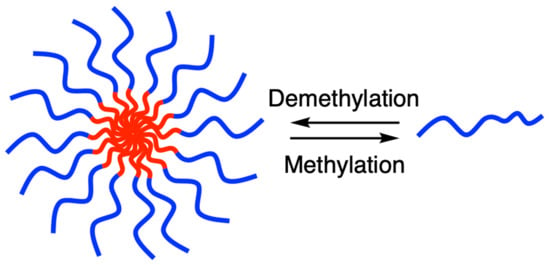
Micellar Assembly and Disassembly of Organoselenium Block Copolymers through Alkylation and Dealkylation Processes
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klose, R.J.; Zhang, Y. Regulation of histone methylation by demethylimination and demethylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Organoselenium chemistry-based polymer synthesis. Org. Chem. Front. 2020, 7, 2815–2841. [Google Scholar] [CrossRef]
- Xia, J.; Li, T.; Lu, C.; Xu, H. Selenium-containing polymers: Perspectives toward diverse applications in both adaptive and biomedical materials. Macromolecules 2018, 51, 7435–7455. [Google Scholar] [CrossRef]
- Ji, S.B.; Xia, J.H.; Xu, H.P. Dynamic chemistry of selenium: Se-N and Se-Se dynamic covalent bonds in polymeric systems. ACS Macro Lett. 2016, 5, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Cao, W.; Zhang, X. Selenium-containing polymers: Promising biomaterials for controlled release and enzyme mimics. Acc. Chem. Res. 2013, 46, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Q.; Driessen, F.; Zhu, X.L.; Du Prez, F.E. Selenolactone as a building block toward dynamic diselenide-containing polymer architectures with controllable topology. ACS Macro Lett. 2017, 6, 89–92. [Google Scholar] [CrossRef]
- Chen, S.; Pan, X.; Zhu, J.; Zhu, X. Synthesis of selenide-containing polymers by multicomponent polymerization based on γ-butyroselenolactone. Polym. Chem. 2019, 10, 6395–6400. [Google Scholar] [CrossRef]
- Zhang, C.J.; Cao, X.H.; Zhang, X.H. Metal-Free alternating copolymerization of nonstrained γ-selenobutyrolactone with epoxides for selenium-rich polyesters. Macromolecules 2020, 53, 203–211. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, X.; Zhang, Z.; Zhu, J.; Pan, X.; Zhu, X. A novel synthesis of poly (ester-Alt.-selenide) s by ring-opening copolymerization of γ-selenobutyrolactone and epoxy monomer. Polymers 2020, 12, 1203. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; An, X.; Gao, F.; Zhu, J.; Zhou, N.; Zhang, Z.; Pan, X.; Zhu, X. Highly efficient chain end derivatization of selenol-ended polystyrenes by nucleophilic substitution reactions. Macromol. Chem. Phys. 2017, 218, 1600485. [Google Scholar] [CrossRef]
- Jiang, H.; Pan, X.; Li, N.; Zhang, Z.; Zhu, J.; Zhu, X. Selenide-containing high refractive index polymer material with adjustable refractive index and Abbe’s number. React. Funct. Polym. 2017, 111, 1–6. [Google Scholar] [CrossRef]
- Han, P.; Ma, N.; Ren, H.; Xu, H.; Li, Z.; Wang, Z.; Zhang, X. Oxidation-responsive micelles based on a selenium-containing polymeric superamphiphile. Langmuir 2010, 26, 14414–14418. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wu, Y.; Ma, N.; Xu, H.; Zhang, X. Side-chain selenium-containing amphiphilic block copolymers: Redox-controlled self-assembly and disassembly. Soft Matter 2012, 8, 1460–1466. [Google Scholar] [CrossRef]
- Eom, T.; Khan, A. Polyselenonium salts: Synthesis through sequential selenium-epoxy ‘click’ chemistry and Se-alkylation. Chem. Commun. 2020, 56, 14271–14274. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Khan, A. Selenonium polyelectrolyte synthesis through post-polymerization modifications of poly(glycidyl methacrylate) scaffolds. Polymers 2020, 12, 2685. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Khan, A. Selenium-epoxy ‘click’ reaction and Se-alkylation—Efficient Access to organo-selenium and selenonium compounds. Chemistry 2020, 2, 827–836. [Google Scholar] [CrossRef]
- Kramer, J.R.; Deming, T.J. Preparation of multifunctional and multireactive polypeptides via methionine alkylation. Biomacromolecules 2012, 13, 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Borguet, Y.P.; Tsarevsky, N.V. Controlled radical polymerization of a styrenic sulfonium monomer and post-polymerization modifications. Polym. Chem. 2013, 4, 2115–2124. [Google Scholar] [CrossRef]
- Gharakhanian, E.G.; Deming, T.J. Chemoselective synthesis of functional homocysteine residues in polypeptides and peptides. Chem. Commun. 2016, 52, 5336–5339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, S.; Stelzer, C.; Khan, A. A general synthetic strategy to prepare poly(ethylene glycol)-based multifunctional copolymers. Polym. Chem. 2012, 3, 2342–2345. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, T.; Khan, A. Micellar Assembly and Disassembly of Organoselenium Block Copolymers through Alkylation and Dealkylation Processes. Polymers 2021, 13, 2456. https://doi.org/10.3390/polym13152456
Eom T, Khan A. Micellar Assembly and Disassembly of Organoselenium Block Copolymers through Alkylation and Dealkylation Processes. Polymers. 2021; 13(15):2456. https://doi.org/10.3390/polym13152456
Chicago/Turabian StyleEom, Taejun, and Anzar Khan. 2021. "Micellar Assembly and Disassembly of Organoselenium Block Copolymers through Alkylation and Dealkylation Processes" Polymers 13, no. 15: 2456. https://doi.org/10.3390/polym13152456
APA StyleEom, T., & Khan, A. (2021). Micellar Assembly and Disassembly of Organoselenium Block Copolymers through Alkylation and Dealkylation Processes. Polymers, 13(15), 2456. https://doi.org/10.3390/polym13152456