Review: Sensors for Biosignal/Health Monitoring in Electronic Skin
Abstract
1. Introduction
2. Types of Sensor Mechanism
2.1. Piezoresistive Sensors
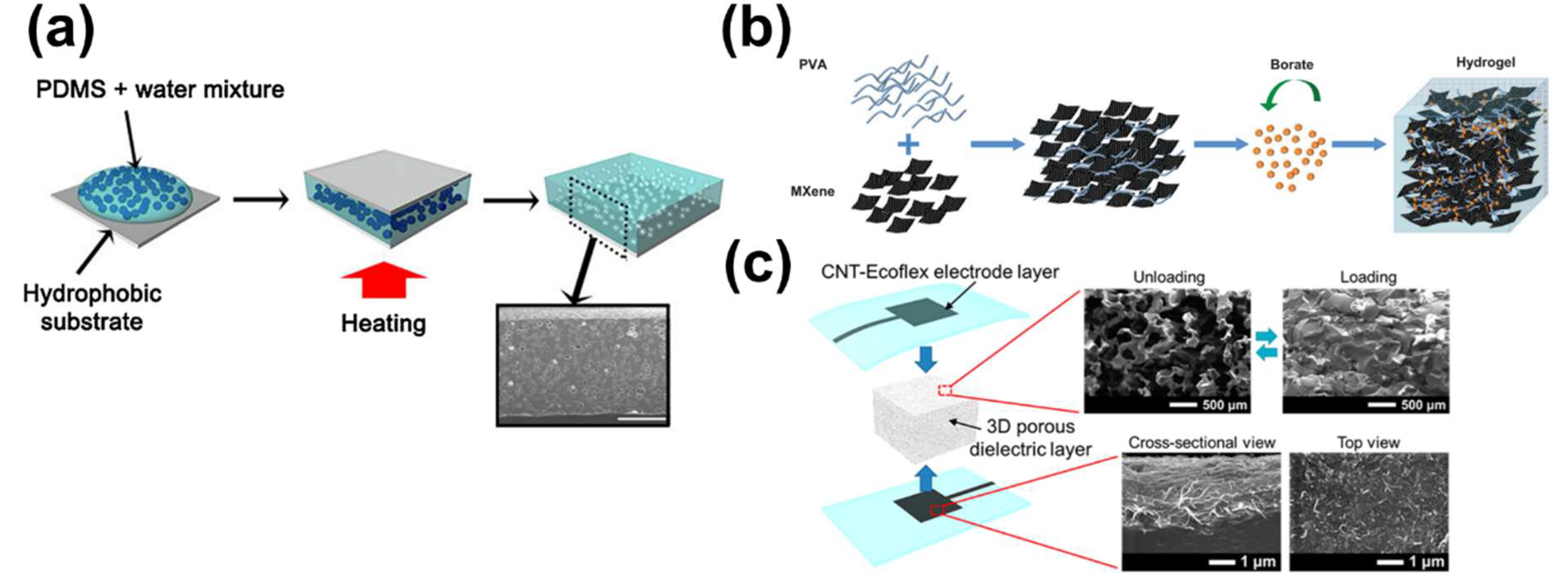
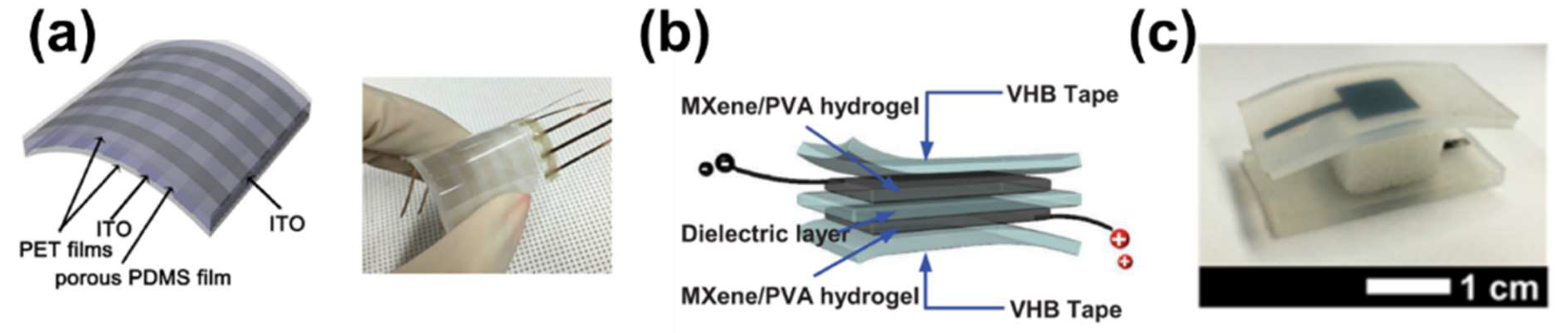
2.2. Capacitive Sensors
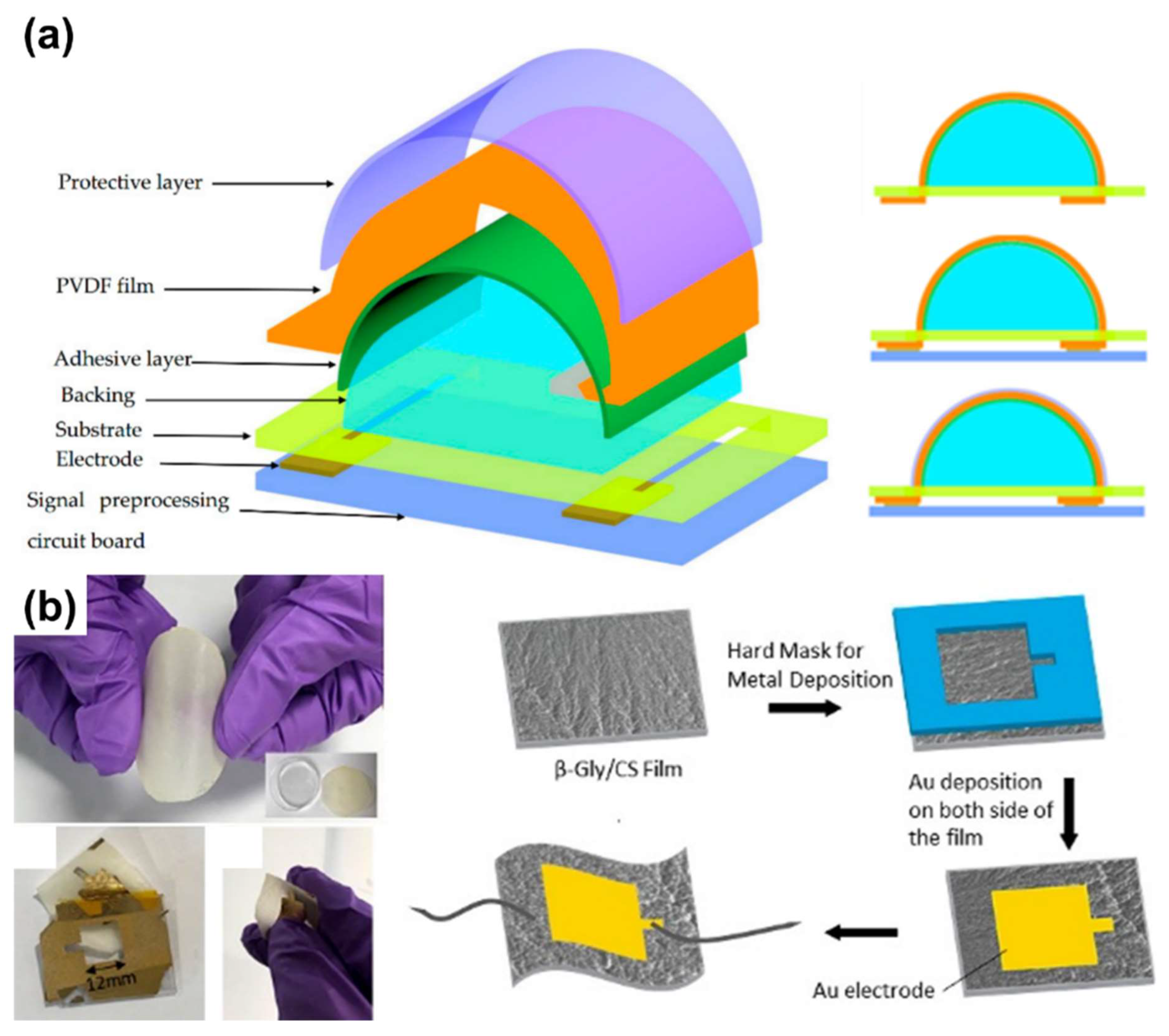
2.3. Piezoelectric Sensors
2.4. Transistor Sensors
3. Classification of Materials
3.1. Active Materials
3.1.1. Metallic Materials (Silver, Gold, and Copper Nanowires)
3.1.2. Carbon-Based Materials (Graphene, CNT)
3.1.3. Conducting Polymers
3.1.4. Metal Oxides
3.2. Flexible Materials
4. Advanced Technologies for E-skin
4.1. Stretchability
4.2. Energy Harvesting
4.3. Biocompatibility
4.4. Biomimetics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bae, G.Y.; Han, J.T.; Lee, G.; Lee, S.; Kim, S.W.; Park, S.; Kwon, J.; Jung, S.; Cho, K. Pressure/Temperature Sensing Bimodal Electronic Skin with Stimulus Discriminability and Linear Sensitivity. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, H.; Lin, X.; Liu, S.; Liu, Y.; Liu, X.; Fei, T.; Zhang, T. Ultrafast Response Polyelectrolyte Humidity Sensor for Respiration Monitoring. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Nguyen, A.; Chortos, A.; To, J.W.F.; Lu, C.; Mei, J.; Kurosawa, T.; Bae, W.G.; Tok, J.B.H.; Bao, Z. A chameleon-inspired stretchable electronic skin with interactive colour changing controlled by tactile sensing. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Li, Y.; Samad, Y.A.; Liao, K. From Cotton to Wearable Pressure Sensor. J. Mater. Chem. A 2015, 3, 2181–2187. [Google Scholar] [CrossRef]
- Sawhney, N.; Schmandt, C. Nomadic Radio: A spatialized audio environment for wearable computing. Int. Symp. Wearable Comput. Dig. Pap. 1997, 171–172. [Google Scholar] [CrossRef]
- Di, J.; Zhang, X.; Yong, Z.; Zhang, Y.; Li, D.; Li, R.; Li, Q. Carbon-Nanotube Fibers for Wearable Devices and Smart Textiles. Adv. Mater. 2016, 28, 10529–10538. [Google Scholar] [CrossRef]
- Yao, J.; Yang, G. Flexible and High-Performance All-2D Photodetector for Wearable Devices. Small 2018, 14, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Kim, C.C.; Sun, J.Y. Stretchable Ionics—A Promising Candidate for Upcoming Wearable Devices. Adv. Mater. 2018, 30, 1–15. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Su, Z.; Zhang, M.; Hu, X.; Wang, Y.; Wang, Z.; Zhong, B.; Zhou, W.; Liu, J.; et al. Highly Flexible and Sensitive Wearable E-Skin Based on Graphite Nanoplatelet and Polyurethane Nanocomposite Films in Mass Industry Production Available. ACS Appl. Mater. Interfaces 2017, 9, 38745–38754. [Google Scholar] [CrossRef]
- Lopes, P.A.; Paisana, H.; De Almeida, A.T.; Majidi, C.; Tavakoli, M. Hydroprinted Electronics: Ultrathin Stretchable Ag-In-Ga E-Skin for Bioelectronics and Human-Machine Interaction. ACS Appl. Mater. Interfaces 2018, 10, 38760–38768. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of Nanomaterials in Wearables: A Review on Wearable Actuators and Sensors. Adv. Mater. 2019, 31, 1–21. [Google Scholar] [CrossRef]
- Stojiljkovic, J.C.Z. Integrated Behavior of Artificial Skin. IEEE Trans. Biomed. Eng. 1977, 891, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Liu, S.; Qu, Q.; Zhang, Y.; Zhao, M.; Liu, J. Bio-inspired Flexible Electronics for Smart E-skin. Acta Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifar, M.; Tahernia, M.; Yang, J.H.; Koh, A.; Choi, S. Biopower-on-Skin: Electricity generation from sweat-eating bacteria for self-powered E-Skins. Nano Energy 2020, 75, 104994. [Google Scholar] [CrossRef]
- Mahanty, B.; Maity, K.; Sarkar, S.; Mandal, D. Human skin interactive self-powered piezoelectric e-skin based on PVDF/MWCNT electrospun nanofibers for non-invasive health care monitoring. Mater. Today Proc. 2020, 21, 1964–1968. [Google Scholar] [CrossRef]
- Fastier-Wooller, J.W.; Dau, V.T.; Dinh, T.; Tran, C.D.; Dao, D.V. Pressure and temperature sensitive e-skin for in situ robotic applications. Mater. Des. 2021, 208, 109886. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Xuan, S.; Zhang, S.; Fan, X.; Jiang, H.; Song, P.; Gong, X. Highly Flexible Multilayered e-Skins for Thermal-Magnetic-Mechanical Triple Sensors and Intelligent Grippers. ACS Appl. Mater. Interfaces 2020, 12, 15675–15685. [Google Scholar] [CrossRef]
- Wang, J.; Lou, H.; Meng, J.; Peng, Z.; Wang, B.; Wan, J. Stretchable energy storage E-skin supercapacitors and body movement sensors. Sens. Actuators B Chem. 2020, 305, 127529. [Google Scholar] [CrossRef]
- Nawrocki, R.A.; Matsuhisa, N.; Yokota, T.; Someya, T. 300-nm Imperceptible, Ultraflexible, and Biocompatible e-Skin Fit with Tactile Sensors and Organic Transistors. Adv. Electron. Mater. 2016, 2, 2–5. [Google Scholar] [CrossRef]
- Guo, X.; Huang, Y.; Cai, X.; Liu, C.; Liu, P. Capacitive wearable tactile sensor based on smart textile substrate with carbon black /silicone rubber composite dielectric. Meas. Sci. Technol. 2016, 27. [Google Scholar] [CrossRef]
- Marchiori, B.; Regal, S.; Arango, Y.; Delattre, R.; Blayac, S.; Ramuz, M. PVDF-TrFE-based stretchable contact and non-contact temperature sensor for E-skin application. Sensors 2020, 20, 623. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, X.; Jian, J.; Wu, Q.; Wei, Y.; Shuai, H.; Hirtz, T.; Zhi, Y.; Deng, G.; Wang, Y.; et al. Substrate-Free Multilayer Graphene Electronic Skin for Intelligent Diagnosis. ACS Appl. Mater. Interfaces 2020, 12, 49945–49956. [Google Scholar] [CrossRef]
- Gong, C.; Hu, K.; Wang, X.; Wangyang, P.; Yan, C.; Chu, J.; Liao, M.; Dai, L.; Zhai, T.; Wang, C.; et al. 2D Nanomaterial Arrays for Electronics and Optoelectronics. Adv. Funct. Mater. 2018, 28, 1–23. [Google Scholar] [CrossRef]
- Wang, B.; Facchetti, A. Mechanically Flexible Conductors for Stretchable and Wearable E-Skin and E-Textile Devices. Adv. Mater. 2019, 31, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Cheng, W. One-Dimensional Nanomaterials for Soft Electronics. Adv. Electron. Mater. 2017, 3. [Google Scholar] [CrossRef]
- Zhang, Y.; Haghighi, P.D.; Burstein, F.; Yap, L.W.; Cheng, W.; Yao, L.; Cicuttini, F. Electronic skin wearable sensors for detecting lumbar–pelvic movements. Sensors 2020, 20, 1510. [Google Scholar] [CrossRef]
- Vaz, S.; Silva, R.; Amaral, M.H.; Martins, E.; Sousa Lobo, J.M.; Silva, A.C. Evaluation of the biocompatibility and skin hydration potential of vitamin E-loaded lipid nanosystems formulations: In vitro and human in vivo studies. Colloids Surf. B Biointerfaces 2019, 179, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Kim, S.O.; Lee, H.J.; Han, C.J.; Lee, C.J.; Yu, Y.T.; Lee, C.R.; Ju, B.K.; Kim, Y.; Kim, J.W. Transparent, pressure-sensitive, and healable e-skin from a UV-cured polymer comprising dynamic urea bonds. J. Mater. Chem. A 2019, 7, 3101–3111. [Google Scholar] [CrossRef]
- Fu, Y.F.; Yi, F.L.; Liu, J.R.; Li, Y.Q.; Wang, Z.Y.; Yang, G.; Huang, P.; Hu, N.; Fu, S.Y. Super soft but strong E-Skin based on carbon fiber/carbon black/silicone composite: Truly mimicking tactile sensing and mechanical behavior of human skin. Compos. Sci. Technol. 2020, 186, 107910. [Google Scholar] [CrossRef]
