Ion Mobility in Thick and Thin Poly-3,4 Ethylenedioxythiophene Films—From EQCM to Actuation
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Electropolymerization
2.3. Actuation Measurements
2.4. EQCM Measurements
2.5. Characterization
3. Results and Discussions
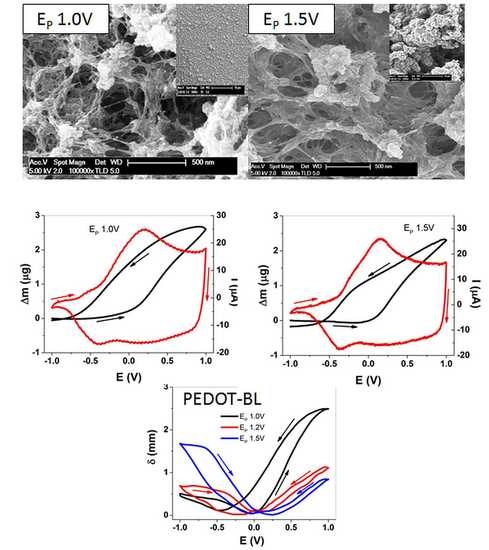
3.1. SEM and EDX Spectroscopy of PEDOT Samples
3.2. Cyclic Voltammetry Driven Ion Exchange
3.2.1. PEDOT Linear and Bending Actuation
3.2.2. EQCM Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alici, G.; Metz, P.; Spinks, G.M. A methodology towards geometry optimization of high performance polypyrrole (PPy) actuators. Smart Mater. Struct. 2006, 15, 243–252. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Physical and chemical awareness from sensing polymeric artificial muscles. Experiments and modeling. Prog. Polym. Sci. 2015, 44, 62–78. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Tanaka, N.; Takashima, W.; Kaneto, K. Artificial fibular muscles with 20% strain based on polypyrrole-metal coil composites. Chem. Lett. 2005, 34, 784–785. [Google Scholar] [CrossRef]
- Aydemir, N.; Kilmartin, P.A.; Travas-Sejdic, J.; Kesküla, A.; Peikolainen, A.L.; Parcell, J.; Harjo, M.; Aabloo, A.; Kiefer, R. Electrolyte and solvent effects in PPy/DBS linear actuators. Sens. Actuators B Chem. 2015, 216, 24–32. [Google Scholar] [CrossRef]
- Harjo, M.; Järvekülg, M.; Tamm, T.; Otero, T.F.; Kiefer, R. Concept of an artificial muscle design on polypyrrole nanofiber scaffolds. PLoS ONE 2020, 15, 1–16. [Google Scholar] [CrossRef]
- Maziz, A.; Concas, A.; Khaldi, A.; Stålhand, J.; Persson, N.-K.; Jager, E.W.H. Knitting and weaving artificial muscles. Sci. Adv. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Jager, E.W.H.; Smela, E.; Ingana, O. Microfabricating Conjugated Polymer Actuators. Science 2000, 290, 1540–1545. [Google Scholar] [CrossRef]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Martinez, J.G.; Richter, K.; Persson, N.K.; Jager, E.W.H. Investigation of electrically conducting yarns for use in textile actuators. Smart Mater. Struct. 2018, 27. [Google Scholar] [CrossRef]
- Yan, B.; Wu, Y.; Guo, L. Recent advances on polypyrrole electroactuators. Polymers 2017, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Entezami, A.A.; Massoumi, B. Artificial muscles, biosensors and drug delivery systems based on conducting polymers: A review. Iran. Polym. J. 2006, 15, 13–30. [Google Scholar]
- Melling, D.; Martinez, J.G.; Jager, E.W.H. Conjugated Polymer Actuators and Devices: Progress and Opportunities. Adv. Mater. 2019, 31, 1808210. [Google Scholar] [CrossRef] [PubMed]
- Põldsalu, I.; Rohtlaid, K.; Nguyen, T.M.G.; Plesse, C.; Vidal, F.; Khorram, M.S.; Peikolainen, A.L.; Tamm, T.; Kiefer, R. Thin ink-jet printed trilayer actuators composed of PEDOT:PSS on interpenetrating polymer networks. Sens. Actuators B Chem. 2018, 258, 1072–1079. [Google Scholar] [CrossRef]
- Tyagi, M.; Spinks, G.M.; Jager, E.W.H. 3D Printing Microactuators for Soft Microrobots. Soft Robot. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Pei, Q.; Zuccarello, G.; Ahlskog, M.; Inganäs, O. Electrochromic and highly stable poly(3,4-ethylenedioxythiophene) switches between opaque blue-black and transparent sky blue. Polymer 1994, 35, 1347–1351. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Y. Effect of electrolyte solvent on the conductivity and structure of as-prepared polypyrrole films. Polymer 1997, 38, 1971–1976. [Google Scholar] [CrossRef]
