.; Leyens, C. Thermal operating window for PEDOT:PSS films and its related thermoelectric properties. Synth. Met. 2017, 225, 49–54. [Google Scholar] [CrossRef]
- Vitoratos, S.; Sakkopoulos, E.; Dalas, N.; Paliatsas, D.; Karageorgopoulos, F.; Petraki, S.; Kennou, S.A. Choulis, Thermal degradation mechanisms of PEDOT:PSS. Org. Electr. 2009, 10, 61–66. [Google Scholar] [CrossRef]
- Diaz, A.J. High-stress study of bioinspired multifunctional PEDOT:PSS/nanoclay nanocomposites using AFM, SEM and numerical simulation. Beilstein J. Nanotechnol. 2017, 8, 2069–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guruswamy, B.; Ravindrachary, V.; Shruthi, C.; Mylarappa, M. Effect of SnO2 Nanoparticle Doping on Structural, Morphological and Thermal Properties of PVA-PVP Polymer Blend. Mater. Sci. Forum 2019, 962, 82–88. [Google Scholar] [CrossRef]
- Esmailzadeh, M.; Daneshmanesh, H.; Zebarjad, S. Role of SnO2 nanoparticles on mechanical and thermal properties of flexible polyurethane foam nanocomposite. J. Porous Mater. 2016, 23, 1381–1388. [Google Scholar] [CrossRef]
- Qu, J.; Ouyang, L.; Kuo, C.C.; Martin, D.C. Stiffness, strength and adhesion characterization of electrochemically deposited conjugated polymer films. Acta Biomater. 2016, 31, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Geer, R. Namomechanical Imaging and Nanoscale Elastic Modulus Measurements of SnO2 Nanobelts. MRS Proc. 2014, 821, 23. [Google Scholar] [CrossRef]
- Odegard, G.M.; Clancy, T.C.; Gates, T.S. Modeling of the mechanical properties of nanoparticle/polymer composites. Polymer 2005, 46, 553–562. [Google Scholar] [CrossRef]
- Fu, S.Y.; Xu, G.; Mai, Y.-W. On the elastic modulus of hybrid Particle/short fiber/polymer composites. Compos. Part B 2002, 33, 291–299. [Google Scholar] [CrossRef]
- Cutolo, A.; Carotenuto, A.R.; Palumbo, S.; Bosia, F.; Pugno, N.M.; Fraldi, M. Unveiling a new shear stress transfer mechanism in composites with helically wound hierarchical fibres. Int. J. Mechan. Sci. 2020, 192, 106135. [Google Scholar] [CrossRef]
- Ou, Y.; Yang, F.; Yu, Z.Z. A new conception on the toughness of nylon 6/silica nanocomposite prepared via in situ polymerization. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 789–795. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, N. Mechanical and thermal behaviour of isotactic polypropylene reinforced with inorganic fullerene-like WS2 nanoparticles: Effect of filler loading and temperature. Mater. Chem. Phys. 2013, 141, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Chang-Jian, C.-W. Thermally conductive polymeric composites incorporating 3D MWCNT/PEDOT:PSS scaffolds. Compos. Part B 2018, 136, 46–54. [Google Scholar] [CrossRef]
- Chang, S.H.; Chiang, C.-H.; Kao, F.S.; Tien, C.-L.; Wu, C.-G. Unraveling the Enhanced Electrical Conductivity of PEDOT:PSS Thin Films for ITO-Free Organic Photovoltaics. IEEE Photon. J. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Byrne, M.T.; Gunko, Y.K. Recent Advances in Research on Carbon Nanotube-Polymer Composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.S.; Singh, J.P.; Singh, R. A novel composite material of graphene and PEDOT:PSS. AIP Conf. Proc. 2016, 1731, 140021. [Google Scholar] [CrossRef]
- Chopra, K.L.; Major, S.; Pandya, D.K. Transparent conductors—A status review. Thin Solid Films 1983, 102, 1–46. [Google Scholar] [CrossRef]










| Sample | Tonset (°C) | T10 (°C) | Tpeak(I,II) (°C) | R (wt%) |
|---|---|---|---|---|
| PEDOT:PSS | 130 | 227 | 249, 479 | 6.8 |
| PEDOT:PSS (1:2.5)/SnO2 (0.5 wt%) | 136 | 225 | 249, 462 | 7.1 |
| PEDOT:PSS(1:2.5)/SnO2 (1.0 wt%) | 147 | 230 | 257, 485 | 7.9 |
| PEDOT:PSS(1:2.5)/SnO2 (2.0 wt%) | 169 | 246 | 270, 527 | 8.4 |
| PEDOT:PSS(1:2.5)/SnO2 (5.0 wt%) | 178 | 252 | 287, 545 | 9.7 |
| PEDOT:PSS(1:2.5)/SnO2 (10 wt%) | 183 | 258 | 282, 539 | 10.3 |
| PEDOT:PSS (1:6)/SnO2 (0.5 wt%) | 149 | 247 | 265, 497 | 7.0 |
| PEDOT:PSS (1:6)/SnO2 (1.0 wt%) | 174 | 259 | 279, 498 | 7.5 |
| PEDOT:PSS (1:6)/SnO2 (2.0 wt%) | 205 | 273 | 296, 534 | 8.0 |
| PEDOT:PSS (1:6)/SnO2 (5.0 wt%) | 201 | 277 | 300, 561 | 8.5 |
| PEDOT:PSS (1:6)/SnO2 (10 wt%) | 190 | 274 | 299, 573 | 9.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Pascual, A.M. Environmentally Friendly Synthesis of Poly(3,4-Ethylenedioxythiophene): Poly(Styrene Sulfonate)/SnO2 Nanocomposites. Polymers 2021, 13, 2445. https://doi.org/10.3390/polym13152445
Díez-Pascual AM. Environmentally Friendly Synthesis of Poly(3,4-Ethylenedioxythiophene): Poly(Styrene Sulfonate)/SnO2 Nanocomposites. Polymers. 2021; 13(15):2445. https://doi.org/10.3390/polym13152445
Chicago/Turabian StyleDíez-Pascual, Ana M. 2021. "Environmentally Friendly Synthesis of Poly(3,4-Ethylenedioxythiophene): Poly(Styrene Sulfonate)/SnO2 Nanocomposites" Polymers 13, no. 15: 2445. https://doi.org/10.3390/polym13152445
APA StyleDíez-Pascual, A. M. (2021). Environmentally Friendly Synthesis of Poly(3,4-Ethylenedioxythiophene): Poly(Styrene Sulfonate)/SnO2 Nanocomposites. Polymers, 13(15), 2445. https://doi.org/10.3390/polym13152445