- Kar, E.; Bose, N.; Dutta, B.; Mukherjee, N.; Mukherjee, S. Ultraviolet-and Microwave-Protecting, Self-Cleaning e-Skin for Efficient Energy Harvesting and Tactile Mechanosensing. ACS Appl. Mater. Interfaces 2019, 11, 17501–17512. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 2013, 4, 1610–1633. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.H. Recent progress in pressure sensors for wearable electronics: From design to applications. Appl. Sci. 2020, 10, 6403. [Google Scholar] [CrossRef]
- Fiorillo, A.S.; Critello, C.D.; Pullano, A.S. Theory, technology and applications of piezoresistive sensors: A review. Sens. Actuators A Phys. 2018, 281, 156–175. [Google Scholar] [CrossRef]
- Di, C.; Zhu, D. Advances of flexible pressure sensors toward artificial intelligence and health care applications. Mater. Horiz. 2015, 2. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.; Tok, J.B.; Bao, Z. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Ryu, C. Understanding the Principles of Wheatstone Bridge Circuit. J. Korean Soc. Explos. Blasting Eng. 2017, 35, 9–17. [Google Scholar]
- Prabhu, S.; Pai, A.B.; Arora, G.S.; Kusshal, M.R.; Pandin, V.; Goutham, M.A. Design of Piezo-Resistive Type Acoustic Vector Sensor using Graphene for Underwater Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1045, 012015. [Google Scholar] [CrossRef]
- Timsit, R.S. Electrical contact resistance: Properties of stationary interfaces. IEEE Trans. Compon. Packag. Technol. 1999, 22, 85–98. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, N.; Shi, Y.; Liu, W.; Yue, Y.; Wang, S.; Ma, Y.; Wen, L.; Li, L.; Long, F.; et al. Piezoresistive Sensor with High Elasticity Based on 3D Hybrid Network of Sponge@CNTs@Ag NPs. ACS Appl. Mater. Interfaces 2016, 8, 22374–22381. [Google Scholar] [CrossRef]
- Kim, K.H.; Hong, S.K.; Jang, N.S.; Ha, S.H.; Lee, H.W.; Kim, J.M. Wearable Resistive Pressure Sensor Based on Highly Flexible Carbon Composite Conductors with Irregular Surface Morphology. ACS Appl. Mater. Interfaces 2017, 9, 17499–17507. [Google Scholar] [CrossRef]
- Huang, C.T.; Shen, C.L.; Tang, C.F.; Chang, S.H. A wearable yarn-based piezo-resistive sensor. Sens. Actuators A Phys. 2008, 141, 396–403. [Google Scholar] [CrossRef]
- Wang, Y.; Ali, S.; Wijekoon, J.; Gong, R.H.; Fernando, A. A wearable piezo-resistive sensor for capturing cardiorespiratory signals. Sens. Actuators A Phys. 2018, 282, 215–229. [Google Scholar] [CrossRef]
- Choudhry, N.A.; Rasheed, A.; Ahmad, S.; Arnold, L.; Wang, L. Design, Development and Characterization of Textile Stitch-Based Piezoresistive Sensors for Wearable Monitoring. IEEE Sens. J. 2020, 20, 10485–10494. [Google Scholar] [CrossRef]
- Puers, R. Capacitive sensors: When and how to use them. Sens. Actuators A Phys. Actuators 1993, 38, 93–105. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, L.; Gao, Y.; Fang, X.; Lu, T.; Pan, L.; Xuan, F. Highly Stretchable and Self-Healable MXene/Polyvinyl Alcohol Hydrogel Electrode for Wearable Capacitive Electronic Skin. Adv. Electron. Mater. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Palaniappan, V.; Masihi, S.; Panahi, M.; Maddipatla, D.; Bose, A.K.; Zhang, X.; Narakathu, B.B.; Bazuin, B.J.; Atashbar, M.Z. Laser-Assisted Fabrication of a Highly Sensitive and Flexible Micro Pyramid-Structured Pressure Sensor for E-Skin Applications. IEEE Sens. J. 2020, 20, 7605–7613. [Google Scholar] [CrossRef]
- You, A.; Be, M.A.Y.; In, I. Piezoelectrically enhanced capacitive strain sensors using GaN metal-insulator- semiconductor diodes. J. Appl. Phys. 2003, 5958. [Google Scholar] [CrossRef]
- O’Dowd, J.; Callanan, A.; Banarie, G.; Company-Bosch, E. Capacitive sensor interfacing using sigma-delta techniques. Proc. IEEE Sens. 2005, 2005, 951–954. [Google Scholar] [CrossRef]
- Santos, A.; Fortunato, E.; Martins, R.; Hugo, Á. Techniques, and Applications of Electronic Skin Pressure Sensors: A Review of Recent Advances. Sensors 2020, 20, 4407. [Google Scholar] [CrossRef]
- Chen, W.; Yan, X. Progress in achieving high-performance piezoresistive and capacitive flexible pressure sensors: A review. J. Mater. Sci. Technol. 2020, 43, 175–188. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kim, J.; Kim, H.; Kim, C.; Lee, S.D. Low-cost flexible pressure sensor based on dielectric elastomer film with micro-pores. Sens. Actuators A Phys. 2016, 240, 103–109. [Google Scholar] [CrossRef]
- Kwon, D.; Lee, T.I.; Shim, J.; Ryu, S.; Kim, M.S.; Kim, S.; Kim, T.S.; Park, I. Highly Sensitive, Flexible, and Wearable Pressure Sensor Based on a Giant Piezocapacitive Effect of Three-Dimensional Microporous Elastomeric Dielectric Layer. ACS Appl. Mater. Interfaces 2016, 8, 16922–16931. [Google Scholar] [CrossRef]
- Heterostructures, H.; Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly Sensitive Glucose Sensor Based on Pt Nanoparticle/Polyaniline Hydrogel Heterostructures. ACS Nano 2013, 3540–3546. [Google Scholar]
- Zhang, Y.Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, N.; Xu, F.; Tong, J.; Chen, Z.; Gui, X.; Fu, Y.; Lao, C. 3D printing technologies for flexible tactile sensors toward wearable electronics and electronic skin. Polymers 2018, 10, 629. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, X.; Chen, S.; Zhao, N. Emerging Technologies of Flexible Pressure Sensors: Materials, Modeling, Devices, and Manufacturing. Adv. Funct. Mater. 2019, 1808509, 1–24. [Google Scholar] [CrossRef]
- Tressler, J.F.; Alkoy, S.; Newnham, R.E. Piezoelectric sensors and sensor materials. J. Electroceramics 1998, 2, 257–272. [Google Scholar] [CrossRef]
- Liu, J.; Gu, H.; Liu, Q.; Ren, L.; Li, G. An intelligent material for tissue reconstruction: The piezoelectric property of polycaprolactone/barium titanate composites. Mater. Lett. 2019, 236, 686–689. [Google Scholar] [CrossRef]
- Liu, J.; He, X.; Wang, F.; Zhou, X.; Li, G. Dielectric and mechanical properties of polycaprolactone/nano-barium titanate piezoelectric composites. Plast. Rubber Compos. 2021, 50, 299–306. [Google Scholar] [CrossRef]
- Nag-Chowdhury, S.; Bellegou, H.; Pillin, I.; Castro, M.; Longrais, P.; Feller, J.F. Non-intrusive health monitoring of infused composites with embedded carbon quantum piezo-resistive sensors. Compos. Sci. Technol. 2016, 123, 286–294. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Mishra, J.; Hatui, G.; Nayak, G.C. Conducting Polymer Nanocomposites: Recent Developments and Future Prospects; Springer Series on Polymer and Composite Materials; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-46456-5. [Google Scholar]
- Sirohi, J.; Chopra, I. Fundamental understanding of piezoelectric strain sensors. J. Intell. Mater. Syst. Struct. 2000, 11, 246–257. [Google Scholar] [CrossRef]
- Sessler, G.M. Piezoelectricity in polyvinylidenefluoride. J. Acoust. Soc. Am. 1981, 70, 1596–1608. [Google Scholar] [CrossRef]
