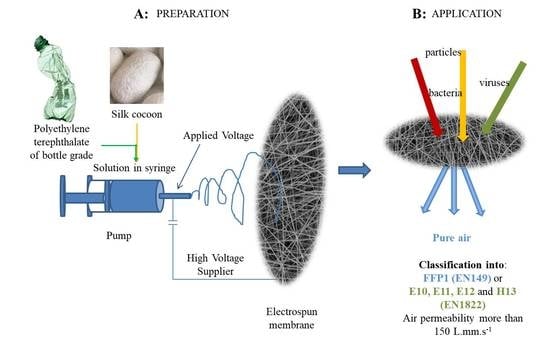
Electrospun Poly(ethylene Terephthalate)/Silk Fibroin Composite for Filtration Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polymer Solutions for Electrospinning
2.2.2. Electrospinning Process (ESP)
2.2.3. Morphology of Fibers and Pores Size
2.2.4. Attenuated Total Reflectance—Fourier Transform Infrared Spectrometry (ATR-FTIR)
2.2.5. Water Contact Angle (WCA)
2.2.6. Mechanical Analysis
2.2.7. Dynamic Mechanical Thermal Analysis (DMTA)
2.2.8. Thermogravimetric Analysis (TGA)
2.2.9. Filtration Efficiency (FE) and Quality Factor (Qf)
2.2.10. Air Permeability (B)
2.2.11. Water Vapor Permeability (WVP)
2.2.12. Antibacterial Activity (R)
2.2.13. Biocompatibility
3. Results and Discussion
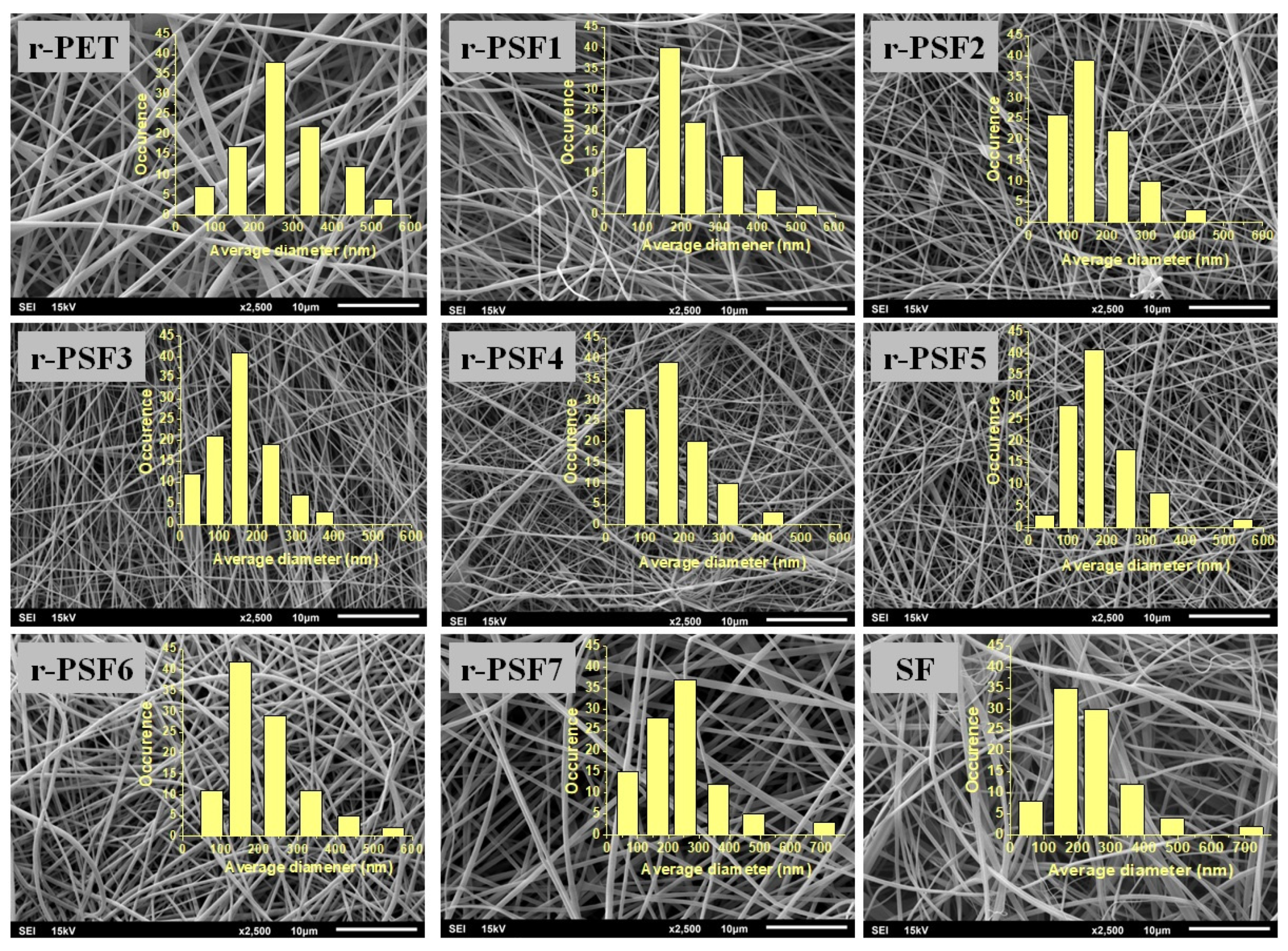
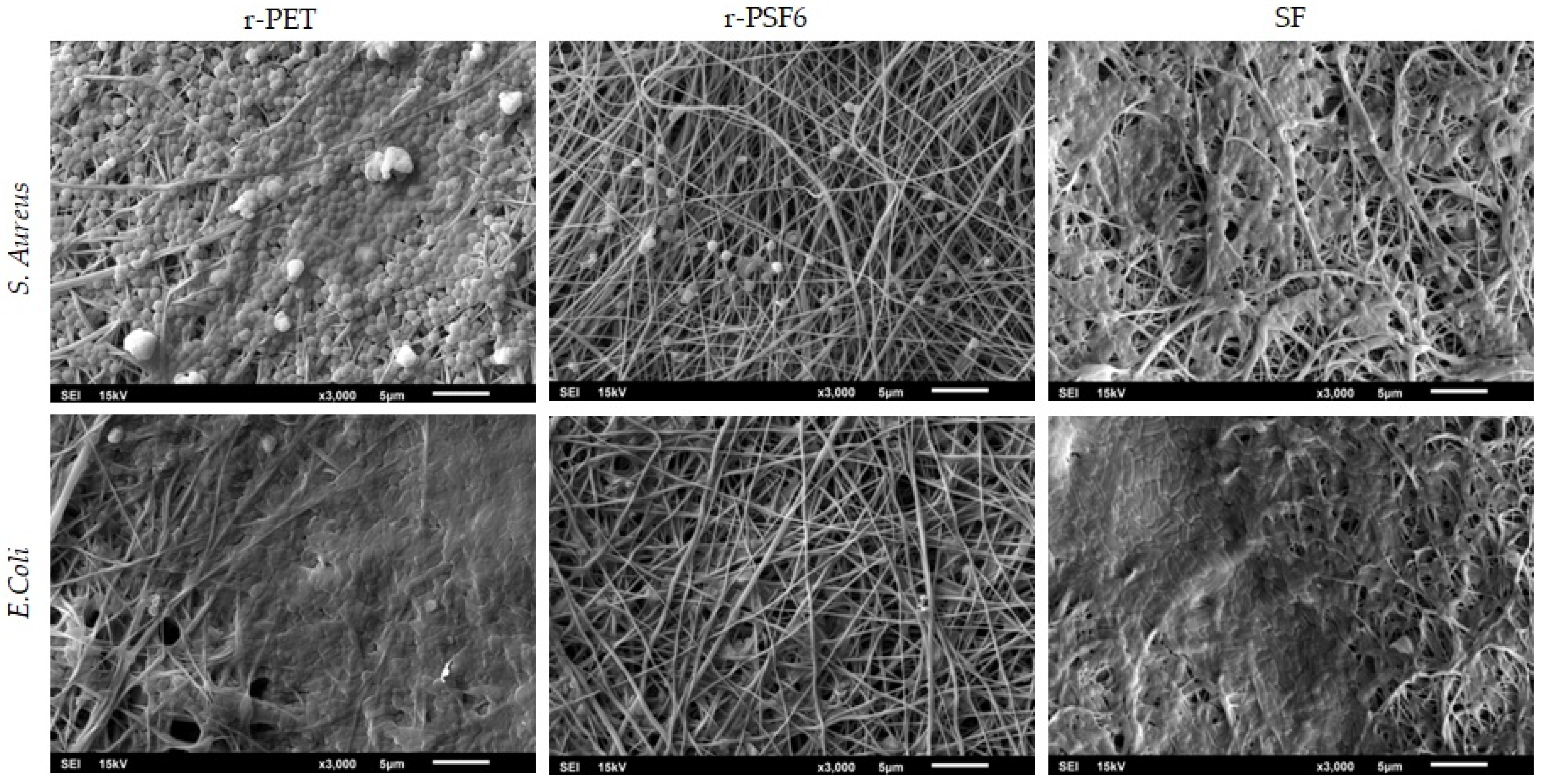
3.1. Morphology of Electrospun Mats and Average Diameter of the Fibers
3.2. Water Contact Angle (WCA)
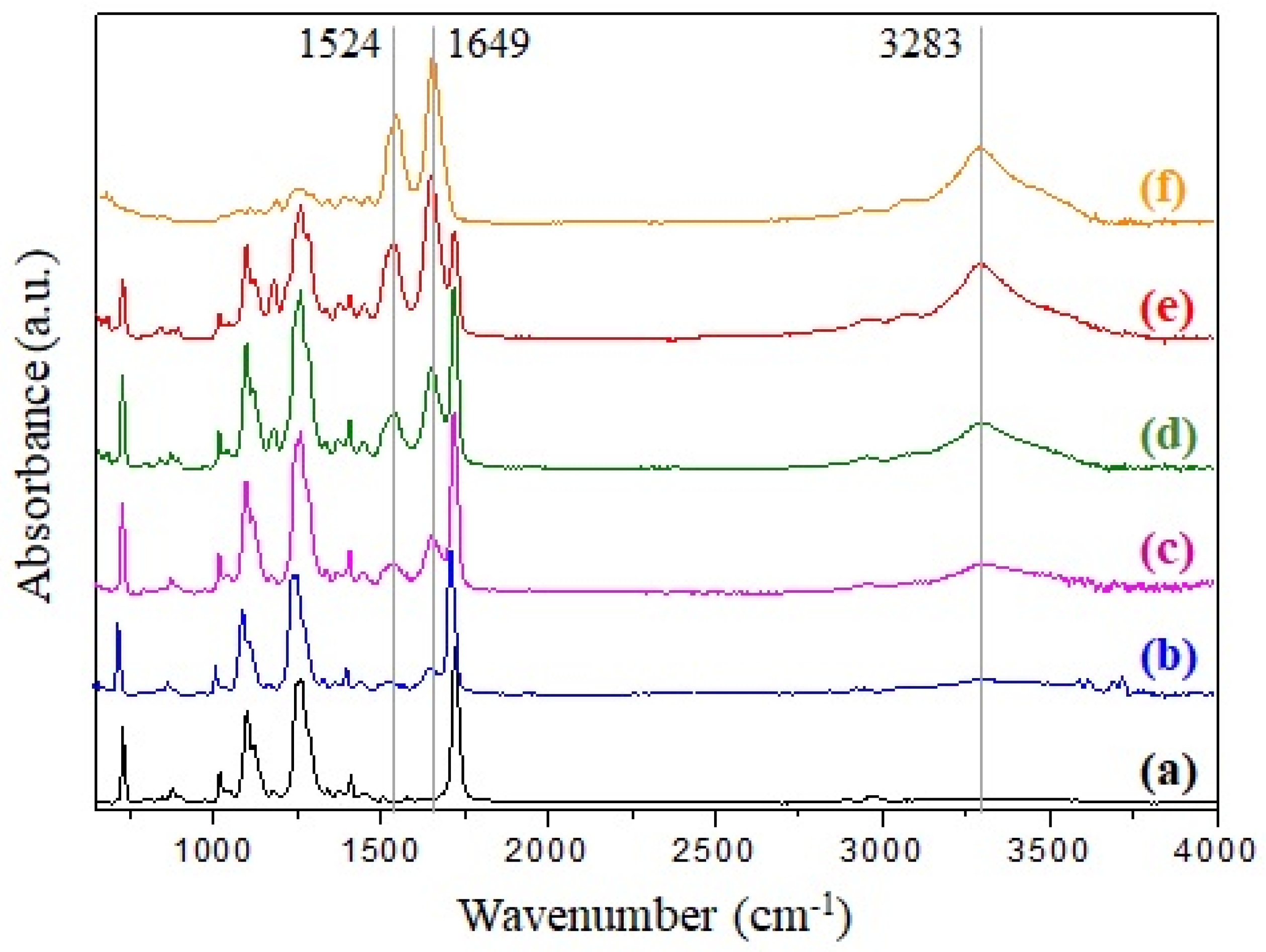
3.3. ATR-FTIR Analysis of Investigated Fibrous Mats
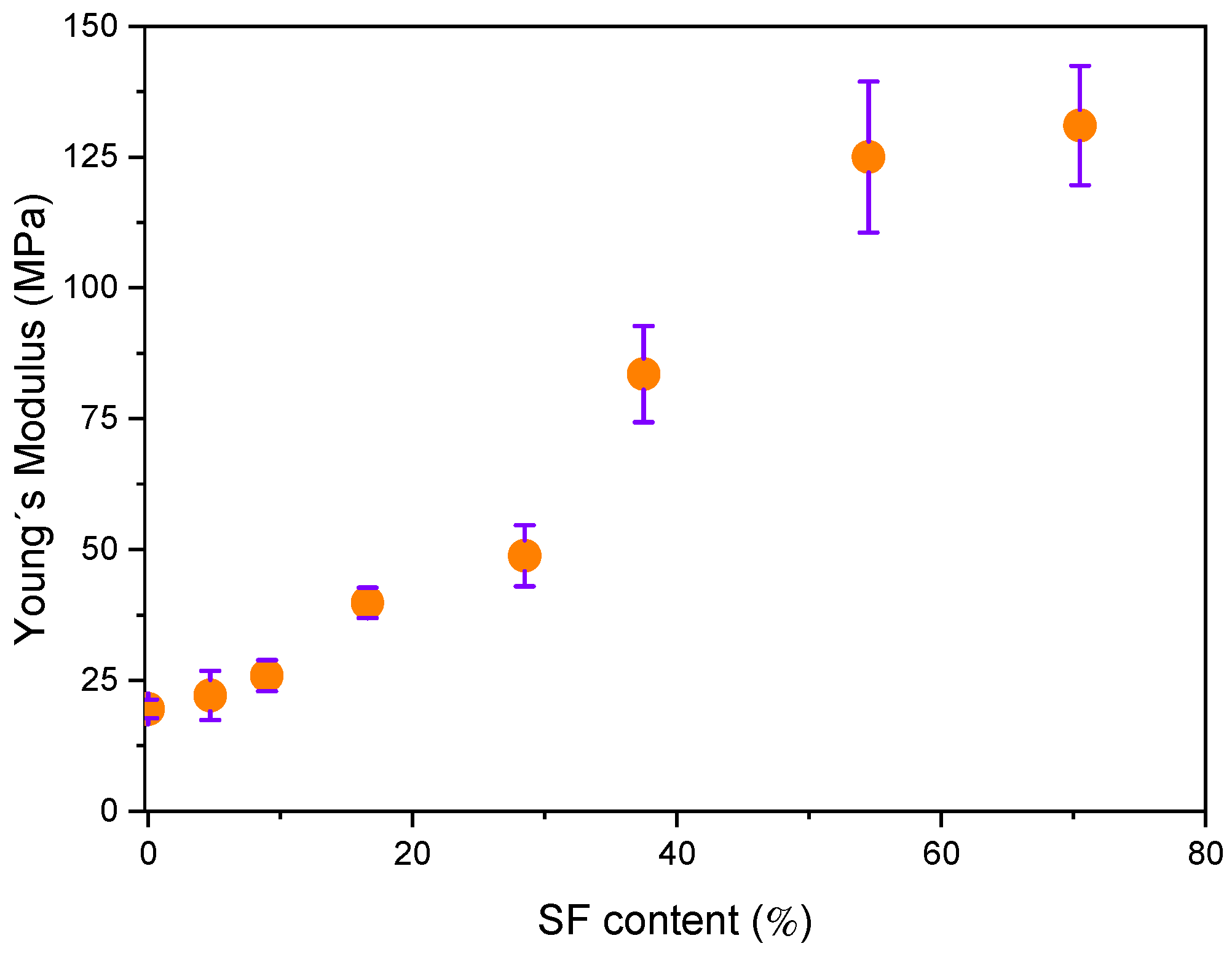