- Maw, S.; Smela, E.; Yoshida, K.; Stein, R.B. Effects of monomer and electrolyte concentrations on actuation of PPy(DBS) bilayers. Synth. Met. 2005, 155, 18–26. [Google Scholar] [CrossRef]
- Khanh, T.T.; Kesküla, A.; Zondaka, Z.; Harjo, M.; Kivilo, A.; Khorram, M.S.; Tamm, T.; Kiefer, R. Role of polymerization temperature on the performance of polypyrrole/dodecylbenzenesulphonate linear actuators. Synth. Met. 2019, 247, 53–58. [Google Scholar] [CrossRef]
- Smela, E.; Gadegaard, N. Surprising volume change in PPy(DBS): An atomic force microscopy study. Adv. Mater. 1999, 11, 953–956. [Google Scholar] [CrossRef]
- Kiefer, R.; Bowmaker, G.A.; Cooney, R.P.; Kilmartin, P.A.; Travas-Sejdic, J. Cation driven actuation for free standing PEDOT films prepared from propylene carbonate electrolytes containing TBACF3SO3. Electrochim. Acta 2008, 53, 2593–2599. [Google Scholar] [CrossRef]
- Aziz, S.; Martinez, J.G.; Salahuddin, B.; Persson, N.K.; Jager, E.W.H. Fast and High-Strain Electrochemically Driven Yarn Actuators in Twisted and Coiled Configurations. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Vandesteeg, N.A.; Madden, P.G.; Madden, J.D.; Anquetil, P.A.; Hunter, I.W. Synthesis and characterization of EDOT-based conducting polymer actuators. Proc. SPIE 2003, 5051, 349–356. [Google Scholar] [CrossRef]
- Weis, D.G.; Kiefer, R.; Zondaka, Z.; Tamm, T.; Urban, G. Polypyrrole and poly (3,4-ethylenedioxythiophene ) on silicon cantilever: Role of formation potential in bending displacement. Synth. Met. 2021, 271, 116653. [Google Scholar] [CrossRef]
- Niu, L.; Kvarnström, C.; Ivaska, A. Mixed ion transfer in redox processes of poly(3,4-ethylenedioxythiophene). J. Electroanal. Chem. 2004, 569, 151–160. [Google Scholar] [CrossRef]
- Hillman, A.R.; Daisley, S.J.; Bruckenstein, S. Kinetics and mechanism of the electrochemical p-doping of PEDOT. Electrochem. Commun. 2007, 9, 1316–1322. [Google Scholar] [CrossRef]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 1054, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Buttry, D.A.; Ward, M.D. Measurement of Interfaclal Processes at Electrode Surfaces with the Electrochemical Quartz Crystal Microbalance. Chem. Rev. 1992, 92, 1355–1379. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Bilger, R.R. Elektrochemische und Gravimetrische in Situ Untersuchungen an Leitfähigen Polymeron; Albert-Ludwigs-University: Freiburg i. Brsg., Germany, 1994. [Google Scholar]
- Kiefer, R.; Weis, D.G.; Travas-Sejdic, J.; Urban, G.; Heinze, J. Effect of electrochemical synthesis conditions on deflection of PEDOT bilayers. Sens. Actuators B Chem. 2007, 123, 379–383. [Google Scholar] [CrossRef]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of actuation in conducting polymers: Osmotic expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Robert Hillman, A.; Daisley, S.J.; Bruckenstein, S. Solvent effects on the electrochemical p-doping of PEDOT. Phys. Chem. Chem. Phys. 2007, 9, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Kesküla, A.; Peikolainen, A.L.; Kiefer, R.; Tamm, T. Consistent response from conducting polymer actuators: Potential window and embedded charges to avoid mixed ion transport. Synth. Met. 2020, 268, 116502. [Google Scholar] [CrossRef]