- Uchino, K. Introduction to Piezoelectric Actuators and Transducers Kenji Uchino; International Center for Actuators and Transducers, Penn State University: State College, PA, USA, 2003. [Google Scholar]
- Ki, M.M.; Taag, C.S.; We-duke, C. A Study on Detection of Hearbeat Signal using Piezoelectric Sensor. Korea Inst. Commun. Sci. 2014, 1–2. [Google Scholar]
- Gu, Y.; Zhang, T.; Chen, H.; Wang, F.; Pu, Y.; Gao, C.; Li, S. Mini Review on Flexible and Wearable Electronics for Monitoring Human Health Information. Nanoscale Res. Lett. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Dahiya, R.S.; Valle, M. Robotic Tactile Sensing: Technologies and System; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Chen, Z.; Wang, Z.; Li, X.; Lin, Y.; Luo, N.; Long, M.; Zhao, N.; Xu, J. Bin Flexible Piezoelectric-Induced Pressure Sensors for Static Measurements Based on Nanowires/Graphene Heterostructures. ACS Nano 2017, 11, 4507–4513. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Tee, B.C.K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kang, W.; Fang, Y.; Xie, L.; Qiu, L.; Jin, T. Piezoelectric poly(vinylidene fluoride) (PVDF) polymer-based sensor for wrist motion signal detection. Appl. Sci. 2018, 8, 836. [Google Scholar] [CrossRef]
- Dong, W.; Xiao, L.; Hu, W.; Zhu, C.; Huang, Y.; Yin, Z. Wearable human-machine interface based on PVDF piezoelectric sensor. Trans. Inst. Meas. Control 2017, 39, 398–403. [Google Scholar] [CrossRef]
- Gu, R.; Ji, M.; Xuan, Y.; Cui, Y.; Yuan, C.; Di Li, W.; Ge, H.; Chen, Y. Stretching-tunable metal gratings fabricated on an elastomeric substrate using a water-soluble sacrificial layer. Appl. Phys. A Mater. Sci. Process. 2015, 121, 335–341. [Google Scholar] [CrossRef]
- Beveren, W. Van Lead-free piezoceramics. Nature 2004, 432, 2–5. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Manjakkal, L.; Shakthivel, D.; Dahiya, R. Glycine-Chitosan-Based Flexible Biodegradable Piezoelectric Pressure Sensor. ACS Appl. Mater. Interfaces 2020, 12, 9008–9016. [Google Scholar] [CrossRef] [PubMed]
- Bardeen, J.; Brattain, W.H. Transistor, a semiconductor triode. Proc. IEEE 1948, 86, 230–231. [Google Scholar] [CrossRef]
- Riordan, M.; Hoddeson, L.; Herring, C. The invention of the transistor. Rev. Mod. Phys. 1999, 71, 336–345. [Google Scholar] [CrossRef]
- Shockley, W. A unipolar “field-effect” Transistor. Proc. IRE 1952, 40, 1365–1376. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F. Organic thin-film transistors for chemical and biological sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Song, J.; Liu, J.; Xu, N.; Wang, Z.L. Piezoelectric field effect transistor and nanoforce sensor based on a single ZnO nanowire. Nano Lett. 2006, 6, 2768–2772. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Jia, Z.; Kymissis, I. A locally amplified strain sensor based on a piezoelectric polymer and organic field-effect transistors. IEEE Trans. Electron Devices 2011, 58, 910–917. [Google Scholar] [CrossRef]
- Someya, T.; Sekitani, T.; Iba, S.; Kato, Y.; Kawaguchi, H.; Sakurai, T. A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc. Natl. Acad. Sci. USA 2004, 101, 9966–9970. [Google Scholar] [CrossRef]
- Lai, S.; Barbaro, M.; Bonfiglio, A. Tailoring the sensing performances of an OFET-based biosensor. Sens. Actuators B Chem. 2016, 233, 314–319. [Google Scholar] [CrossRef]
- Zhu, M.; Ali, M.U.; Zou, C.; Xie, W.; Li, S.; Meng, H. Tactile and temperature sensors based on organic transistors: Towards e-skin fabrication. Front. Phys. 2021, 16, 1–13. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Bettinger, C.J.; Bao, Z. Chemical and Engineering Approaches To Enable Organic Field-Effect Transistors for Electronic Skin Applications. Acc. Chem. Res 2012, 45, 361–371. [Google Scholar] [CrossRef]
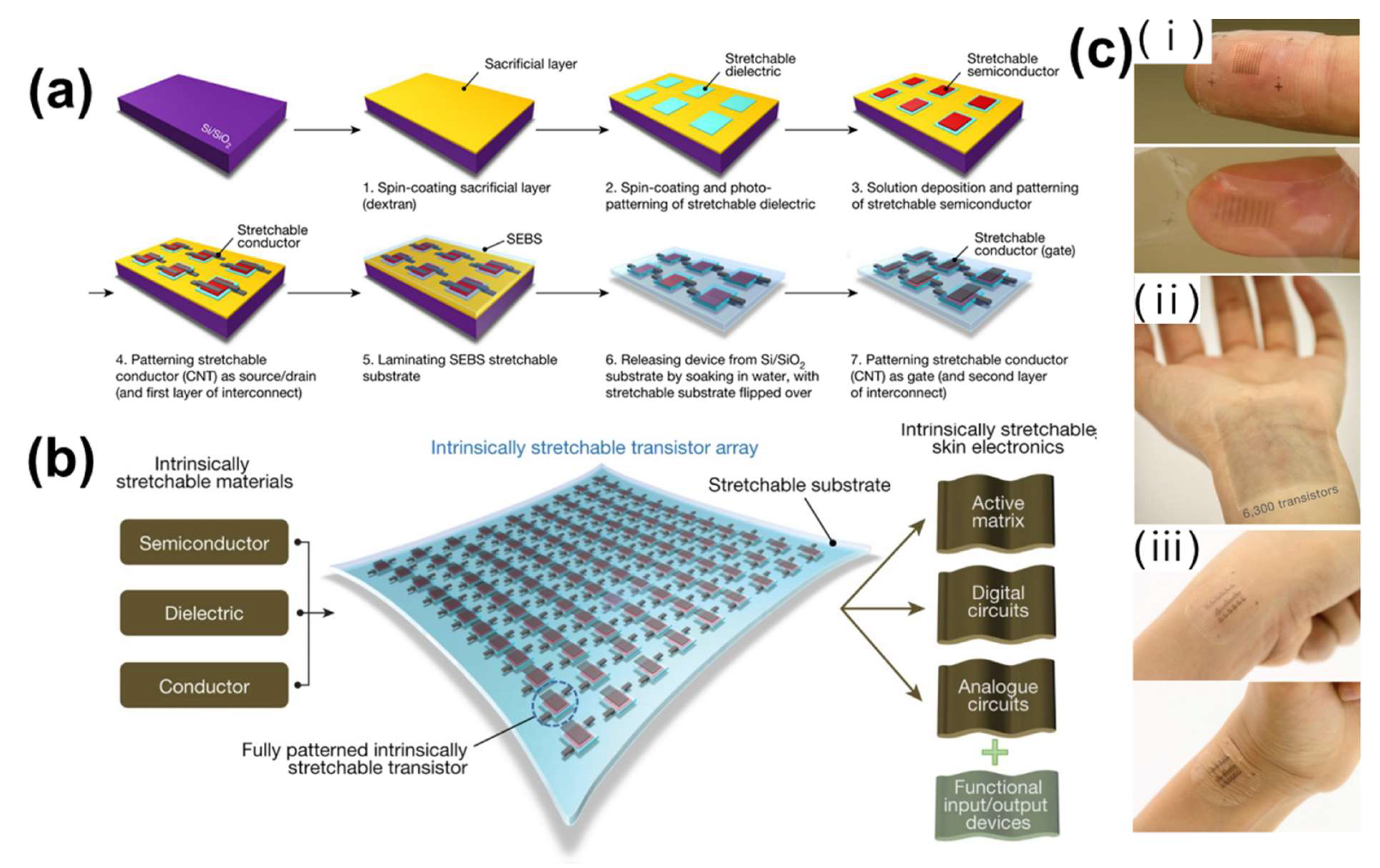
- Wang, S.; Xu, J.; Wang, W.; Wang, G.J.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.; Feig, V.R.; Lopez, J.; et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 2018, 555, 83–88. [Google Scholar] [CrossRef]
- Zhang, Q.; Leonardi, F.; Pfattner, R.; Mas-Torrent, M. A Solid-State Aqueous Electrolyte-Gated Field-Effect Transistor as a Low-Voltage Operation Pressure-Sensitive Platform. Adv. Mater. Interfaces 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Jang, J.; Jun, Y.S.; Seo, H.; Kim, M.; Park, J.U. Motion detection using tactile sensors based on pressure-sensitive transistor arrays. Sensors 2020, 20, 3624. [Google Scholar] [CrossRef]
- Dutta, S.; Dodabalapur, A. Zinc tin oxide thin film transistor sensor. Sens. Actuators B Chem. 2009, 143, 50–55. [Google Scholar] [CrossRef]
- Rullyani, C.; Singh, M.; Li, S.; Sung, C.; Lin, H. Stimuli-responsive polymer as gate dielectric for organic transistor sensors. Org. Electron. 2020, 85, 105818. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.R.; Kil, H.J.; Kim, Y.C.; Park, J.W. Highly Conformable, Transparent Electrodes for Epidermal Electronics. Nano Lett. 2018, 18, 4531–4540. [Google Scholar] [CrossRef]
- Hong, S.; Lee, S.; Kim, D.H. Materials and Design Strategies of Stretchable Electrodes for Electronic Skin and its Applications. Proc. IEEE 2019, 107, 2185–2197. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Xia, Z.; Cai, K. A review of electronic skin: Soft electronics and sensors for human health. J. Mater. Chem. B 2020, 8, 852–862. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Hyun, J.K.; Zhang, S.; Lauhon, L.J. Nanowire heterostructures. Annu. Rev. Mater. Res. 2013, 43, 451–479. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, J.M.; Stucky, G.D. Preparation of noble metal nanowires using hexagonal mesoporous silica SBA-15. Chem. Mater. 2000, 12, 2068–2069. [Google Scholar] [CrossRef]
- Charles, R. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar]
- Walter, E.C.; Zach, M.P.; Favier, F.; Murray, B.J.; Inazu, K.; Hemminger, J.C.; Penner, R.M. Metal nanowire arrays by electrodeposition. ChemPhysChem 2003, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kwon, H.; Kwon, H.J.; Pyo, J.B.; Oh, J.; Hong, S.Y.; Park, J.H.; Char, K.; Ha, J.S.; Son, J.G.; et al. Buckling Instability Control of 1D Nanowire Networks for a Large-Area Stretchable and Transparent Electrode. Adv. Funct. Mater. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Huczko, A. Template-based synthesis of nanomaterials. Appl. Phys. A Mater. Sci. Process. 2000, 70, 365–376. [Google Scholar] [CrossRef]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-processed metal nanowire mesh transparent electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef]
- Bellew, A.T.; Manning, H.G.; Gomes da Rocha, C.; Ferreira, M.S.; Boland, J.J. Resistance of Single Ag Nanowire Junctions and Their Role in the Conductivity of Nanowire Networks. ACS Nano 2015, 9, 11422–11429. [Google Scholar] [CrossRef]
- Reed, M.A.; Zhou, C.; Muller, C.J.; Burgin, T.P.; Tour, J.M. Conductance of a molecular junction. Science 1997, 278, 252–254. [Google Scholar] [CrossRef]