3.4. Mechanical Properties
3.5. Dynamic Mechanical Thermal Analysis
3.6. Thermogravimetric Analysis (TGA)
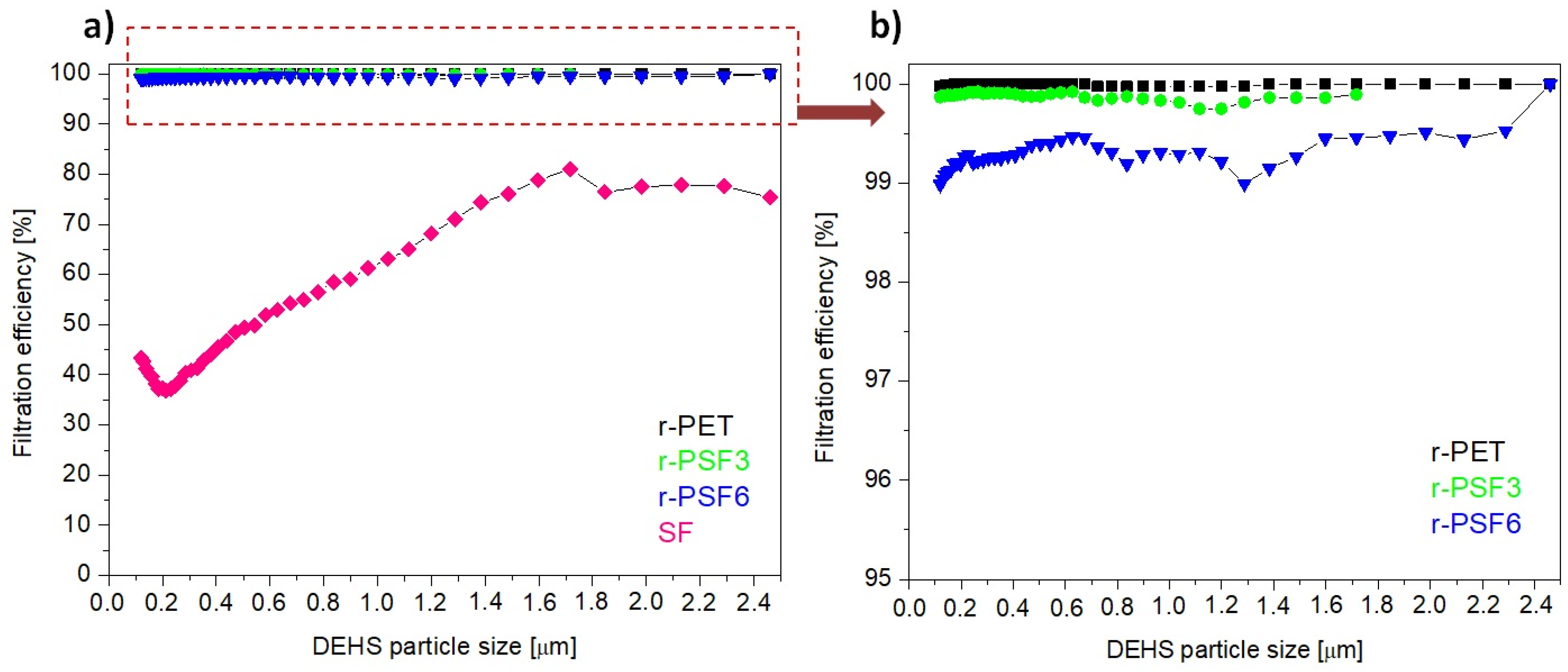
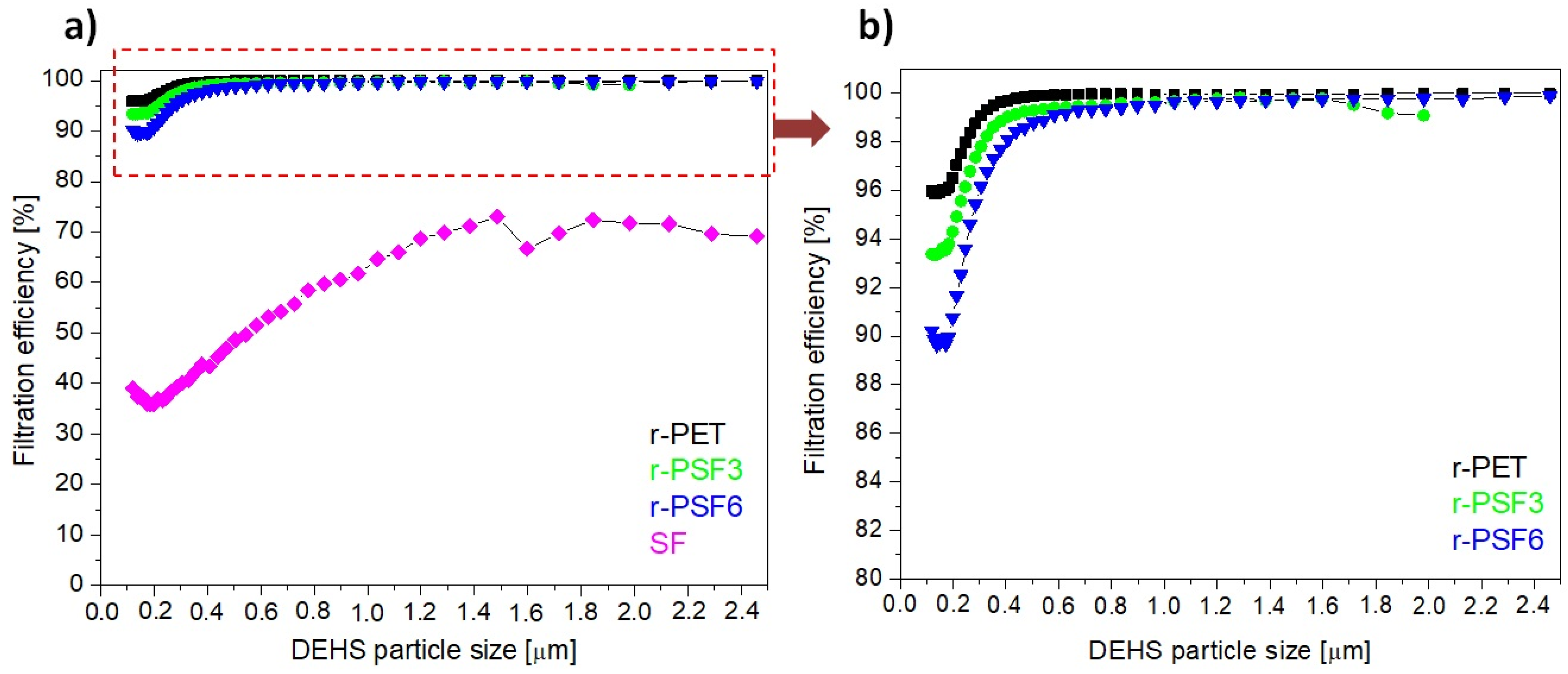
3.7. Filtration Efficiency and Comfort Properties
3.8. Air and Water Vapor Permeability
3.9. Antibacterial Activity
3.10. Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scudellari, M. How the Pandemic Might Play Out in 2021 an Beyond. News Feature. Available online: https://www.nature.com/articles/d41586-020-02278-5 (accessed on 12 July 2021).