- Mocaër, A.; Pillier, F.; Pailleret, A. Switching of the ion exchange behaviour of PEDOT thin films during a potential cycling: An electrochemical atomic force microscopy study. Electrochim. Acta 2021, 138651. in press. [Google Scholar] [CrossRef]
- Kiefer, R.; Nguyen, N.T.; Le, Q.B.; Anbarjafari, G.; Tamm, T. Antagonist concepts of polypyrrole actuators: Bending hybrid actuator and mirrored trilayer linear actuator. Polymers 2021, 13, 861. [Google Scholar] [CrossRef]
- Kvarnström, C.; Neugebauer, H.; Blomquist, S.; Ahonen, H.J.; Kankare, J.; Ivaska, A. In situ spectroelectrochemical characterization of poly (3, 4-ethylenedioxythiophene ). Electrochim. Acta 1999, 44, 2739–2750. [Google Scholar] [CrossRef]
- Hillman, A.R.; Daisley, S.J.; Bruckenstein, S. Ion and solvent transfers and trapping phenomena during n-doping of PEDOT films. Electrochim. Acta 2008, 53, 3763–3771. [Google Scholar] [CrossRef]






| EP (V) | tp (min) | Q (mC cm−2) | j (mA cm−2) | d (μm) | ||||
|---|---|---|---|---|---|---|---|---|
| BL | FF | BL | FF | BL | FF | BL | FF | |
| 1.0 | 228 ± 14 | 327 ±22 | 333 ± 22 | 356 ± 21 | 0.05 ± 0.003 | 0.06 ± 0.004 | 4.3 ± 0.4 | 33 ± 3 |
| 1.2 | 5.2 ± 0.3 | 8.8 ± 0.4 | 365 ± 22 | 378 ± 27 | 1.46 ± 0.1 | 1.6 ± 0.1 | 4.8 ± 0.5 | 34 ± 2 |
| 1.5 | 0.9 ± 0.1 | 1.2 ± 0.1 | 367 ± 30 | 386 ± 32 | 6.73 ± 0.4 | 7.8 ± 0.5 | 5.2 ± 0.4 | 38 ± 3 |
| EP | PEDOT-FF (S cm−1) | PEDOT-BL (S cm−1) |
|---|---|---|
| 1.0 V | 6.4 ± 0.6 | 15.8 ± 1.2 |
| 1.2 V | 3.5 ± 0.3 | 8.8 ± 0.7 |
| 1.5 V | 2.5 ± 0.2 | 5.9 ± 0.5 |
| EP | PEDOT-FF | PEDOT-BL | ||||
|---|---|---|---|---|---|---|
| ε (%) +1 V | ε (%) −1 V | Q (C cm−3) | δ (mm) +1 V | δ (mm) −1 V | Q (C cm−3) | |
| 1.0 V | 0.92 ± 0.08 | 0.16 ± 0.01 | 98 ± 8 | 2.5 ± 0.2 | 0.50 ± 0.03 | 91 ± 8 |
| 1.2 V | 0.73 ± 0.06 | 0.56 ± 0.04 | 87 ± 8 | 1.1 ± 0.1 | 0.70 ± 0.05 | 85 ± 7 |
| 1.5 V | 0.55 ± 0.05 | 0.82 ± 0.07 | 72 ± 7 | 0.80 ± 0.06 | 1.7 ± 0.1 | 74 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiefer, R.; Weis, D.G.; Velmurugan, B.K.; Tamm, T.; Urban, G. Ion Mobility in Thick and Thin Poly-3,4 Ethylenedioxythiophene Films—From EQCM to Actuation. Polymers 2021, 13, 2448. https://doi.org/10.3390/polym13152448
Kiefer R, Weis DG, Velmurugan BK, Tamm T, Urban G. Ion Mobility in Thick and Thin Poly-3,4 Ethylenedioxythiophene Films—From EQCM to Actuation. Polymers. 2021; 13(15):2448. https://doi.org/10.3390/polym13152448
Chicago/Turabian StyleKiefer, Rudolf, Daniel Georg Weis, Bharath Kumar Velmurugan, Tarmo Tamm, and Gerald Urban. 2021. "Ion Mobility in Thick and Thin Poly-3,4 Ethylenedioxythiophene Films—From EQCM to Actuation" Polymers 13, no. 15: 2448. https://doi.org/10.3390/polym13152448
APA StyleKiefer, R., Weis, D. G., Velmurugan, B. K., Tamm, T., & Urban, G. (2021). Ion Mobility in Thick and Thin Poly-3,4 Ethylenedioxythiophene Films—From EQCM to Actuation. Polymers, 13(15), 2448. https://doi.org/10.3390/polym13152448