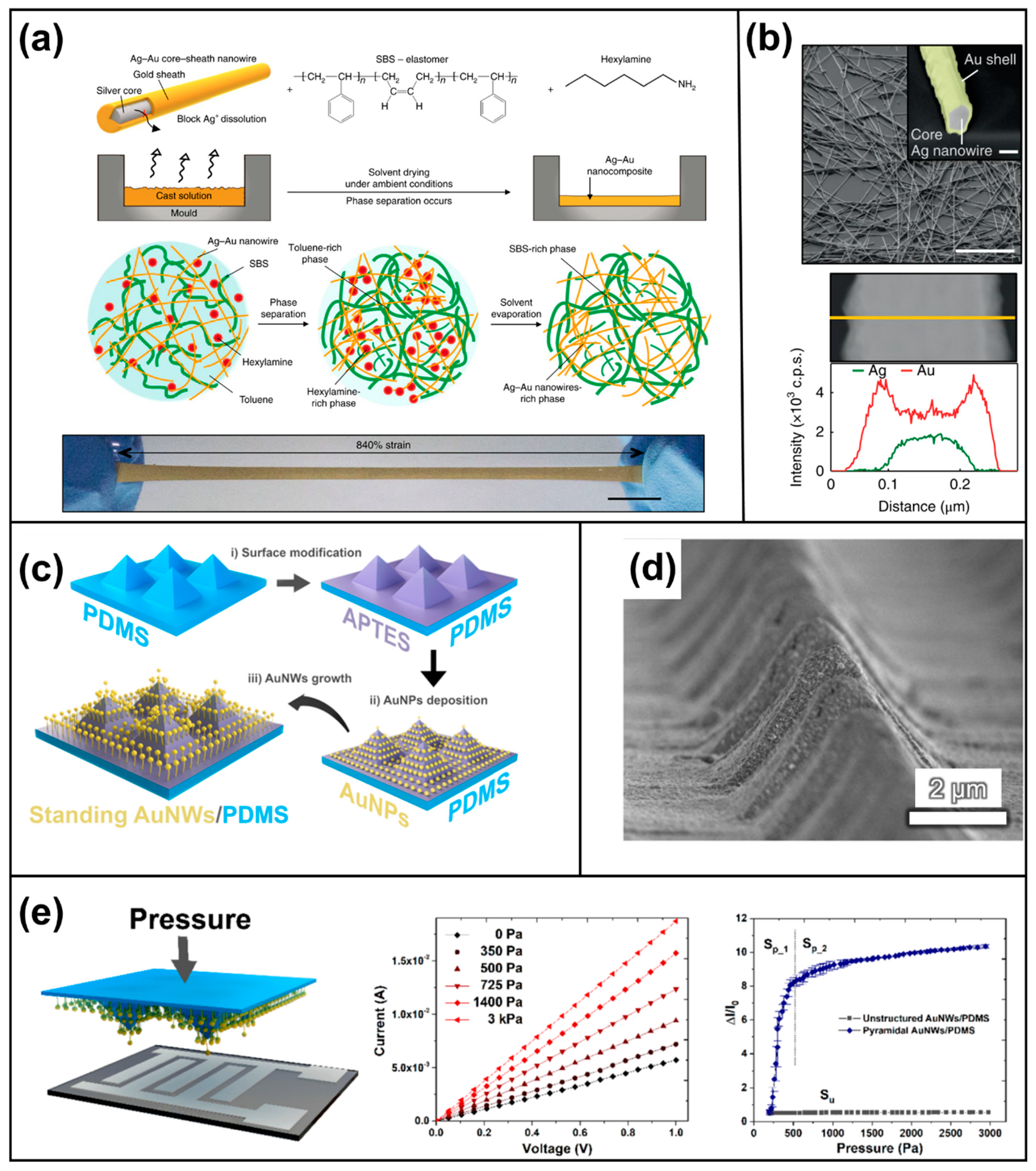
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y.; et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 2018, 13, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, D.M.; Nakahira, A.; Ying, J.Y.; Laoteppitaks, C.; Ambrosini, A.; Poeppelmeier, K.R. Ligand-assisted liquid crystal templating in mesoporous niobium oxide molecular sieves. Chemtracts 1999, 12, 118–120. [Google Scholar] [CrossRef]
- Choi, S.; Park, S.; Yu, J. Ligand-assisted etching: The stability of silver nanoparticles and the generation of luminescent silver nanodots. Chem. Commun. 2014, 50, 15098–15100. [Google Scholar] [CrossRef]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar] [CrossRef]
- Sun,, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Metal coating on suspended carbon nanotubes and its implication to metal-tube interaction. ACS Appl. Mater. Interfaces 2017, 11, 1–8. [Google Scholar] [CrossRef]
- Dunbar, K.; Blanchette, I. The in vivo/in vitro approach to cognition: The case of analogy. Trends Cogn. Sci. 2001, 5, 334–339. [Google Scholar] [CrossRef]
- Đurović, M.D.; Bugarčić, Ž.D.; van Eldik, R. Stability and reactivity of gold compounds—From fundamental aspects to applications. Coord. Chem. Rev. 2017, 338, 186–206. [Google Scholar] [CrossRef]
- Messing, M.E.; Hillerich, K.; Johansson, J.; Deppert, K.; Dick, K.A. The use of gold for fabrication of nanowire structures. Gold Bull. 2009, 42. [Google Scholar] [CrossRef]
- Zhu, B.; Ling, Y.; Yap, L.W.; Yang, M.; Lin, F.; Gong, S.; Wang, Y.; An, T.; Zhao, Y.; Cheng, W. Hierarchically Structured Vertical Gold Nanowire Array-Based Wearable Pressure Sensors for Wireless Health Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 29014–29021. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Kim, J.O.; Oh, J.; Kwon, S.Y.; Sim, J.Y.; Kim, D.W.; Choi, H.B.; Park, S. Microstructured Porous Pyramid-Based Ultrahigh Sensitive Pressure Sensor Insensitive to Strain and Temperature. ACS Appl. Mater. Interfaces 2019, 11, 19472–19480. [Google Scholar] [CrossRef]
- Choong, C.L.; Shim, M.B.; Lee, B.S.; Jeon, S.; Ko, D.S.; Kang, T.H.; Bae, J.; Lee, S.H.; Byun, K.E.; Im, J.; et al. Highly stretchable resistive pressure sensors using a conductive elastomeric composite on a micropyramid array. Adv. Mater. 2014, 26, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Ong, S.H.; Tang, L.C. Robust loop parameters for ball neck strength enhancement. Proc. Electron. Packag. Technol. Conf. EPTC 1998, 313–317. [Google Scholar]
- Zhong, Z.W. Wire bonding using copper wire. Microelectron. Int. 2009, 26, 10–16. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Xu, J.; Zeng, H. Superstable transparent conductive Cu@Cu4Ni nanowire elastomer composites against oxidation, bending, stretching, and twisting for flexible and stretchable optoelectronics. Nano Lett. 2014, 14, 6298–6305. [Google Scholar] [CrossRef]
- Shouyu, H.; Chunjing, H.; Chunqing, W. Experimental research of copper wire ball bonding. In Proceedings of the 2005 6th International Conference on Electronic Packaging Technology, Shenzhen, China, 30 August 2005. [Google Scholar] [CrossRef]
- Ye, S.; Rathmell, A.R.; Ha, Y.C.; Wilson, A.R.; Wiley, B.J. The role of cuprous oxide seeds in the one-pot and seeded syntheses of copper nanowires. Small 2014, 10, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhen, H.; Niu, L.; Fang, X.; Zhang, Y.; Guo, R.; Yu, Y.; Yan, F.; Li, H.; Zheng, Z. Full-Solution Processed Flexible Organic Solar Cells Using Low-Cost Printable Copper Electrodes. Adv. Mater. 2014, 26, 7271–7278. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Satishkumar, B.C.; Govindaraj, A.; Nath, M. Nanotubes. J. Chem. Inf. Model. 2001, 2, 78–105. [Google Scholar] [CrossRef]
- Novoselov, K.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the new nanocarbon. J. Mater. Chem. 2009, 19, 2457–2469. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1535. [Google Scholar] [CrossRef]
- Goroff, N.S. Mechanism of fullerene formation. Acc. Chem. Res. 1996, 29, 77–83. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Yun, W.S.; Kim, J.; Park, K.-H.; Ha, J.S.; Ko, Y.-J.; Park, K.; Kim, S.K.; Doh, Y.-J.; Lee, H.-J.; Salvetat, J.-P.; et al. Fabrication of metal nanowire using carbon nanotube as a mask. J. Vac. Sci. Technol. A Vac. Surf. Film. 2000, 18, 1329–1332. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Zhao, X.; Ando, Y.; Liu, Y.; Jinno, M.; Suzuki, T. Carbon Nanowire Made of a Long Linear Carbon Chain Inserted Inside a Multiwalled Carbon Nanotube. Phys. Rev. Lett. 2003, 90, 4. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Shimoyama, H.; Sakai, G.; Kaneto, K. Physical properties of multiwalled carbon nanotubes. Int. J. Inorg. Mater. 1999, 1, 77–82. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, T.S.; Do Choi, H.; Kwon, J.H.; Chung, Y.C.; Yoon, H.G. Electrical conductivity of chemically modified multiwalled carbon nanotube/epoxy composites. Carbon N. Y. 2005, 43, 23–30. [Google Scholar] [CrossRef]
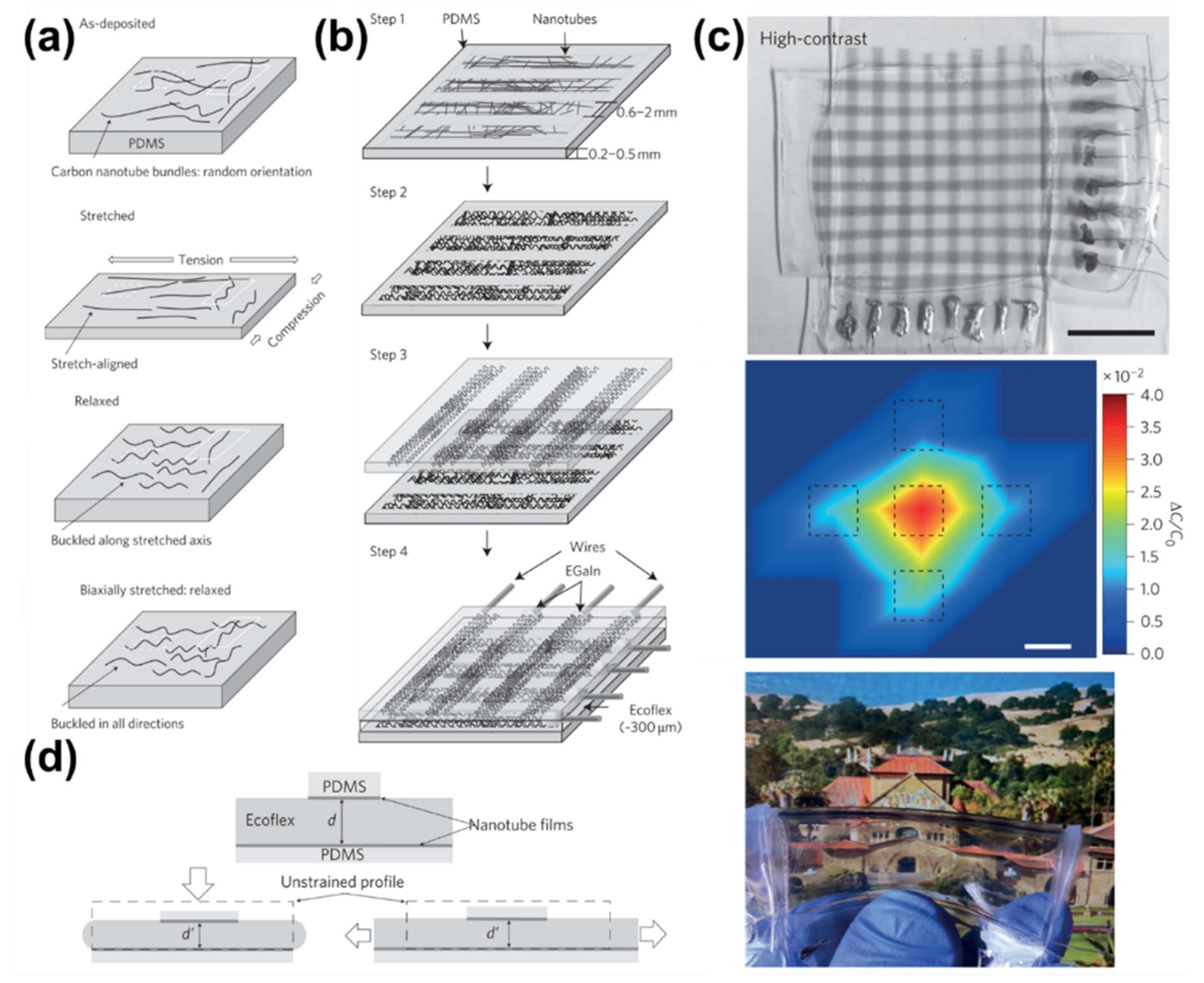
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Jiang, X.; Bukhari, H.; Zhang, Z.; Han, W.; Xie, E. Simple and efficient pressure sensor based on PDMS wrapped CNT arrays. Carbon N. Y. 2019, 155, 71–76. [Google Scholar] [CrossRef]
- Rasmussen, S.C. Electrically conducting plastics: Revising the history of conjugated organic polymers. ACS Symp. Ser. 2011, 1080, 147–163. [Google Scholar] [CrossRef]
- Chung, M.H.; Kim, S.; Yoo, D.; Kim, J.H. Materials and characteristics of emerging transparent electrodes. Appl. Chem. Eng. 2014, 25, 242–248. [Google Scholar] [CrossRef][Green Version]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer (synthesis, properties, and applications). Usp. Khim. 1997, 66, 502–505. [Google Scholar] [CrossRef]
- Efimov, O.N.; Chem, R. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443. [Google Scholar]
- Kim, Y.; Seonmyeong Noh, H.Y. Nanostructured Conducting Polymers Based Electrode Materials. Polym. Sci. Technol. 2016, 27, 233–244. [Google Scholar]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Yogeswaran, N.; Dang, W.; Navaraj, W.T.; Shakthivel, D.; Khan, S.; Polat, E.O.; Gupta, S.; Heidari, H.; Kaboli, M.; Lorenzelli, L.; et al. New materials and advances in making electronic skin for interactive robots. Adv. Robot. 2015, 29, 1359–1373. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, Y.R.; Lee, G.; Jin, S.W.; Lee, Y.H.; Hong, S.Y.; Park, H.; Kim, J.W.; Lee, S.S.; Ha, J.S. Highly Conductive, Stretchable, and Transparent PEDOT:PSS Electrodes Fabricated with Triblock Copolymer Additives and Acid Treatment. ACS Appl. Mater. Interfaces 2018, 10, 28027–28035. [Google Scholar] [CrossRef]
- Jin Young, O.; Kim, S.; Baik, H.K.; Jeong, U. Conducting Polymer Dough for Deformable Electronics. Adv. Mater. 2016, 28, 4455–4461. [Google Scholar] [CrossRef]