- Statement on the Seventh Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Coronavirus Deseade (COVID-19) Pandemic. Available online: https://www.who.int/news/item/19-04-2021-statement-on-the-seventh-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 20 June 2021).
- The facemask global value chain in the COVID-19 outbreak: Evidence and policy lessons. Available online: https://www.oecd.org/coronavirus/policy-responses/the-face-mask-global-value-chain-in-the-COVID-19-outbreak-evidence-and-policy-lessons-a4df866d/ (accessed on 23 July 2021).
- Ivanoska-Dacikj, A.; Stachewicz, U. Smart textiles and wearable technologies—Opportunities offered in the fight against pandemics in relation to current COVID-19 state. Rev. Adv. Mater. Sci. 2020, 59, 487–505. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S. Electrospun nanofibers-based face masks. Adv. Fiber Mater. 2020, 2, 161–166. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, X.; Zhang, W.; Xu, R.; Kim, S.C.; Cui, Y.; Howard, T.T.; Wu, E.; Cui, Y. Air-filtering masks for respiratory protection from PM2.5 and pandemic pathogens. OneEarth 2020, 3, 574–589. [Google Scholar] [CrossRef]
- Leung, W.W.F.; Sun, Q. Charged PVDF multilayer nanofiber filter in filtering simulated airbone novel coronavirus (COVID-19) using ambient nano-aerosols. Sep. Purif. Technol. 2020, 245, 116887. [Google Scholar] [CrossRef]
- Yin, X.; Yu, J.; Ding, B. Electrospun fibers for filtration. In Hanbook of Fibrous Materials, 1st ed.; Hu, J., Kumar, B., Lu, J., Eds.; Wiley-VCH: Weinheim, Germany, 2020; Volume 2, pp. 175–206. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Zahmatkeshan, M.; Adel, M.; Bahrami, S.; Esmaeili, F.; Rezayat, A.M.; Saeedi, Y.; Mehravi, B.; Jameie, S.B.; Ashtari, K. Polymer based nanofibers: Preparation, Fabrication, and applications. In Hanbook of Nanofibers, 1st ed.; Barhoum, A., Bechalany, M., Makhlouf, A.S.H., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–47. [Google Scholar] [CrossRef]
- Aragón, J.; Costa, C.; Coelhoso, I.; Mendoza, G.; Aguilar-Ricardo, A.; Irusta, S. Electrospun asymmetric membranes for wound dressing applications. Mater. Sci. Eng. C 2019, 103, 109822. [Google Scholar] [CrossRef] [PubMed]
- Locilento, D.A.; Mercante, L.A.; Andre, R.S.; Mattosi, L.H.C.; Luna, G.L.F.; Brassolatti, P.; Anibal, F.d.F.; Correa, D.S. Biocompatibile and biodegradable electrospun nanofibrous membranes loaded with grape seed extract for wound dressing application. J. Nanomater. 2019, 2472964. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A review on properties of natural and synthetic based electrospun fibrous materials for bone tissue engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, L.P.; Rodrigues, A.A.; Macedo, M.; Jardini, A.L.; Filho, R.M. Electrospun polyurethane membranes for tissue engineering applications. Mater. Sci Eng. C 2017, 72, 113–117. [Google Scholar] [CrossRef]
- Gorrasi, G.; Longo, R.; Viscusi, G. Fabrication and characterization of electrospun membranes based on poly(ε-caprolactone), poly(3-hydroxybutyrate) and their blend for tunable drug delivery of curcumin. Polymers 2020, 12, 2239. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kweon, O.Y.; Lee, S.J.; Oh, J.H. Wearable high-performance pressure sensors based on three-dimensional electrospun conductive nanofibers. NPG Asia Mater. 2018, 10, 540–551. [Google Scholar] [CrossRef]
- Aliheidari, N.; Aliahmad, N.; Agarwal, M.; Dalir, H. Electrospun nanofibers for label-free sensor application. Sensor 2019, 19, 3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Z.; Jiang, J.; Wang, X.; Li, W.; Fang, L.; Zhen, G. Self-powered electrospun composite nanofiber membrane for highly efficient air filtration. Nanomaterials 2020, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Elbahri, M. Nanocomposite electrospun nanofiber membranes for environmental remediation. Materials 2014, 7, 1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.; Xu, Y.; Chen, D.; Xie, Y.; Chen, L.; Que, M.; Hou, Y. Deformamble and flexible electrospun nanofiber-supported crosslinked gel polymer electrolyte membranes for high safety lithium-ion batteries. RSC Adv. 2017, 7, 22728–22734. [Google Scholar] [CrossRef] [Green Version]
- Arifeen, W.U.; Kim, M.; Ting, D.; Kurniawan, R.; Choi, J.; Kisoo, Y.; Ko, T.J. Hybrid thermal resistant electrospun polymer membrane as the separator of lithium ion batteries. Mater. Chem. Phys. 2020, 245, 122780. [Google Scholar] [CrossRef]
- Thomas, M.; Rajiv, S. Dye-sensitized solar cells based on an electrospun polymer nanocomposite membrane as electrolyte. New J. Chem. 2019, 43, 4444–4454. [Google Scholar] [CrossRef]
- Kaschuk, J.J.; Miettunen, K.; Borghei, M.; Frollini, E.; Rojas, O.J. Electrolyte membranes based on ultrafine fibers of acetylated cellulose for improved and long-lasting dye-sensitized solar cells. Cellulose 2019, 26, 6151–6163. [Google Scholar] [CrossRef] [Green Version]
- Bonincontro, D.; Fraschetti, F.; Squarzoni, C.; Mazzocchetti, L.; Maccaferri, E.; Giorgini, L.; Zucchelli, A.; Gualandi, C.; Focarete, M.L.; Albonetti, S. Pd/Au based catalyst immobilization in polymeric nanofibrous membranes via electrospinning for the selective oxidation of 5-hydroxymethylfurfural. Processes 2020, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.; Jankovic, J.; Susac, D.; Saha, M.S.; Tam, M.; Yang, H.; Ko, F. Electrospun carbon nanofiber catalyst layers for polymer electrolyte membrane fuel cells: Fabrication and optimization. J. Mater. Sci. 2018, 53, 11633–11647. [Google Scholar] [CrossRef]
- Gorji, M.; Jeddi, A.A.A.; Gharehaghaji, A.A. Fabrication and characterization of polyurethane electrospun nanofiber membranes for protective clothing applications. J. Appl. Polym. Sci. 2012, 125, 4135–4141. [Google Scholar] [CrossRef]
- Yu, X.; Wu, X.; Si, Y.; Wang, X.; Yu, J.; Ding, B. Waterproof and breathable electrospun nanofibrous membranes. Macromol. Rapid Commun. 2019, 40, e1800931. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Abdulhamid, M.A.; Nunes, S.P.; Szekely, G. Hierarchically porous electrospun nanofibrous mats produced from intrinsically microporous fluorinated polyimide for the removal of oils and non-polar solvents. Environ. Sci. Nano 2020, 7, 1365–1372. [Google Scholar] [CrossRef]
- Tian, Z.; Chee, T.S.; Zhang, X.; Lei, L.; Xiao, C. Novel bismuth-based electrospinning materials for highly efficient capture of radioiodine. Chem. Eng. J. 2021, 415, 128687. [Google Scholar] [CrossRef]
- Topuz, F.; Holtzl, T.; Szekely, G. Scavenging organic micropollutants from water with nanofibrous hypercrosslinked cyclodextrin membranes derived from green resources. Chem. Eng. J. 2021, 419, 129443. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Frajová, J.; Nosko, M. Recycling of poly(ethylene therephthalate) by electrospinning to enhanced the filtration efficiency. Mater. Lett. 2020, 278, 128426. [Google Scholar] [CrossRef]
- Leung, W.W.F.; Sun, Q. Electrostatic charged nanofiber filter for filtering airbone novel coronavirus (COVID-19) and nano-aerosols. Sep. Purif. Technol. 2020, 250, 116886. [Google Scholar] [CrossRef]
