Waterborne Cross-Linkable Polyacrylate Latex Coatings with Good Water Resistance and Strength Stabilized by Modified Hectorite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
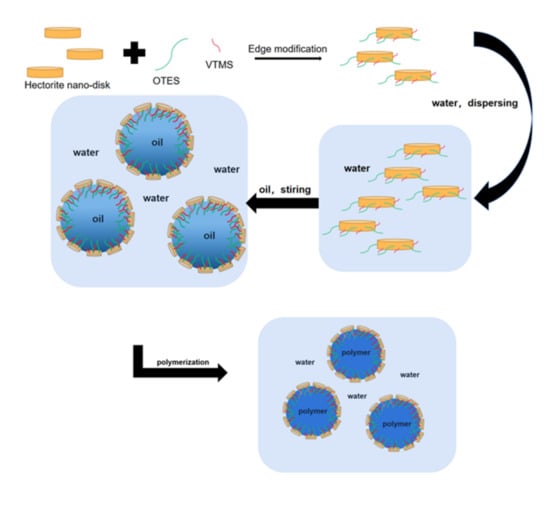
2.2. Modification of Hectorite
2.3. Preparation of O/W Emulsions and Synthesis of Polyacrylate Latexes
2.4. Characterization
2.5. Swelling Test
2.6. Water Absorption Test
3. Results and Discussion
3.1. Modification of Hectorite
3.2. Pickering Emulsion Stabilized by Hectorite
3.3. Films Obtained from the Polyacrylate Latexes
3.3.1. Swelling Ratio and Crosslinking Degree
3.3.2. Mechanical Strength
3.3.3. Water Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tığlı, R.S.; Evren, V. Synthesis and characterization of pure poly(acrylate) latexes. Prog. Org. Coat. 2005, 52, 144–150. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Miao, L.; Yang, C.-L.; Lu, M.-G. Synthesis of ambient temperature self-crosslinking VTES-based core–shell polyacrylate emulsion via modified micro-emulsion polymerization process. Polym. Bull. 2012, 70, 1631–1645. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Morphology evolution of functionalized styrene and methyl methacrylate copolymer latex nanoparticles by one-step emulsifier-free emulsion polymerization. Eur. Polym. J. 2020, 133, 109790. [Google Scholar] [CrossRef]
- Cao, Q.; Heil, T.; Kumru, B.; Antonietti, M.; Schmidt, B.V. Visible-light induced emulsion photopolymerization with carbon nitride as a stabilizer and photoinitiator. Polym. Chem. 2019, 10, 5315–5323. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Liang, Y.; He, Y. On the Pickering emulsions stabilized by calcium carbonate particles with various morphologies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123722. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Liu, P.; Ma, J.; Zhang, W.; Liu, C.; Simion, D. Novel fabrication of stable Pickering emulsion and latex by hollow silica nanoparticles. J. Colloid Interface Sci. 2019, 553, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, G.; Liu, W.; Li, Y.; Song, Z.; Wang, H.; Guan, F.; Chen, X. A fluorescent pickering-emulsion stabilizer prepared using carbon nitride quantum dots and laponite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 310–317. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Wu, D.; Wu, M.; Tian, Y.; Niu, Z.; Huang, Y. Edge-modified amphiphilic Laponite nano-discs for stabilizing Pickering emulsions. J. Colloid Interface Sci. 2013, 410, 27–32. [Google Scholar] [CrossRef]
- Garcia, P.C.; Whitby, C.P. Laponite-stabilised oil-in-water emulsions: Viscoelasticity and thixotropy. Soft Matter 2012, 8, 1609–1615. [Google Scholar] [CrossRef]
- Teixeira, R.F.A.; McKenzie, H.S.; Boyd, A.A.; Bon, S.A.F. Pickering Emulsion Polymerization Using Laponite Clay as Stabilizer To Prepare Armored “Soft” Polymer Latexes. Macromolecules 2011, 44, 7415–7422. [Google Scholar] [CrossRef]
- Li, W.; Yu, L.; Liu, G.; Tan, J.; Liu, S.; Sun, D. Oil-in-water emulsions stabilized by Laponite particles modified with short-chain aliphatic amines. Colloids Surf. A Physicochem. Eng. Asp. 2012, 400, 44–51. [Google Scholar] [CrossRef]