- Alemu Mengistie, D.; Wang, P.C.; Chu, C.W. Effect of molecular weight of additives on the conductivity of PEDOT:PSS and efficiency for ITO-free organic solar cells. J. Mater. Chem. A 2013, 1, 9907–9915. [Google Scholar] [CrossRef]
- Lee, I.; Kim, G.W.; Yang, M.; Kim, T.S. Simultaneously Enhancing the Cohesion and Electrical Conductivity of PEDOT:PSS Conductive Polymer Films using DMSO Additives. ACS Appl. Mater. Interfaces 2016, 8, 302–310. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Lee, J.A.; Vosgueritchian, M.; Tee, B.C.; Bolander, J.A. Electronic Properties of Transparent Conductive Films of PEDOT:PSS on Stretchable Substrates. Chem. Mater. 2012, 24, 373–382. [Google Scholar] [CrossRef]
- Li, Z.; Qin, F.; Liu, T.; Ge, R.; Meng, W.; Tong, J.; Xiong, S.; Zhou, Y. Optical properties and conductivity of PEDOT:PSS films treated by polyethylenimine solution for organic solar cells. Org. Electron. 2015, 21, 144–148. [Google Scholar] [CrossRef]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and Characterization of Polypyrrole (PPy) Thin Films. Soft Nanosci. Lett. 2011, 01, 6–10. [Google Scholar] [CrossRef]
- Duchet, J.; Legras, R.; Demoustier-Champagne, S. Chemical synthesis of polypyrrole: Structure-properties relationship. Synth. Met. 1998, 98, 113–122. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Pringle, J.M.; Efthimiadis, J.; Howlett, P.C.; Efthimiadis, J.; MacFarlane, D.R.; Chaplin, A.B.; Hall, S.B.; Officer, D.L.; Wallace, G.G.; Forsyth, M. Electrochemical synthesis of polypyrrole in ionic liquids. Polymer (Guildf) 2004, 45, 1447–1453. [Google Scholar] [CrossRef]
- Vidal, J.C.; García, E.; Castillo, J.R. In situ preparation of a cholesterol biosensor: Entrapment of cholesterol oxidase in an overoxidized polypyrrole film electrodeposited in a flow system. Determination of total cholesterol in serum. Anal. Chim. Acta 1999, 385, 213–222. [Google Scholar] [CrossRef]
- Li, J.; Cui, L.; Zhang, X. Preparation and electrochemistry of one-dimensional nanostructured MnO2/PPy composite for electrochemical capacitor. Appl. Surf. Sci. 2010, 256, 4339–4343. [Google Scholar] [CrossRef]
- Smela, E. Microfabrication of PPy microactuators and other conjugated polymer devices. J. Micromechanics Microeng. 1999, 9, 1–18. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, S.H.; Byun, S.W.; Lee, J.Y.; Joo, J.S.; Jeong, S.H.; Park, M.J. Electrical properties and EMI shielding characteristics of polypyrrole-nylon 6 composite fabrics. J. Appl. Polym. Sci. 2003, 87, 1969–1974. [Google Scholar] [CrossRef]
- Li, X.; Vandezande, P.; Vankelecom, I.F.J. Polypyrrole modified solvent resistant nanofiltration membranes. J. Memb. Sci. 2008, 320, 143–150. [Google Scholar] [CrossRef]
- Wu, T.M.; Chang, H.L.; Lin, Y.W. Synthesis and characterization of conductive polypyrrole/multi-walled carbon nanotubes composites with improved solubility and conductivity. Compos. Sci. Technol. 2009, 69, 639–644. [Google Scholar] [CrossRef]
- Stejskal, J. Colloidal dispersions of conducting polymers. J. Polym. Mater. 2001, 18, 225–258. [Google Scholar]
- Lee, J.Y.; Kim, D.Y.; Kim, C.Y. Synthesis of soluble polypyrrole of the doped state in organic solvents. Synth. Met. 1995, 74, 103–106. [Google Scholar] [CrossRef]
- Trung, T.Q.; Ramasundaram, S.; Hwang, B.U.; Lee, N.E. An All-Elastomeric Transparent and Stretchable Temperature Sensor for Body-Attachable Wearable Electronics. Adv. Mater. 2016, 28, 502–509. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Ma, C.; Wang, Y.; Li, X.; Yu, G. A Conductive Self-Healing Hybrid Gel Enabled by Metal-Ligand Supramolecule and Nanostructured Conductive Polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef]
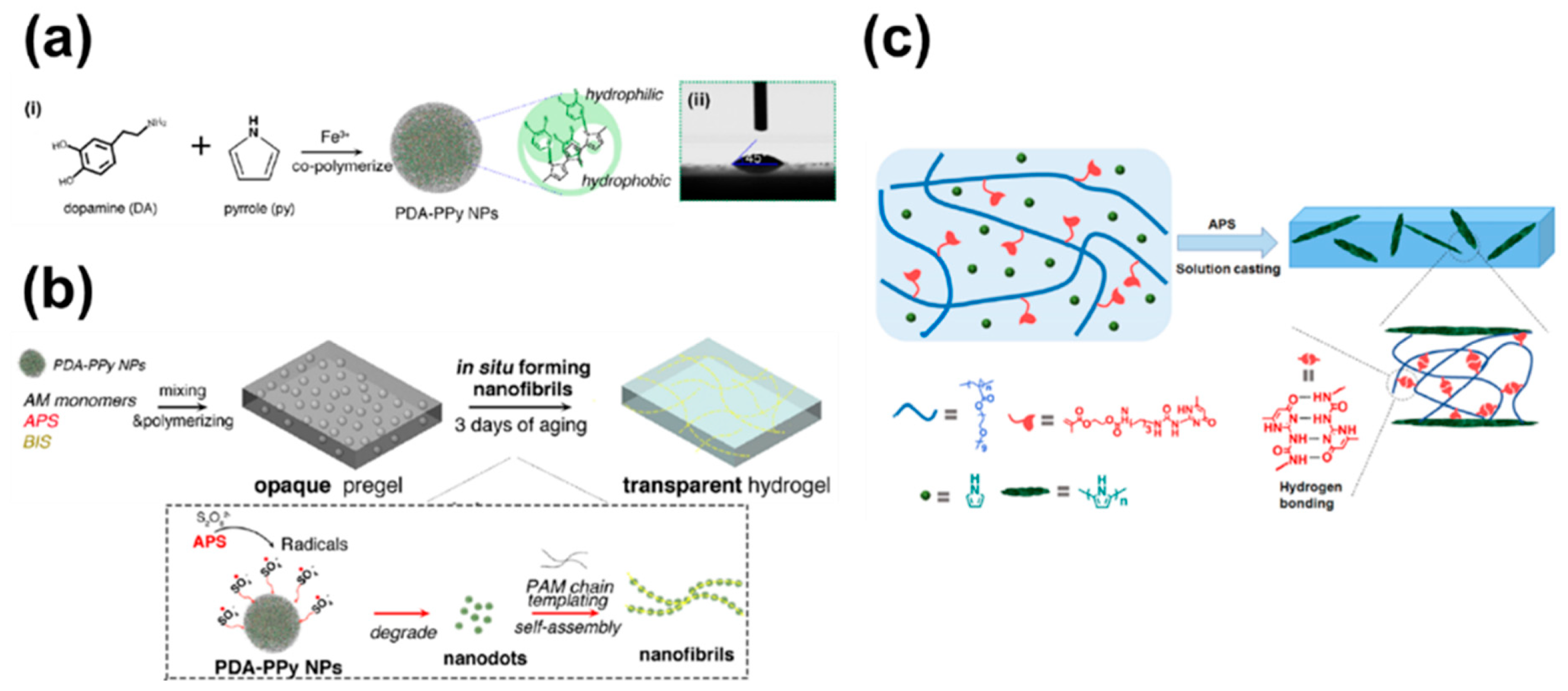
- Han, L.; Yan, L.; Wang, M.; Wang, K.; Fang, L.; Zhou, J.; Fang, J.; Ren, F.; Lu, X. Transparent, Adhesive, and Conductive Hydrogel for Soft Bioelectronics Based on Light-Transmitting Polydopamine-Doped Polypyrrole Nanofibrils. Chem. Mater. 2018, 30, 5561–5572. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Thundat, T.; Zeng, H. Polypyrrole-Doped Conductive Supramolecular Elastomer with Stretchability, Rapid Self-Healing, and Adhesive Property for Flexible Electronic Sensors. ACS Appl. Mater. Interfaces 2019, 11, 18720–18729. [Google Scholar] [CrossRef]
- Hong, S.Y.; Oh, J.H.; Park, H.; Yun, J.Y.; Jin, S.W.; Sun, L.; Zi, G.; Ha, J.S. Polyurethane foam coated with a multi-walled carbon nanotube/polyaniline nanocomposite for a skin-like stretchable array of multi-functional sensors. NPG Asia Mater. 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Sobha, A.P.; Narayanankutty, S.K. Electrical and thermoelectric properties of functionalized multiwalled carbon nanotube/polyaniline composites prepared by different methods. IEEE Trans. Nanotechnol. 2014, 13, 835–841. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, R.C. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lee, Y.H.; Park, H.; Jin, S.W.; Jeong, Y.R.; Yun, J.; You, I.; Zi, G.; Ha, J.S. Stretchable Active Matrix Temperature Sensor Array of Polyaniline Nanofibers for Electronic Skin. Adv. Mater. 2016, 28, 930–935. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Fan, S. A promising approach to enhanced thermoelectric properties using carbon nanotube networks. Adv. Mater. 2010, 22, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Gong, S.; Lai, D.T.H.; Wang, Y.; Yap, L.W.; Si, K.J.; Shi, Q.; Jason, N.N.; Sridhar, T.; Uddin, H.; Cheng, W. Tattoolike Polyaniline Microparticle-Doped Gold Nanowire Patches as Highly Durable Wearable Sensors. ACS Appl. Mater. Interfaces 2015, 7, 19700–19708. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Washizu, Y.; Hayashi, N.; Ishii, H.; Matsuie, N.; Tsuboi, K.; Ouchi, Y.; Harima, Y.; Yamashita, K.; Seki, K. Spontaneous buildup of giant surface potential by vacuum deposition of Alq3 and its removal by visible light irradiation. J. Appl. Phys. 2002, 92, 7306–7310. [Google Scholar] [CrossRef]
- Shigesato, Y.; Paine, D.C. A microstructural study of low resistivity tin-doped indium oxide prepared by d.c. magnetron sputtering. Thin Solid Film. 1994, 238, 44–50. [Google Scholar] [CrossRef]
- Jang, H.D.; Seong, C.M.; Chang, H.K.; Kim, H.C. Synthesis and characterization of indium-tin oxide (ITO) nanoparticles. Curr. Appl. Phys. 2006, 6, 1044–1047. [Google Scholar] [CrossRef]
- Yu, D.; Wang, D.; Yu, W.; Qian, Y. Synthesis of ITO nanowires and nanorods with corundum structure by a co-precipitation-anneal method. Mater. Lett. 2004, 58, 84–87. [Google Scholar] [CrossRef]
- Shahidi, S.; Moazzenchi, B.; Ghoranneviss, M. A review-application of physical vapor deposition (PVD) and related methods in the textile industry. EPJ Appl. Phys. 2015, 71, 1–14. [Google Scholar] [CrossRef]
- Patel, N.G.; Patel, P.D.; Vaishnav, V.S. Indium tin oxide (ITO) thin film gas sensor for detection of methanol at room temperature. Sens. Actuators B Chem. 2003, 96, 180–189. [Google Scholar] [CrossRef]