- Bortolassi, A.C.C.; Nagarajan, S.; de Araújo Lima, B.; Guerra, V.G.; Lopes Aguiar, M.; Huon, V.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Efficient nanoparticles removal and bactericidal action of electrospun nanofibers membranes for air filtration. Mater. Sci. Eng. C 2019, 102, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Molnár, K.; Mészáros, L. The role of electrospun nanofibers in the fight against the COVID-19. Express Polym. Lett. 2020, 14, 605. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliucininkas, L.; Prasauskas, T.; Buivydiene, D.; Martuzevicius, D. The comparative study of aerosol filtration by electrospun polyamide, polyvinyl acetate, polyacrylonitrile and cellulose acetate nanofiber media. J. Aerosol Sci. 2016, 92, 27–37. [Google Scholar] [CrossRef]
- Min, K.; Kim, S.; Kim, S. Silk protein nanofibers for highly efficient, eco-friendly, optically translucent, and multifunctional air filters. Sci. Rep. 2018, 8, 9598. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Zhang, H.; Pan, Z. Nanoporous PLA/(chitosan nanoparticle) composite fibrous membranes with excellent air filtration and antibacterial performance. Polymers 2018, 10, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Li, Q.; Young, T.M.; Harper, D.P.; Wang, S. A novel method for fabricating an electrospun poly(vinyl alcohol)/cellulose nanocrystals composite nanofibrous filter with low air resistance for high-efficiency filtration of particulate matter. ACS Sustain. Chem. Eng. 2019, 7, 8706–8714. [Google Scholar] [CrossRef]
- Yun, K.M.; Hogan, C.J., Jr.; Matsubayashi, Y.; Kawabe, M.; Iskandar, F.; Okuyama, K. Nanoparticle filtration by electrospun polymer fibers. Chem. Eng. Sci. 2007, 62, 4751–5759. [Google Scholar] [CrossRef]
- Pardo-Figuerez, M.; Chiva-Flor, A.; Figueroa-Lopez, K.; Prieto, C.; Lagaron, J.M. Antimicrobial nanofiber based filters for high filtration efficiency respirators. Nanomaterials 2021, 11, 900. [Google Scholar] [CrossRef]
- Victor, F.S.; Kugarajah, V.; Bangaru, M.; Ranjan, S.; Dharmalingam, S. Electrospun nanofibers of polyvinylidene fluoride incorporated with titanium nanotubes for purifying air with bacterial contamination. Environ. Sci. Pollut. Res. 2021, 28, 37520–37533. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, S.P.F.; Cruz, F.G.S.; Bretas, R.E.S.; Guerra, V.G.; Aguiar, M.L. A sustainable recycling alternative: Electrospun PET-membranes for air nanofiltration. Polymers 2021, 13, 1166. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, K.S.; Zhang, F.; et al. Electrospun nanofibers membranes for effective air filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Bagheri, M.H.; Khalaji, I.; Azizi, A.; Loibl, R.T.; Basualdo, N.; Manzo, S.; Gorrepati, M.L.; Mehendale, S.; Mohr, C.; Schiffers, S.N. Filtration efficiency, breathability, and reusability of improvides materials for face masks. Aerosol Sci. Technol. 2021, 55, 817–827. [Google Scholar] [CrossRef]
- Ryberg, M.; Laurent, A.; Hauschild, M. Mapping of Global Plastics Value Chain and Plastics Losses to the Environment (with a Particular Focus on Marine Environment); United Nations Environment Programme: Nairobi, Kenya, 2018; Available online: http://wedocs.unep.org/bitstream/handle/20.500.11822/26745/mapping_plastics.pdf (accessed on 27 May 2021).
- McKeen, L.W. Effect of radiation on the properties of polyester polymers. In The Effect of Radiation on Properties of Polymers; Plastic design Library; William Andrew: Norwich, NY, USA, 2020; Chapter 4; pp. 93–128. [Google Scholar] [CrossRef]
- Begum, S.A.; Rane, A.V.; Kanny, K. Application of compatibilized polymer blends in automobile industry. In Compatibilization of Polymer Blends: Micro and Nano Scale Phase Morphologies, Interphase Characterization and Properties; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 20; pp. 563–593. [Google Scholar] [CrossRef]
- Caykara, T.; Sande, M.G.; Azoia, N.; Rodrigues, L.R.; Silva, C.J. Exploring the potential of polyethylene terephthalate in design of antibacterial surfaces. Med. Microbiol. Immunol. 2020, 209, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, B.; Marvafi, Y.; AliAkbari, R.; Kowsari, E.; Ajdari, F.B.; Ramakrishna, S. Recent studies on recycled PET fibers: Production and applications: A review. Mater. Circ. Econ. 2021, 3, 4. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Stoica, A.E.; Dima-Balcescu, M.S.; Chircov, C.; Gharbia, S.; Balta, C.; Rosu, M.; Herman, H.; Holban, A.M.; Ficai, A.; et al. Electrospun polyethylene terephthalate nanofibers loaded with silver nanoparticles: Novel approach in anti-infective therapy. J. Clin. Med. 2019, 8, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.P.; Nguyen, Q.V.; Nhuyen, V.H.; Le, T.H.; Huynh, V.Q.N.; Vo, D.V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk fibroin-based bioamterials for biomedical applications: A review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calamak, S.; Erdogdu, C.; Ozalp, M.; Ulubayram, K. Silk fibroin based antibacterial bionanotextiles as wound dressing materials. Mater. Sci. Eng. C 2014, 43, 11–20. [Google Scholar] [CrossRef]
- Amiraliyan, N.; Nouri, M.; Haghighat Kish, M. Structural characterization and mechanical properties of electrospun silk fibroin nanofiber mats. Polym. Sci. Ser. A 2010, 52, 407–412. [Google Scholar] [CrossRef]
- Zhou, C.J.; Li, Y.; Yao, S.W.; He, J.H. Silkworm-based silk fibers by electrospinning. Res. Phys. 2019, 15, 102646. [Google Scholar] [CrossRef]
- Shanmugam, V.; Babu, K.; Garrison, T.F.; Capezza, A.J.; Olsson, R.T.; Ramakrishna, S.; Hedenqvist, M.S.; Singha, S.; Bartoli, M.; Giorcelli, M.; et al. Potential natural polymer-based nanofibers for the development of facemasks in countering viral outbreaks. J. Appl. Polym. Sci. 2021, 138, 50658. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Kozma, E.; Opálek, A.; Kroneková, Z.; Kleinová, A.; Nagy, Š.; Kronek, J.; Rydz, J.; Eckstein Andicsová, A. Diclofenac embedded in silk fibroin fibers as a drug delivery system. Materials 2020, 13, 3580. [Google Scholar] [CrossRef]
- Bandeira, M.; Borges, V.; Gomes, J.P.; Duarte, A.; Jordao, J. Insight on Klebsiella pneumoniae biofilms assembled on different surfaces using phenotypic and genotypic approaches. Microorganisms 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Al-Attabi, E.; Dumée, L.F.; Kong, L.; Schütz, J.A.; Morsi, Y. High efficiency poly(acrylonitrile) electrospun nanofiber membranes for airborne nanomaterials filtration. Adv. Eng. Mater. 2017, 20, 1700572. [Google Scholar] [CrossRef]
- Hes, L. Non-destructive determinantion of comfort parameters during marketing of functional garments and clothing. Indian J. Fibre Text. Res. 2008, 33, 239–245. [Google Scholar]
- Razzaque, A.; Tesinova, P.; Hes, L.; Salacova, J.; Abid, H.A. Investigation on hydrostatic resistance and thermal performance of layeres waterproof breathable fabrics. Fibers Polym. 2017, 18, 1924–1930. [Google Scholar] [CrossRef]
- ISO 22196. Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Prices for Most Recycled Plastics Continue to Rise. Available online: https://resource-recycling.com/recycling/2021/02/16/prices-for-most-recycled-plastics-continue-to-rise/ (accessed on 23 June 2021).