- Józefczak, A.; Wlazło, R. Ultrasonic Studies of Emulsion Stability in the Presence of Magnetic Nanoparticles. Adv. Condens. Matter Phys. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Briggs, N.; Raman, A.K.Y.; Barrett, L.; Brown, C.; Li, B.; Leavitt, D.; Aichele, C.P.; Crossley, S. Stable pickering emulsions using multi-walled carbon nanotubes of varying wettability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 227–235. [Google Scholar] [CrossRef]
- Godfrin, M.P.; Tiwari, A.; Bose, A.; Tripathi, A. Phase and steady shear behavior of dilute carbon black suspensions and carbon black stabilized emulsions. Langmuir 2014, 30, 15400–15407. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, P.A.; Wang, J.Z.; Baker, J.; Mathias, L.J. Synthesis and characterization of covalently functionalized laponite clay. Chem. Mater. 2005, 17, 3012–3018. [Google Scholar] [CrossRef]
- Lu, Z.; Guan, W.; Tang, L. High performances polyurethane-urea polyacrylate hybrid emulsion coatings with multiple crosslinking structures. Prog. Org. Coat. 2019, 132, 328–335. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, A.; Gan, F. Improvement of polyacrylate coating by filling modified nano-TiO2. Appl. Surf. Sci. 2006, 252, 8635–8640. [Google Scholar] [CrossRef]
- Binks, B.P.; Clint, J.H. Solid wettability from surface energy components: Relevance to pickering emulsions. Langmuir 2002, 18, 1270–1273. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Pickering emulsions stabilized by monodisperse latex particles: Effects of particle size. Langmuir 2001, 17, 4540–4547. [Google Scholar] [CrossRef]













| Type of Modified Particle | Quantity of Modifier (wt%) | ||
|---|---|---|---|
| OTES | VTMS | ||
| 1 | hectorite-C18 | 10 | 0 |
| 2 | hectorite-C18-V | 10 | 10 |
| 3 | hectorite-2C18 | 20 | 0 |
| 4 | hectorite-2C18-V | 20 | 10 |
| 5 | hectorite-2C18-2V | 20 | 20 |
| Type of Modifiers | Mean Droplet Diameter of Pickering Emulsions (µm) | |
|---|---|---|
| a | SDS | 10 ± 4 |
| b | unmodified hectorite | 54 ± 10 |
| c | hectorite-C18 | 50 ± 11 |
| d | hectorite-C18-V | 45 ± 15 |
| e | hectorite-2C18 | 30 ± 6 |
| f | hectorite-2C18-V | 25 ± 5 |
| g | hectorite-2C18-2V | 13 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chen, J.; Ma, G.; Yang, D. Waterborne Cross-Linkable Polyacrylate Latex Coatings with Good Water Resistance and Strength Stabilized by Modified Hectorite. Polymers 2021, 13, 2470. https://doi.org/10.3390/polym13152470
Yang Y, Chen J, Ma G, Yang D. Waterborne Cross-Linkable Polyacrylate Latex Coatings with Good Water Resistance and Strength Stabilized by Modified Hectorite. Polymers. 2021; 13(15):2470. https://doi.org/10.3390/polym13152470
Chicago/Turabian StyleYang, Yunfan, Jinyang Chen, Guoli Ma, and Dingqing Yang. 2021. "Waterborne Cross-Linkable Polyacrylate Latex Coatings with Good Water Resistance and Strength Stabilized by Modified Hectorite" Polymers 13, no. 15: 2470. https://doi.org/10.3390/polym13152470