- Vaishnav, V.S.; Patel, P.D.; Patel, N.G. Indium tin oxide thin film gas sensors for detection of ethanol vapours. Thin Solid Film. 2005, 490, 94–100. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, J.; Noh, Y.J.; Na, S.I.; Kim, H.K. Ag nanowire-embedded ITO films as a near-infrared transparent and flexible anode for flexible organic solar cells. Sol. Energy Mater. Sol. Cells 2013, 110, 147–153. [Google Scholar] [CrossRef]
- Song, M.; Kim, H.J.; Kim, C.S.; Jeong, J.H.; Cho, C.; Lee, J.Y.; Jin, S.H.; Choi, D.G.; Kim, D.H. ITO-free highly bendable and efficient organic solar cells with Ag nanomesh/ZnO hybrid electrodes. J. Mater. Chem. A 2015, 3, 65–70. [Google Scholar] [CrossRef]
- Emmott, C.J.M.; Urbina, A.; Nelson, J. Environmental and economic assessment of ITO-free electrodes for organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 97, 14–21. [Google Scholar] [CrossRef]
- Cong, H.; Pan, T. Photopatternable conductive PDMS materials for microfabrication. Adv. Funct. Mater. 2008, 18, 1912–1921. [Google Scholar] [CrossRef]
- Lei, K.F.; Lee, K.F.; Lee, M.Y. A flexible PDMS capacitive tactile sensor with adjustable measurement range for plantar pressure measurement. Microsyst. Technol. 2014, 20, 1351–1358. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; Tan, J.; Xuan, F. Sandpaper-molded wearable pressure sensor for electronic skins. Sens. Actuators A Phys. 2018, 280, 205–209. [Google Scholar] [CrossRef]
- Akther, F.; Yakob, S.B.; Nguyen, N.T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Materials and devices for transparent stretchable electronics. J. Mater. Chem. C 2017, 5, 2202–2222. [Google Scholar] [CrossRef]
- Wang, D.Y.; Kim, C.L.; Kim, D.E. Development of flexible polymer sheet with high surface durability using discretely embedded micro-balls. CIRP Ann. Manuf. Technol. 2017, 66, 527–530. [Google Scholar] [CrossRef]
- Kudo, H.; Sawada, T.; Kazawa, E.; Yoshida, H.; Iwasaki, Y.; Mitsubayashi, K. A flexible and wearable glucose sensor based on functional polymers with Soft-MEMS techniques. Biosens. Bioelectron. 2006, 22, 558–562. [Google Scholar] [CrossRef]
- Choi, M.C.; Kim, Y.; Ha, C.S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Sataloff, R.T.; Johns, M.M.; Kost, K.M. When PDMS isn’t the best. Anal. Chem. 2007, 79, 3249–3253. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Formation of more stable hydrophilic surfaces of PDMS by plasma and chemical treatments. Microelectron. Eng. 2006, 83, 1277–1279. [Google Scholar] [CrossRef]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface modification of Sylgard-184 poly(dimethyl siloxane) networks by ultraviolet and ultraviolet/ozone treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ren, X.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N.L. Tailoring the surface properties of poly(dimethylsiloxane) microfluidic devices. Langmuir 2004, 20, 5569–5574. [Google Scholar] [CrossRef]
- Eddington, D.T.; Puccinelli, J.P.; Beebe, D.J. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sens. Actuators B Chem. 2006, 114, 170–172. [Google Scholar] [CrossRef]
- Maheshwari, N.; Kottantharayil, A.; Kumar, M.; Mukherji, S. Long term hydrophilic coating on poly(dimethylsiloxane) substrates for microfluidic applications. Appl. Surf. Sci. 2010, 257, 451–457. [Google Scholar] [CrossRef]
- Gillmor, S.D.; Larson, B.J.; Braun, J.M.; Mason, C.E.; Cruz-Barba, L.E.; Denes, F.; Lagally, M.G. Low-contact-angle polydimethyl siloxane (PDMS) membranes for fabricating micro-bioarrays. In Proceedings of the 2nd Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology, Madison, WI, USA, 2–4 May 2002; pp. 51–56. [Google Scholar] [CrossRef]
- Sulym, I.; Goncharuk, O.; Sternik, D.; Terpilowski, K.; Derylo-Marczewska, A.; Borysenko, M.V.; Gun’ko, V.M. Nanooxide/Polymer Composites with Silica@PDMS and Ceria–Zirconia–Silica@PDMS: Textural, Morphological, and Hydrophilic/Hydrophobic Features. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef]
- Jin, M.; Feng, X.; Xi, J.; Zhai, J.; Cho, K.; Feng, L.; Jiang, L. Super-hydrophobic PDMS surface with ultra-low adhesive force. Macromol. Rapid Commun. 2005, 26, 1805–1809. [Google Scholar] [CrossRef]
- Skupin, G.; Yamamoto, M.; Siegenthaler, K.O.; Künkel, A. Performance-Enabling Plastics. Adv. Polym. Sci. 2012, 91–136. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Z.; Leow, W.R.; Chen, X. Elastic substrates for stretchable devices. MRS Bull. 2017, 42, 103–107. [Google Scholar] [CrossRef]
- Kumaresan, Y.; Ozioko, O.; Dahiya, R. Multifunctional Electronic Skin with a stack of Temperature and Pressure Sensor Arrays. IEEE Sens. J. 2021, XX. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, B.; Chen, Y.; Feng, X. Biocompatible and ultra-flexible inorganic strain sensors attached to skin for long-term vital signs monitoring. IEEE Electron Device Lett. 2016, 37, 496–499. [Google Scholar] [CrossRef]
- Jo, M.; Min, K.; Roy, B.; Kim, S.; Lee, S.; Park, J.Y.; Kim, S. Protein-Based Electronic Skin Akin to Biological Tissues. ACS Nano 2018, 12, 5637–5645. [Google Scholar] [CrossRef]
- Kansal, A.; Hsu, J.; Zahedi, S.; Srivastava, M.B. Power Management in Energy Harvesting Sensor Networks. ACM Trans. Embed. Comput. Syst. 2007, 6, 32. [Google Scholar] [CrossRef]
- Lee, P.; Lee, J.; Lee, H.; Yeo, J.; Hong, S.; Nam, K.H.; Lee, D.; Lee, S.S.; Ko, S.H. Highly stretchable and highly conductive metal electrode by very long metal nanowire percolation network. Adv. Mater. 2012, 24, 3326–3332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, F.; Wang, X.; Zhu, Y. Wavy ribbons of carbon nanotubes for stretchable conductors. Adv. Funct. Mater. 2012, 22, 1279–1283. [Google Scholar] [CrossRef]
- Kim, A.; Ahn, J.; Hwang, H.; Lee, E.; Moon, J. A pre-strain strategy for developing a highly stretchable and foldable one-dimensional conductive cord based on a Ag nanowire network. Nanoscale 2017, 9, 5773–5778. [Google Scholar] [CrossRef]
- Kim, D.H.; Xiao, J.; Song, J.; Huang, Y.; Rogers, J.A. Stretchable, curvilinear electronics based on inorganic materials. Adv. Mater. 2010, 22, 2108–2124. [Google Scholar] [CrossRef]
- Hu, W.; Niu, X.; Zhao, R.; Pei, Q. Elastomeric transparent capacitive sensors based on an interpenetrating composite of silver nanowires and polyurethane. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. High-performance stretchable transparent electrodes based on silver nanowires synthesized via an eco-friendly halogen-free method. J. Mater. Chem. C 2014. [Google Scholar] [CrossRef]
- Kim, K.K.; Hong, S.; Cho, H.M.; Lee, J.; Suh, Y.D.; Ham, J.; Ko, S.H. Highly Sensitive and Stretchable Multidimensional Strain Sensor with Prestrained Anisotropic Metal Nanowire Percolation Networks. Nano Lett. 2015, 15, 5240–5247. [Google Scholar] [CrossRef] [PubMed]
- Hohman, M.M.; Shin, M.; Rutledge, G.; Brenner, M.P. Electrospinning and electrically forced jets. I. Stability theory. Phys. Fluids 2001, 13, 2201–2220. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. IEEE Ind. Appl. Soc. 1993, 3, 1698–1703. [Google Scholar] [CrossRef]