- Good Quality Carpet Yarn Spun Silk Pure White for Knitting and Weaving. Available online: www.alibaba.com/product-detail/Good-quality-carpet-yarn-spun-silk_60711054364.html?spm=a2700.pc_countrysearch.main07.81.c05239bbQjN4SS (accessed on 23 June 2021).
- Valeirinho, B.; Rei, M.F.; Lopes-Da-Silva, A. Solvent and concentration effects on the properties of electrospun poly(ethylene terephrthalate) nanofiber mats. J. Polym. Sci. B Polym. Phys. 2008, 45, 460–471. [Google Scholar] [CrossRef]
- Guo, Y.; He, W.; Liu, J. Electrospinning polyethylene terephthalate/SiO2 nanofiber composite needle felt for enhanced filtration performance. J. Appl. Polym. Sci. 2019, 137, 48282. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Bramson, M.T.K.; Puhl, D.L.; Johnson, J.; Corr, D.T.; Gilbert, R.J. Solvent retention in electrospun fibers affects scaffold mechanical properties. Electrospinning 2018, 2, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, Y.; Shao, H.; Hu, X. Electrospun ultra-fine silk fibroin fibers from aqueous solutions. J. Mater. Sci. 2005, 40, 5359–5363. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kobashi, T.; Yamanaka, S.; Morikawa, H.; Tamada, Y. Comparisons between silk fibroin nonwoven electrospun fabric using aqueous and formic acid solutions. Int. J. Polym. Mater. Polym. Biomater. 2018, 57, 462–467. [Google Scholar] [CrossRef]
- Chen, J.P.; Chen, S.H.; Lai, G.J. Preparation and characterization of biomimetic silk fibroin/chitosan composite nanofibers by electrospinning for osteoblasts culture. Nanoscale Res. Lett. 2012, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlova, E.; Nikishin, I.; Bogdanova, A.; Klinov, D.; Bagrov, D. The miscibility and spatial distribution of the components in electrospun polymer-protein mats. RSC. Adv. 2020, 10, 4672–4680. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. Role of electrospinning parametrs on poly(lactic-co-glycolic acid) and poly(caprolactone-co-glycolic acid) membranes. Polymers 2021, 13, 695. [Google Scholar] [CrossRef] [PubMed]
- Cheremisinoff, N. Industrial Solvents Handbook, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2008; pp. 51–53. [Google Scholar]
- Angel, N.; Guo, L.; Yan, F.; Wang, H.; Kong, L. Effect of processing parameters on the electrospinning of cellulose acetate studied by response surface methodology. J. Agric. Food Res. 2020, 2, 100015. [Google Scholar] [CrossRef]
- Abbas, J.A.; Said, I.A.; Mohamed, M.A.; Yasin, S.A.; Ali, Z.A.; Ahmed, H. Electrospinning of polyethylene terephthalate (PET) nanofibers: Optimization study using taguchi design of experiment. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 454, p. 012130. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Saito, Y.; Haider, M.K.; Bie, X.; Wada, K.; Kim, I.S. Carboxymethyl cellulose (CMC) based electrospun composite nanofiber mats for food packaging. Polymers 2020, 13, 302. [Google Scholar] [CrossRef]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messinry, M. Efect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly(vinyl chloride) nanofiber mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef] [Green Version]
- Mirtič, J.; Balažic, H.; Zupanič, Š.; Kristl, J. Effect of solution composition variables on tlectrospun alginate nanofibers: Response surface analysis. Polymers 2019, 11, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karahalìloğlu, Z. Cell-compatible PHB/silk fibroin composite nanofiber mat for tissue engineering applications. Turk. J. Biol. 2017, 41, 503–513. [Google Scholar] [CrossRef]
- Keirouz, A.; Zakharova, M.; Kwon, J.; Robert, C.; Koutsos, V.; Callanan, A.; Chen, X.; Fortunato, G.; Radacsi, N. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater. Sci. Eng. C 2020, 112, 110939. [Google Scholar] [CrossRef]
- Chen, F.; Ji, Z.; Qi, Q. Effect of pore size and layers on filtration performance of coalescing filters with different wettabilities. Sep. Purif. Technol. 2018, 201, 71–78. [Google Scholar] [CrossRef]
- Fauzi, A.; Hapidin, D.A.; Munir, M.M.; Iskandar, F.; Khairurrijal, J. A superhydrophilic bilayer structure of a nylon 6 anofiber/cellulose membrane and its characterization as potential water filtration media. RSC Adv. 2020, 10, 17205–17216. [Google Scholar] [CrossRef]
- Dou, H.; Yu, Z.; Zuo, B. Structure and antibacterial activity of silk fibroin/chitosan nanofibrous mats using an electrospinning technique. Adv. Mater. Res. 2011, 332, 967–972. [Google Scholar] [CrossRef]
- Ovalle-Sánchez, A.A.; Elizondo-Martínez, P.; Pérez-Rodrígez, N.A.; Hernández-Fernández, E.; Sánchez-Anguiano, M.G. Degradation of poly(ethyleneterephthalate) waste to obtain oligomers uzing a zinc complex as catalyst. J. Chil. Chem. Soc. 2017, 62, 3741–3745. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos Pereira, A.P.; Prado da Silva, M.H.; Pereira Lima Júnior, É.; dos Santos Paula, A.; Tommasini, F.J. Processing and characterization of PET composites reinforced with geopolymer concrete waste. Mat. Res. 2017, 20, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadehmoghadam, S.; Dong, Y. Fabrication and characterization of electrospun silk fibroin/gelatin scaffolds crosslinked with glutaraldehyde vapor. Front. Mater. 2019, 6, 91. [Google Scholar] [CrossRef]
- Kamalha, E.; Zheng, Y.; Zeng, Y. Analysis of the secondary crystalline structure of regenerated bombyx mori fibroin. Res. Rev. Biosci. 2013, 7, 76–83. [Google Scholar]
- Calhoun, M.A.; Chowdhury, S.S.; Nelson, M.T.; Lannutti, J.L.; Dupaix, R.B.; Winter, J.O. Effect of electrospun fiber mat thickness and support method on cell morphology. Nanomaterials 2019, 9, 644. [Google Scholar] [CrossRef] [Green Version]
- Gobin, A.S.; Froude, V.E.; Mathur, A.B. Structural and mechanical characteristics of silk fibroin and chitosan blend scaffolds for tissue regeneration. J. Biomed. Mater. Res. A 2005, 74A, 465–473. [Google Scholar] [CrossRef]
- Mosnáčková, K.; Opálková Šišková, A.; Kleinová, A.; Danko, M.; Mosnáček, J. Properties and degradation of novel fully biodegradable PLA/PHB blends filled with keratin. Int. J. Mol. Sci. 2020, 21, 9678. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation study of waste polyethylene terephthalate (PET) under inert and oxidative environments. Thermochim. Acta 2019, 679, 178340. [Google Scholar] [CrossRef]
- Motta, A.; Fambri, L.; Migliaresi, C. Regenerated silk fibroin films: Thermal and dynamic mechanical analysis. Macromol. Chem. Phys. 2002, 203, 1658–1665. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Ma, C. Thermal analysis and kinetic study of native silk. J. Therm. Anal. Calorim. 2020, 139, 589–595. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Abbasnezhad, N.; Seydani, M.Z.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An overview of filtration efficiency through the masks: Mechanisms of the aerosol penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef]
- EN1822-1. High Efficiency Air Filters (EPA, HEPA and ULPA)—Part 1: Classification, Performance Testing, Marking. Available online: https://www.en-standard.eu/csn-en-1822-1-high-efficiency-air-filters-epa-hepa-and-ulpa-part-1-classification-performance-testing-marking-3/ (accessed on 10 June 2021).