- Al-Saygh, A.; Ponnamma, D.; AlMaadeed, M.A.A.; Poornima Vijayan, P.; Karim, A.; Hassan, M.K. Flexible pressure sensor based on PVDF nanocomposites containing reduced graphene oxide-titania hybrid nanolayers. Polymers 2017, 9, 33. [Google Scholar] [CrossRef]
- Hosoda, K.; Tada, Y.; Asada, M. Anthropomorphic robotic soft fingertip with randomly distributed receptors. Rob. Auton. Syst. 2006, 54, 104–109. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Xu, C. Silver nanowire dopant enhancing piezoelectricity of electrospun PVDF nanofiber web. Fourth Int. Conf. Smart Mater. Nanotechnol. Eng. 2013, 8793, 879314. [Google Scholar] [CrossRef]
- Li, B.; Xu, C.; Zheng, J.; Xu, C. Sensitivity of pressure sensors enhanced by doping silver nanowires. Sensors 2014, 14, 9889–9899. [Google Scholar] [CrossRef]
- Kim, G.H.; Nam, H.; Choi, W.S.; An, T.; Lim, G. Electrospinning Nanofiber on an Insulating Surface with a Patterned Functional Electrolyte Electrode. Adv. Mater. Interfaces 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Ku, M.L.; Li, W.; Chen, Y.; Ray Liu, K.J. Advances in Energy Harvesting Communications: Past, Present, and Future Challenges. IEEE Commun. Surv. Tutor. 2016, 18, 1384–1412. [Google Scholar] [CrossRef]
- Stephen, N.G. On energy harvesting from ambient vibration. J. Sound Vib. 2006, 293, 409–425. [Google Scholar] [CrossRef]
- Weisskopf, V.F. On the self-energy and the electromagnetic field of the electron. Phys. Rev. 1939, 56, 72–85. [Google Scholar] [CrossRef]
- Wang, Q.M.; Du, X.H.; Xu, B.; Eric Cross, L. Electromechanical coupling and output efficiency of piezoelectric bending actuators. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1999, 46, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Roundy, S. On the effectiveness of vibration-based energy harvesting. J. Intell. Mater. Syst. Struct. 2005, 16, 809–823. [Google Scholar] [CrossRef]
- Kansal, A.; Srivastava, M.B. An environmental energy harvesting framework for sensor networks. ISLPDE 2003, 481. [Google Scholar] [CrossRef]
- Zhang, X.S.; Di Han, M.; Wang, R.X.; Zhu, F.Y.; Li, Z.H.; Wang, W.; Zhang, H.X. Frequency-multiplication high-output triboelectric nanogenerator for sustainably powering biomedical microsystems. Nano Lett. 2013, 13, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.R.; Tian, Z.Q.; Lin Wang, Z. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Cao, X.; Jie, Y.; Wang, N.; Wang, Z.L. Triboelectric Nanogenerators Driven Self-Powered Electrochemical Processes for Energy and Environmental Science. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Zhou, K.; Zhao, Y.; Sun, X.; Yuan, Z.; Zheng, G.; Dai, K.; Mi, L.; Pan, C.; Liu, C.; Shen, C. Ultra-stretchable triboelectric nanogenerator as high-sensitive and self-powered electronic skins for energy harvesting and tactile sensing. Nano Energy 2020, 70, 104546. [Google Scholar] [CrossRef]
- Zi, Y.; Niu, S.; Wang, J.; Wen, Z.; Tang, W.; Wang, Z.L. Standards and figure-of-merits for quantifying the performance of triboelectric nanogenerators. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Wang, G.; Liu, G.; Chen, J.; Pu, X.; Xi, Y.; Wang, X.; Guo, H.; Hu, C.; et al. Integrated charge excitation triboelectric nanogenerator. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Zi, Y.; Wang, J.; Wang, S.; Li, S.; Wen, Z.; Guo, H.; Wang, Z.L. Effective energy storage from a triboelectric nanogenerator. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, J.; Huang, J.; Sun, J.; Wang, Z.L.; Seo, S.; Sun, Q. Stretchable Energy-Harvesting Tactile Interactive Interface with Liquid-Metal-Nanoparticle-Based Electrodes. Adv. Funct. Mater. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Sardini, E.; Serpelloni, M. Self-powered wireless sensor for air temperature and velocity measurements with energy harvesting capability. IEEE Trans. Instrum. Meas. 2011, 60, 1838–1844. [Google Scholar] [CrossRef]
- Wang, Z.L. Self-powered nanosensors and nanosystems. Adv. Mater. 2012, 24, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Qin, Y.; Xu, C.; Wei, Y.; Yang, R.; Wang, Z.L. Self-powered nanowire devices. Nat. Nanotechnol. 2010, 5, 366–373. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Zhao, Z.; Zhou, L.; Yin, X.; Li, X.; Gao, Y.; Zhang, C.; Zhang, Q.; Wang, J.; et al. A Fully Self-Powered Vibration Monitoring System Driven by Dual-Mode Triboelectric Nanogenerators. ACS Nano 2020, 14, 2475–2482. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, B.; Ouyang, H.; Fan, Y.; Wang, Z.L.; Li, Z. Emerging Implantable Energy Harvesters and Self-Powered Implantable Medical Electronics. ACS Nano 2020, 14, 6436–6448. [Google Scholar] [CrossRef]
- Rajesh, R.; Sharma, V.; Viswanath, P. Information capacity of energy harvesting sensor nodes. IEEE Int. Symp. Inf. Theory Proc. 2011, 2363–2367. [Google Scholar] [CrossRef]
- Bacharowski, W. Energy Scavenging for Mobile and Wireless Electronics. IEEE Pervasive Comput. 2005, 53, 18–27. [Google Scholar] [CrossRef]
- Varíola, F.; Vetrone, F.; Richert, L.; Jedrzejowski, P.; Yi, J.H.; Zalzal, S.; Clair, S.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: An overview of strategies, fabrication methods, and challenges. Small 2009, 5, 996–1006. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon coatings as biocompatible materials—An overview. Diam. Relat. Mater. 2003, 12, 166–170. [Google Scholar] [CrossRef]
- Oh, J.Y.; Bao, Z. Second Skin Enabled by Advanced Electronics. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Hou, C.; Xu, Z.; Qiu, W.; Wu, R.; Wang, Y.; Xu, Q.; Liu, X.Y.; Guo, W. A Biodegradable and Stretchable Protein-Based Sensor as Artificial Electronic Skin for Human Motion Detection. Small 2019, 15, 1–8. [Google Scholar] [CrossRef]
- Pang, C.; Lee, C.; Suh, K.Y. Recent advances in flexible sensors for wearable and implantable devices. J. Appl. Polym. Sci. 2013, 130, 1429–1441. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Synthesis, Characterization, and Electrical Properties of Polypyrrole/Multiwalled Carbon Nanotube Composites. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S., II; Kalaichelvan, P.T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crops Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Lin, Y.; Yang, Z.; Liu, L. Cu-Ag core-shell nanowires for electronic skin with a petal molded microstructure. J. Mater. Chem. C 2015, 3, 9594–9602. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Hwang, S.W.; Su, Y.; Kim, S.; Cheng, H.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.; Rogers, J.A. Transient, biocompatible electronics and energy harvesters based on ZnO. Small 2013, 9, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Karikkineth, B.C.; Nagamine, K.; Kaji, H.; Torimitsu, K.; Nishizawa, M. Highly Conductive Stretchable and Biocompatible Electrode-Hydrogel Hybrids for Advanced Tissue Engineering. Adv. Healthc. Mater. 2014, 3, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Lai, D.T.H.; Su, B.; Si, K.J.; Ma, Z.; Yap, L.W.; Guo, P.; Cheng, W. Highly Stretchy Black Gold E-Skin Nanopatches as Highly Sensitive Wearable Biomedical Sensors. Adv. Electron. Mater. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Wu, W.Y.; Zhong, X.; Wang, W.; Miao, Q.; Zhu, J.J. Flexible PDMS-based three-electrode sensor. Electrochem. Commun. 2010, 12, 1600–1604. [Google Scholar] [CrossRef]