- EN 149:2001+A1:2009. Respiratory Protective Device—Filtering Half Masks to Protect against Particles—Requirements, Testing, Marking. Available online: https://www.en-standard.eu/bs-en-149-2001-a1-2009-respiratory-protective-devices-filtering-half-masks-to-protect-against-particles-requirements-testing-marking/ (accessed on 10 June 2021).
- Liu, Y.Q.; Feng, J.W.; Zhang, C.C.; Teng, Y.; Liu, Z.; He, J.H. Air permeability of nanofiber membrane with hierarchical structure. Therm. Sci. 2018, 22, 1637–1643. [Google Scholar] [CrossRef]
- Abuzade, R.A.; Zadhoush, A.; Gharehaghaji, A.A. Air permeability of electrospun polyacrylonitrile nanoweb. J Appl. Polym. Sci. 2012, 126, 232–243. [Google Scholar] [CrossRef]
- McPherson, L. Correlation of Electrospun Polyvinylpyrrolidone Fiber Mat Thickness with Basis Weight, Fiber Diameter, Pore Size Distribution, and Air Permeability. Honor Research Project 662. 2018. Available online: http://ideaexchange.uakron.edu/honors_research_projects/662 (accessed on 30 May 2021).
- Wang, R.; Liu, Y.; Li, B.; Hsiao, B.S.; Chu, B. Electrospun nanofibrous membranes for high flux microfiltration. J. Membr. Sci. 2012, 392, 167–174. [Google Scholar] [CrossRef]
- Patel, S.U.; Manzo, G.M.; Patel, S.U.; Kulkarni, P.S.; Chase, G.G. Permeability of electrospun superhydrophobic nanofiber mats. J. Nanotechnol. 2012, 483976. [Google Scholar] [CrossRef] [Green Version]
- Nadiger, V.G.; Shukla, S.R. Antibacterial properties of silk fabric treated with aloe vera and silver nanoparticles. J. Text. Inst. 2017, 108, 385–396. [Google Scholar] [CrossRef]
- Kaur, J.; Rajkhowa, R.; Afrin, T.; Tsuzuki, T.; Wang, X. Facts and myths of antibacterial properties of silk. Biopolymers 2014, 101, 237–245. [Google Scholar] [CrossRef]
- Yi, S.; Wu, Y.; Zhang, Y.; Zou, Y.; Dai, F.; Si, Y. Antibacterial activity of photoactive silk fibroin/cellulose acetate blend nanofibrous membranes against Escherichia coli. ACS Sustain. Chem. Eng. 2020, 8, 16775–16780. [Google Scholar] [CrossRef]
- Ungur, G.; Hrůza, J. Modified nanofoibrous filters with durable antibacterial properties. Molecules 2021, 26, 1255. [Google Scholar] [CrossRef]
- Calamak, S.; Alsoy, E.A.; Ertas, N.; Erdogdu, C.; Sagiroglu, M.; Ulubayram, K. Ag/silk fibroin nanofibers: Effect of fibroin morphology on Ag+ release and antibacterial activity. Eur. Polym. J. 2015, 67, 99–112. [Google Scholar] [CrossRef]
- Khan, A.U.R.; Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. Exploration of the antibacterial and wound healing potential of a PLAG/silk fibroin based electrospun membrane loaded with zinc oxide nanoparticles. J. Mater. Chem. B 2021, 9, 1452–1465. [Google Scholar] [CrossRef]
- Kargar, M.; Wang, J.; Nain, A.S.; Behkam, B. Controlling bacterial adhesion to surface using topographical cues: A study of the interaction of Pseudomonas aeruginosa with nanofiber-textured surfaces. Soft Matter 2012, 8, 10254–10259. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Zhang, C.; Feng, C.; Jiang, L. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef]
- Kaushik, S.; Thungon, P.D.; Goswami, P. Silk fibroin: An emerging biocompatible material for application of enzymes and whole cells in bioelectronics and bioanalytical sciences. ACS Biomater. Sci. Eng. 2020, 6, 4337–4355. [Google Scholar] [CrossRef]
- Xu, S.; Yan, X.; Zhao, Y.; Wang, W.; Yang, Y. In vitro biocompatibility of electrospun silk fibroin mats with Schwann cells. J. Appl. Polym. Sci. 2010, 119, 3490–3494. [Google Scholar] [CrossRef]
- Yonesi, M.; Garcia-Nieto, M.; Guinea, G.V.; Panetsos, F.; Perez-Rigueiro, J.; Gonzalez-Nieto, D. Silk Fibroin: An ancient material for repairing the injured nervous systems. Pharmaceutics 2021, 13, 429. [Google Scholar] [CrossRef]
- Mejia-Sauza, M.L.; Moncada, M.E.; Ossa-Orozco, C.P. Characterization of electrospun silk fibroin scaffolds for bone tissue engineering: A review. TecnoLógicas 2020, 23, 228–246. [Google Scholar]
- Hodgkinson, T.; Yuan, X.F.; Bayat, A. Electrospun silk fibroin fiber diameter influences in vitro dermal fibroblast behavior and promotes healing of ex vivo wound models. J. Tiss. Eng. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, D.; Lin, S.; Han, F.; Wang, K.; Jiang, D.; Wu, J.; Mo, X.; Wang, H. Green electrospun silk fibroin nanofibers loaded with cationic ethosomes for transdermal drug delivery. Chem. Res. Chin. Univ. 2021, 37, 488–495. [Google Scholar] [CrossRef]
- Choudhury, A.J.; Gogoi, D.; Chutia, J.; Kandimalla, R.; Kalita, S.; Kotoky, J.; Chaudhari, Y.B.; Khan, M.R.; Kalita, K. Controlled antibiotic-releasing antheraea assama silk fibroin suture for infection prevention and fast wound healing. Surgery 2016, 159, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Lee, C. Immortalization of primary keratinocytes and its application to skin research. Biomol. Ther. 2015, 23, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc in keratinocytes and langerhans cells: Relevance to the epidermal homeostasis. J. Immunol. Res. 2018, 2018, 5404093. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Salekdeh, S.S.H.; Solouk, A.; Yousefzadeh, M. Electrospun polyethylene terephthalate (PET) nanofibrous conduit for biomedical application. Polym. Adv. Technol. 2019, 31, 284–296. [Google Scholar] [CrossRef]




| Sample | 10% (m/V) of r-PET [mL] | 8% (m/V) of SF [mL] | SF Content in % (V/V) | Total Concentration (%) |
|---|---|---|---|---|
| r-PET | 4 | 0 | 0 | 10 |
| r-PSF1 | 4 | 0.2 | 4.7 | 9.9 |
| r-PSF2 | 4 | 0.4 | 9 | 9.8 |
| r-PSF3 | 4 | 0.8 | 16.6 | 9.6 |
| r-PSF4 | 4 | 1.6 | 28.5 | 9.4 |
| r-PSF5 | 4 | 2.4 | 37.5 | 9.2 |
| r-PSF6 | 4 | 4.8 | 54.5 | 8.9 |
| r-PSF7 | 4 | 9.6 | 70.5 | 8.5 |
| SF | 0 | 4 | 100 | 8 |
| Sample | AD ± SD (nm) | Mean Pore Size ± SD (nm) | Contact Angle (°) |
|---|---|---|---|
| r-PET | 232 ± 118 | 850 ± 331 | 95 ± 3 |
| r-PSF1 | 168 ± 83 | 654 ± 344 | 94 ± 2 |
| r-PSF2 | 134 ± 63 | 352 ± 124 | 92 ± 3 |
| r-PSF3 | 127 ± 50 | 348 ± 121 | 86 ± 1 |
| r-PSF4 | 139 ± 76 | 398 ± 104 | 61 ± 4 |
| r-PSF5 | 146 ± 103 | 427 ± 140 | 51 ± 9 |
| r-PSF6 | 149 ± 89 | 480 ± 188 | 43 ± 4 |
| r-PSF7 | 253 ± 125 | 975 ± 345 | 31 ± 5 |
| SF | 202 ± 128 | 828 ± 380 | 10 ± 1 |
| Sample | Mechanical Analysis | |
|---|---|---|
| σTS ± S σ [MPa] | εB ± S ε [%] | |
| r-PET | 1.62 ± 0.11 | 64.90 ± 14.8 |
| r-PSF1 | 1.28 ± 0.19 | 67.30 ± 16.2 |
| r-PSF2 | 1.32 ± 0.21 | 59.50 ± 6.82 |
| r-PSF3 | 1.49 ± 0.13 | 40.50 ± 8.10 |
| r-PSF4 | 1.85 ± 0.26 | 31.00 ± 6.68 |
| r-PSF5 | 2.75 ± 0.29 | 36.80 ± 8.10 |
| r-PSF6 | 3.45 ± 0.50 | 33.50 ± 4.30 |
| r-PSF7 | 3.10 ± 0.37 | 25.80 ± 4.60 |
| SF | 1.35 ± 0.34 | 1.89 ± 0.60 |
| Sample | Basis Weight (g·m−2) | Thickness (mm) | * EMPPS (%) | ΔP (Pa) | Qf (Pa−1) | Filter Class According to EN149 | Filter Class According to EN1822 |
|---|---|---|---|---|---|---|---|
| r-PET | 12.01±0.01 | 0.11 ± 0.002 | 99.97 ± 0.2 | 414 ± 21 | 0.019 ± 0.001 | High ΔP | H13 |
| r-PSF3 | 11.15 ± 0.01 | 0.11 ± 0.002 | 99.85 ± 0.4 | 272 ± 14 | 0.024 ± 0.001 | High ΔP | E12 |
| r-PSF6 | 10.25 ± 0.01 | 0.10 ± 0.001 | 98.99 ± 1.9 | 315 ± 15 | 0.015 ± 0.001 | High ΔP | E11 |
| SF | 11.00 ± 0.02 | 0.11 ± 0.002 | 43.34 ± 6.4 | 47 ± 8 | 0.012 ± 0.002 | No classification | No classification |
| Filtration Class According to the EN149 | Minimal Efficiency of MPPA (%) | Recommended Pressure Drop (Pa) (at 30 L·min−1) | Protection |
| FFP1 | ≥80 | 60 | Solid inert particles, aerosols without particular toxicity, e.g., calcium carbonate, plaster, brick dust, pollen, and fur. |
| FFP 2 | ≥94 | 70 | Biological and carcinogenic compounds, harmful solid particles, toxic or irritating aqueous aerosols, e.g., silica, sodium carbonate, iron, wood and glass dust, water-soluble pesticides, grain, mold, fungi, exhaust gasses. |
| FFP3 | ≥99 | 100 | Biological compounds, toxic solids, aqueous aerosols, e.g., TBC bacteria, asbestos, radioactive beryllium particles. |
| Filtration Class According to the EN1822 | Minimal Efficiency of MPPA (%) | Recommended Final Pressure Drop (Pa) | Protection |
| E10 | ≥85 | 250–1000 * | germs, bacteria, metallic-oxide smoke |
| E11 | ≥95 | ||
| E12 | ≥99.5 | viruses, tobacco smoke, soot | |
| H13 | ≥99.95 | oil fumes, radioactive suspended particulates | |
| H14 | ≥99.995 | aerosols |
| Sample | Basis Weight (g·m−2) | Thickness (mm) (μm) | *EMPPS (%) | ΔP (Pa) | Qf (Pa−1) | Filter Class According to EN149 | Filter Class According to EN1822 |
|---|---|---|---|---|---|---|---|
| r-PET | 3.23 ± 0.02 | 0.08 ± 0.003 | 95.98 ± 0.2 | 123 ± 4.4 | 0.026 ± 0.001 | High ΔP | E11 |
| r-PSF3 | 2.08 ± 0.02 | 0.07 ± 0.001 | 93.38 ± 0.9 | 92 ± 2.6 | 0.030 ± 0.001 | High ΔP | E10 |
| r-PSF6 | 2.65 ± 0.01 | 0.08 ± 0.001 | 90.23 ± 2.7 | 59 ± 2.2 | 0.039 ± 0.001 | FFP1 | E10 |
| SF | 1.75 ± 0.03 | 0.07 ± 0.001 | 39.01 ± 6.4 | 27 ± 9.6 | 0.018 ± 0.002 | No classification | No classification |
| Sample | Basis Weight | 1.75–3.23 (g·m−2) | 10–12 (g·m−2) | ||
|---|---|---|---|---|---|
| B (L·mm·s−1) | WVP (%) | B (L·mm·s−1) | WVP (%) | ||
| r-PET | 53.4 ± 2.7 | 89.2 ± 1.5 | 39.8 ± 2.4 | 94.7 ± 1.0 | |
| r-PSF3 | 117.0 ± 4.2 | 86.5 ± 1.7 | 54.6 ± 3.9 | 89.2 ± 1.4 | |
| r-PSF6 | 149.9 ± 5.7 | 88.9 ± 1.7 | 85.0 ± 7.0 | 89.9 ± 1.3 | |
| SF | 201.3 ± 8.3 | 89.3 ± 2.0 | 150.0 ± 9.1 | 89.5 ± 1.9 | |
| Tested Microorganism | Sample | The Number of Bacteria Recovered at 24 h Contact Time (CFU·cm−2) | Log of the Number of Bacteria Recovered at 24 h Contact Time (CFU·cm−2) | Antimicrobial Activity (R) | Reduction (%) |
|---|---|---|---|---|---|
| S. aureus CCM 3953 | r-PET | 390,000 ± 18,000 | 5.59 ± 0.26 | - | - |
| r-PSF6 | 21,000 ± 1200 | 4.26 ± 0.24 | 1.3 ± 0.02 | 94.62 ± 6.6 | |
| SF | 350,000 ± 16,400 | 5.54 ± 0.26 | - | - | |
| E. coli CCM 3988 | r-PET | 550,000 ± 25,000 | 5.74 ± 0.26 | - | - |
| r-PSF6 | 30,000 ± 1600 | 4.48 ± 0.24 | 1.3 ± 0.02 | 94.55 ± 6.4 | |
| SF | 480,000 ± 21,300 | 5.68 ± 0.25 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opálková Šišková, A.; Mosnáčková, K.; Hrůza, J.; Frajová, J.; Opálek, A.; Bučková, M.; Kozics, K.; Peer, P.; Eckstein Andicsová, A. Electrospun Poly(ethylene Terephthalate)/Silk Fibroin Composite for Filtration Application. Polymers 2021, 13, 2499. https://doi.org/10.3390/polym13152499
Opálková Šišková A, Mosnáčková K, Hrůza J, Frajová J, Opálek A, Bučková M, Kozics K, Peer P, Eckstein Andicsová A. Electrospun Poly(ethylene Terephthalate)/Silk Fibroin Composite for Filtration Application. Polymers. 2021; 13(15):2499. https://doi.org/10.3390/polym13152499
Chicago/Turabian StyleOpálková Šišková, Alena, Katarína Mosnáčková, Jakub Hrůza, Jaroslava Frajová, Andrej Opálek, Mária Bučková, Katarína Kozics, Petra Peer, and Anita Eckstein Andicsová. 2021. "Electrospun Poly(ethylene Terephthalate)/Silk Fibroin Composite for Filtration Application" Polymers 13, no. 15: 2499. https://doi.org/10.3390/polym13152499
APA StyleOpálková Šišková, A., Mosnáčková, K., Hrůza, J., Frajová, J., Opálek, A., Bučková, M., Kozics, K., Peer, P., & Eckstein Andicsová, A. (2021). Electrospun Poly(ethylene Terephthalate)/Silk Fibroin Composite for Filtration Application. Polymers, 13(15), 2499. https://doi.org/10.3390/polym13152499