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Heuer, A.H.; Fink, D.J.; Laraia, V.J.; Arias, J.L.; Calvert, P.D.; Kendall, K.; Messing, G.L.; Blackwell, J.; Rieke, P.C.; Thompson, D.H.; et al. Innovative materials processing strategies: A biomimetic approach. Science 1992, 255, 1098–1105. [Google Scholar] [CrossRef]
- Ozin, G.A. Morphogenesis of Biomineral and Morphosynthesis of Biomimetic Forms. Acc. Chem. Res. 1997, 30, 17–27. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Mahata, C.; Algadi, H.; Lee, J.; Kim, S.; Lee, T. Biomimetic-inspired micro-nano hierarchical structures for capacitive pressure sensor applications. Meas. J. Int. Meas. Confed. 2020, 151, 107095. [Google Scholar] [CrossRef]
- Kang, D.; Pikhitsa, P.V.; Choi, Y.W.; Lee, C.; Shin, S.S.; Piao, L.; Park, B.; Suh, K.Y.; Kim, T., II; Choi, M. Ultrasensitive mechanical crack-based sensor inspired by the spider sensory system. Nature 2014, 516, 222–226. [Google Scholar] [CrossRef]
- Khuyen, N.Q.; Kiefer, R.; Elhi, F.; Anbarjafari, G.; Martinez, J.G.; Tamm, T. A biomimetic approach to increasing soft actuator performance by friction reduction. Polymers 2020, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Lee, S.H.; Ko, H.; Lee, D.; Bae, W.G.; Kim, T., II; Hwang, D.S.; Jeong, H.E. Ultra-Adaptable and Wearable Photonic Skin Based on a Shape-Memory, Responsive Cellulose Derivative. Adv. Funct. Mater. 2019, 29, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Z.; Li, T.; Sun, F.; Bai, Y.; Lu, Q.; Wang, S.; Yang, X.; Hao, M.; Lan, N.; et al. Highly Selective Biomimetic Flexible Tactile Sensor for Neuroprosthetics. Research 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Xu, L.; Cheng, X.; Li, Y.; Huang, X.; Guo, W.; Liu, S.; Wang, Z.L.; Wu, H. Bioinspired Triboelectric Nanogenerators as Self-Powered Electronic Skin for Robotic Tactile Sensing. Adv. Funct. Mater. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, J.H. Biomimetic Tactile Sensors Based on Nanomaterials. ACS Nano 2020, 14, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Guan, Q.; Zhang, X. Biomimetic multifunctional E-skins integrated with mechanoluminescence and chemical sensing abilities. J. Mater. Chem. C 2021, 9, 2815–2822. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, J.; Yang, K.; Peng, H.; Yin, F. High-Performance and Multifunctional Skinlike Strain Sensors Based on Graphene/Springlike Mesh Network. ACS Appl. Mater. Interfaces 2018, 10, 19906–19913. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Wang, M.J.; Liu, H.C.; Zhang, Y.L.; Lin, Q.H.; Chen, T.; Sun, L.N. Multifunctional Self-Powered E-Skin with Tactile Sensing and Visual Warning for Detecting Robot Safety. Adv. Mater. Interfaces 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Cui, X.; Wang, S.; Liu, Z.; Deng, L.; Qi, A.; Qiao, X.; Li, L.; Pan, C.; et al. Piezoelectric Polyacrylonitrile Nanofiber Film-Based Dual-Function Self-Powered Flexible Sensor. ACS Appl. Mater. Interfaces 2018, 10, 15855–15863. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Ye, C.; Jiang, Y.; Zhai, S.; Cheng, R.; Liu, D.; Gao, X.; Wang, J.; Wang, Z.L. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Tee, C.K.; Chortos, A.; Berndt, A.; Nguyen, A.K.; Tom, A.; McGuire, A.; Lin, Z.C.; Tien, K.; Bae, W.-G.; Wang, H.; et al. A skin-inspired organic digital mechanoreceptor. Science 2015, 350, 313–316. [Google Scholar] [CrossRef]
- Choi, J.H.; Wang, H.; Oh, S.J.; Paik, T.; Jo, P.S.; Sung, J.; Ye, X.; Zhao, T.; Diroll, B.T.; Murray, C.B.; et al. Exploiting the colloidal nanocrystal library to construct electronic devices. Science 2016, 352, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, H.; Nishimura, S.; Murai, K.; Hayashi, Y.; Suzuki, T.; Nakayama, T.; Jiang, W.; Yamazaki, A.; Seki, K.; Niihara, K. Pulsed wire discharge apparatus for mass production of copper nanopowders. Rev. Sci. Instrum. 2007, 78. [Google Scholar] [CrossRef] [PubMed]
- Vishinkin, R.; Haick, H. Nanoscale Sensor Technologies for Disease Detection via Volatolomics. Small 2015, 11, 6142–6164. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Kim, Y.S.; Ornelas, G.; Sinha, M.; Naidu, K.; Coleman, T.P. Scalable microfabrication procedures for adhesive-integrated flexible and stretchable electronic sensors. Sensors 2015, 15, 23459–23476. [Google Scholar] [CrossRef]
- Magnusson, M.H.; Ohlsson, B.J.; Björk, M.T.; Dick, K.A.; Borgström, M.T.; Deppert, K.; Samuelson, L. Semiconductor nanostructures enabled by aerosol technology. Front. Phys. 2014, 9, 398–418. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.J.; Kim, J.H.; Choi, D.G.; Choi, J.H.; Jung, J.Y.; Jeon, S.; Lee, E.S.; Jeong, J.H.; Lee, J. Rapid Low-Temperature 3D Integration of Silicon Nanowires on Flexible Substrates. Small 2015, 11, 3995–4001. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sul, H.; Jung, Y.; Kim, H.; Han, S.; Choi, J.; Shin, J.; Kim, D.; Jung, J.; Hong, S.; et al. Thermally Controlled, Active Imperceptible Artificial Skin in Visible-to-Infrared Range. Adv. Funct. Mater. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Cai, Y.W.; Zhang, X.N.; Wang, G.G.; Li, G.Z.; Zhao, D.Q.; Sun, N.; Li, F.; Zhang, H.Y.; Han, J.C.; Yang, Y. A flexible ultra-sensitive triboelectric tactile sensor of wrinkled PDMS/MXene composite films for E-skin. Nano Energy 2021, 81, 105663. [Google Scholar] [CrossRef]
- Yu, X.; Xie, Z.; Yu, Y.; Lee, J.; Vazquez-Guardado, A.; Luan, H.; Ruban, J.; Ning, X.; Akhtar, A.; Li, D.; et al. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 2019, 575, 473–479. [Google Scholar] [CrossRef]
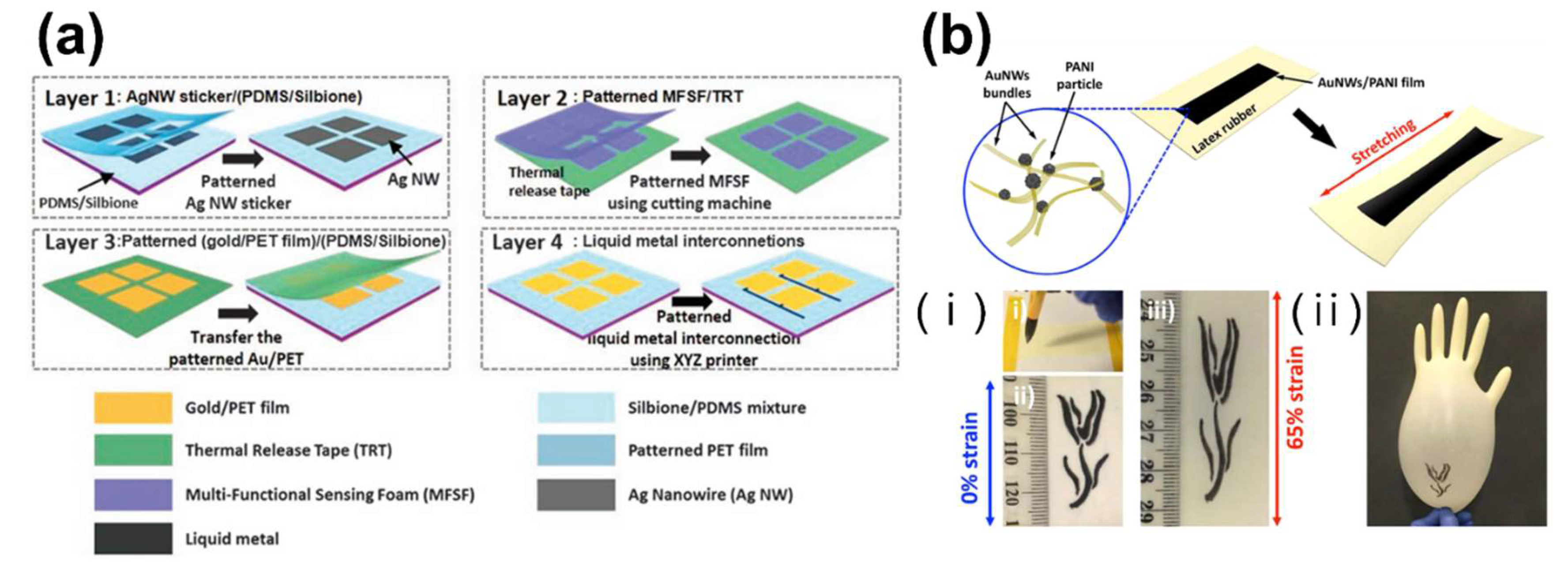
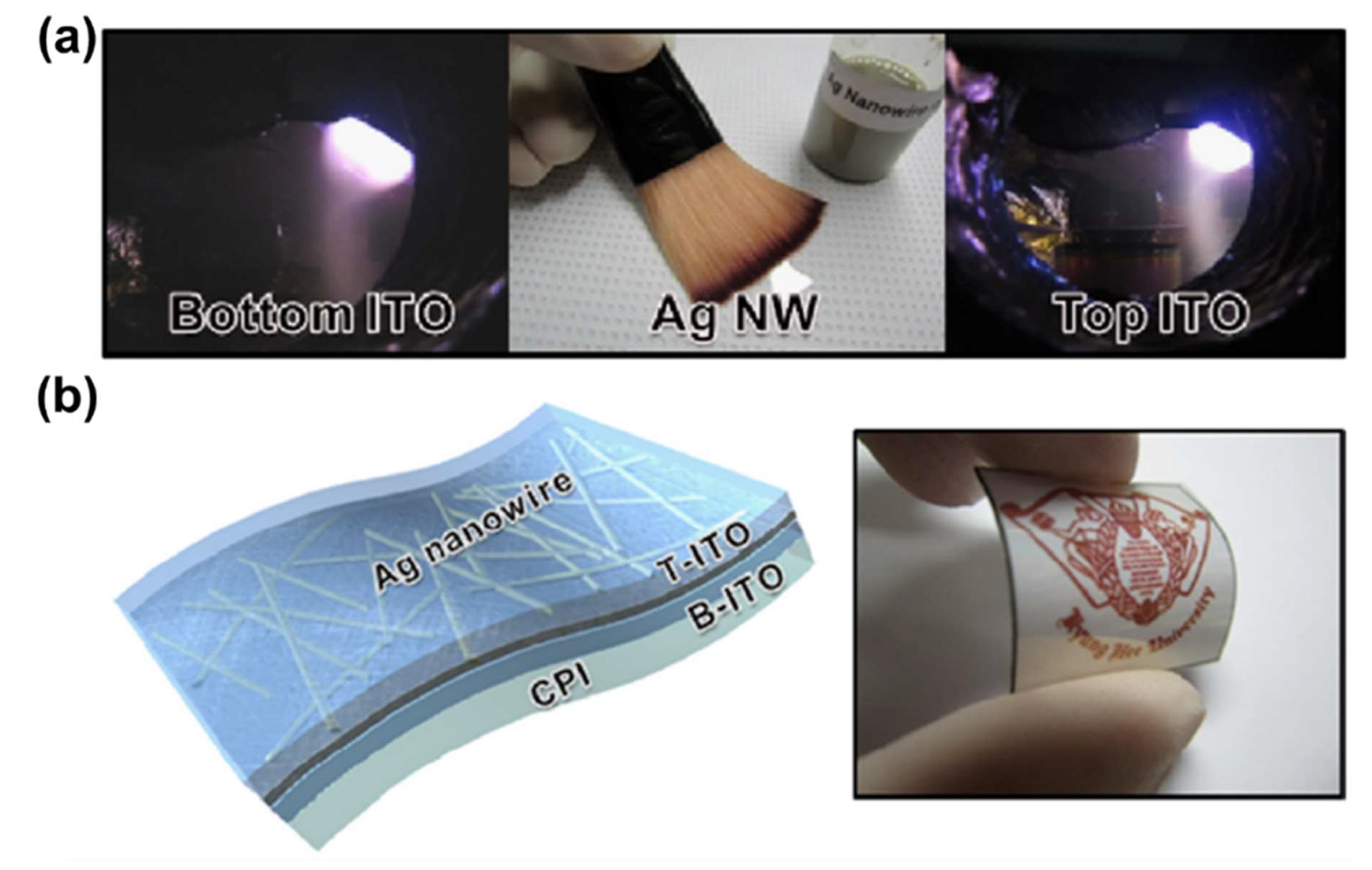
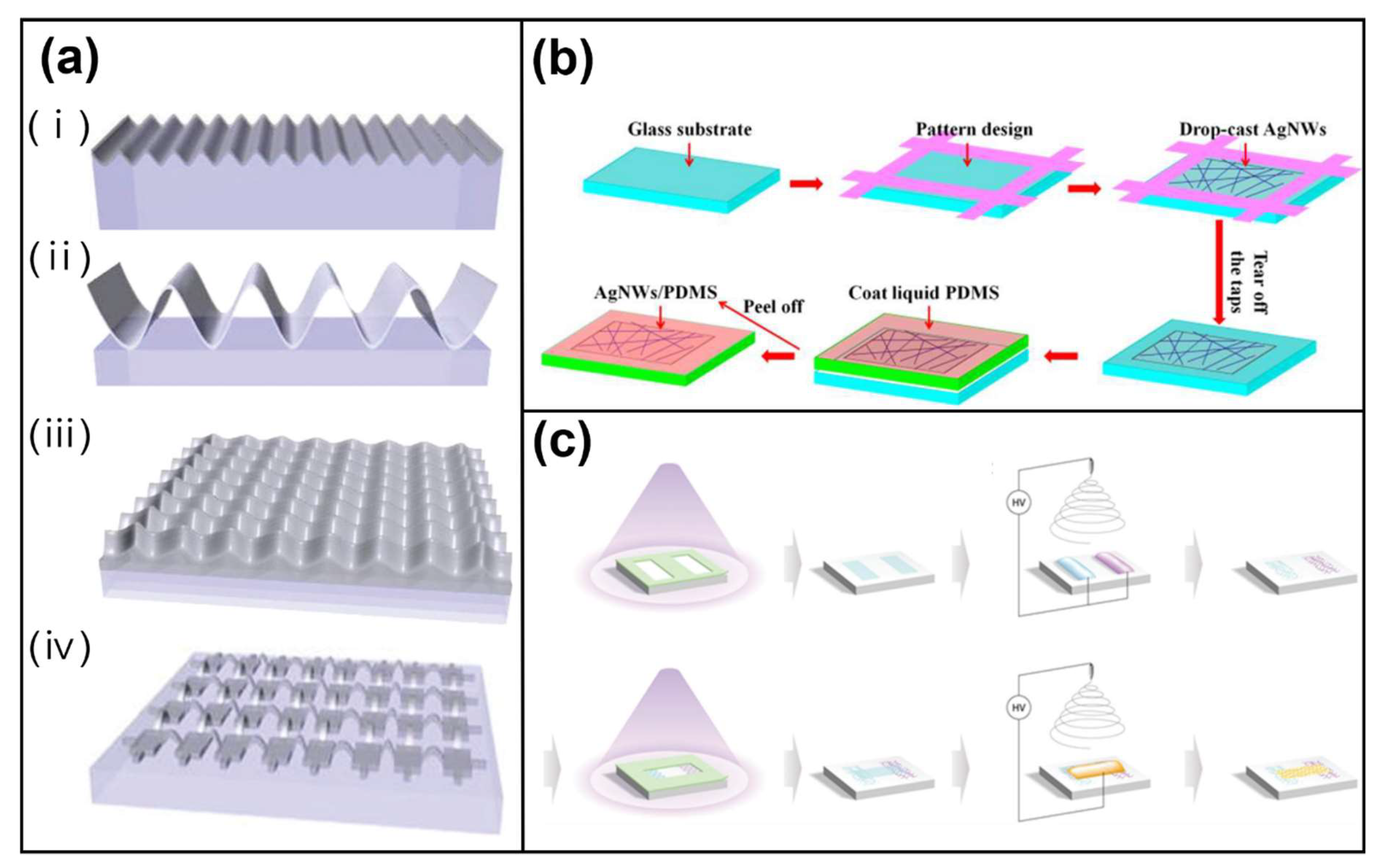
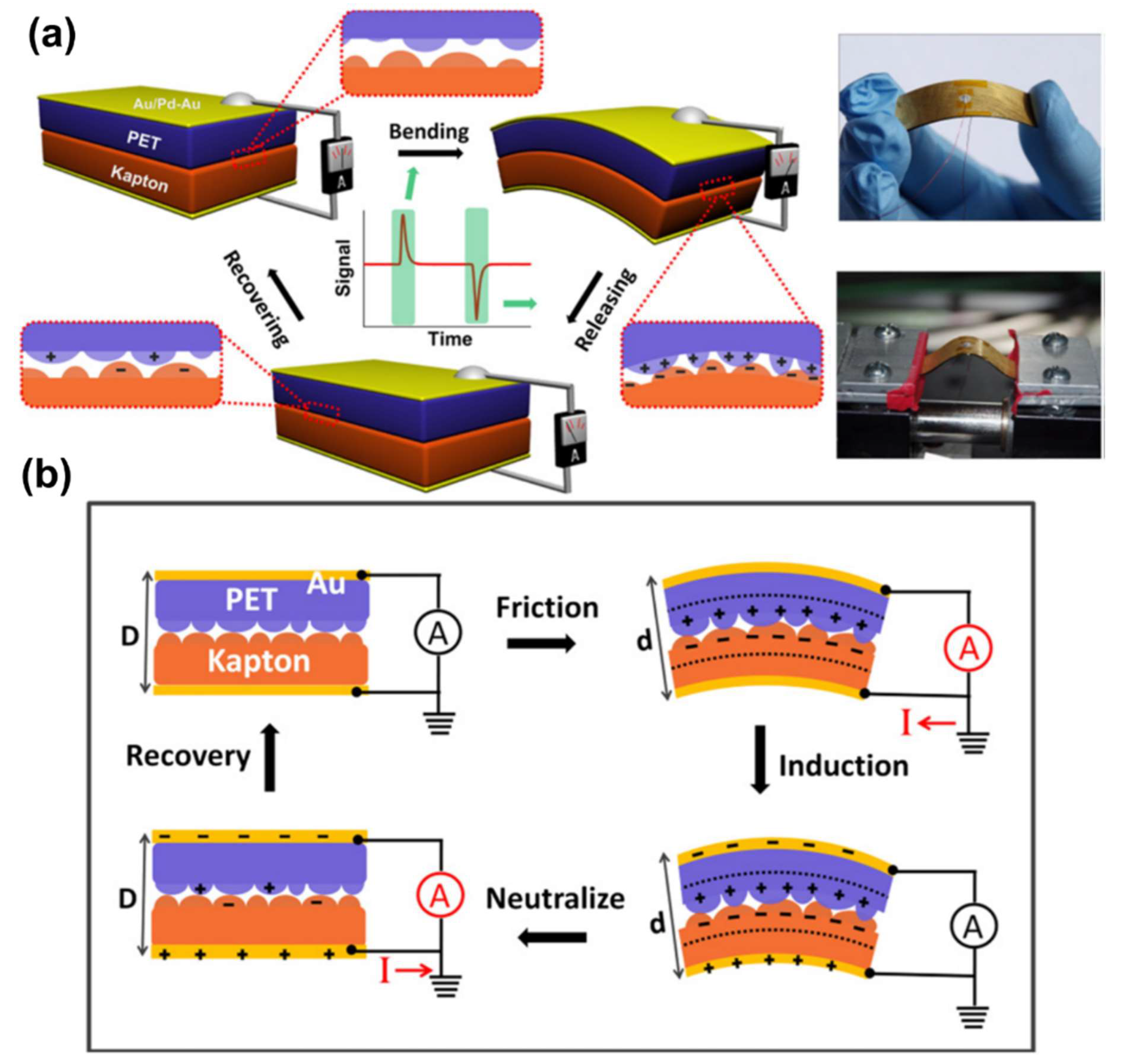


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.S.; Lee, C.H.; Kim, N.K.; An, T.; Kim, G.H. Review: Sensors for Biosignal/Health Monitoring in Electronic Skin. Polymers 2021, 13, 2478. https://doi.org/10.3390/polym13152478
Oh HS, Lee CH, Kim NK, An T, Kim GH. Review: Sensors for Biosignal/Health Monitoring in Electronic Skin. Polymers. 2021; 13(15):2478. https://doi.org/10.3390/polym13152478
Chicago/Turabian StyleOh, Hyeon Seok, Chung Hyeon Lee, Na Kyoung Kim, Taechang An, and Geon Hwee Kim. 2021. "Review: Sensors for Biosignal/Health Monitoring in Electronic Skin" Polymers 13, no. 15: 2478. https://doi.org/10.3390/polym13152478
APA StyleOh, H. S., Lee, C. H., Kim, N. K., An, T., & Kim, G. H. (2021). Review: Sensors for Biosignal/Health Monitoring in Electronic Skin. Polymers, 13(15), 2478. https://doi.org/10.3390/polym13152478