Combining Sandblasting, Alkaline Etching, and Collagen Immobilization to Promote Cell Growth on Biomedical Titanium Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Characteristics
2.3. Cell Adhesion
2.4. Cell Proliferation
2.5. Cell Differentiation and Mineralization
2.6. Statistical Analysis
3. Results
3.1. Surface Characteristics
3.2. Cell Adhesion
3.3. Cell Proliferation
3.4. Cell Differentiation and Mineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabor, R.; Doubkova, M.; Gorosova, S.; Malanik, K.; Vandrovcova, M.; Cvrcek, L.; Drobikova, K.; Mamulova Kutlakova, K.; Bacakova, L. Preparation of highly wettable coatings on Ti-6Al-4V ELI alloy for traumatological implants using micro-arc oxidation in an alkaline electrolyte. Sci. Rep. 2020, 10, 19780. [Google Scholar] [CrossRef]
- Khodaei, M.; Amini, K.; Valanezhad, A.; Watanabe, I. Surface treatment of titanium dental implant with H2O2 solution. Int. J. Miner. Metall. Mater. 2020, 27, 1281–1286. [Google Scholar] [CrossRef]
- Konovalov, S.; Osintsev, K.; Golubeva, A.; Smelov, V.; Ivanov, Y.; Chen, X.; Komissarova, I. Surface modification of Ti-based alloy by selective laser melting of Ni-based superalloy powder. J. Mater. Res. Technol. 2020, 9, 8796–8807. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Grobe, A.; Heiland, M.; Ebker, T. Impact of dental Implant surface modifications on osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanovska, J.; Matejka, R.; Rosina, J.; Bacakova, L.; Kolarova, H. Treatments for enhancing the biocompatibility of titanium implants. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2020, 164, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Yeo, I.L. Modifications of dental implant surfaces at the micro- and nano-level for enhanced osseointegration. Materials 2020, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.; Pei Ting, O.; See Yee, T.; Pushparajan, S.; Swaminathan, D.; Kutty, M.G. Biomimetic coating of modified titanium surfaces with hydroxyapatite using simulated body fluid. Adv. Mater. Sci. Eng. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Balza, J.C.; Zujur, D.; Gil, L.; Subero, R.; Dominguez, E.; Delvasto, P.; Alvarez, J. Sandblasting as a surface modification technique on titanium alloys for biomedical applications: Abrasive particle behavior. IOP. Conf. Ser. Mater. Sci. Eng. 2013, 45, 12004. [Google Scholar] [CrossRef]
- Cornelius Timothius, C.J.; Lung, C.Y.K.; Seneviratne, C.J.; Tsoi, J.K.H.; Matinlinna, J.P. Resin and osteoblastic adhesion on zirconia and titanium implant materials blasted with various grits. PeerJ Prepr. 2015, 3, e1189v1182. [Google Scholar]
- Gehrke, S.A.; Taschieri, S.; Del Fabbro, M.; Coelho, P.G. Positive biomechanical effects of titanium oxide for sandblasting implant surface as an alternative to aluminium oxide. J. Oral Implantol. 2015, 41, 515–522. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.; Han, Y.; Xu, K. Effects of a modified sandblasting surface treatment on topographic and chemical properties of titanium surface. Implant. Dent. 2001, 10, 59–64. [Google Scholar] [CrossRef]
- Park, J.B.; Kim, Y.S.; Lee, G.; Yun, B.G.; Kim, C.H. The effect of surface treatment of titanium with sand-blasting/acid-etching or hydroxyapatite-coating and application of bone morphogenetic protein-2 on attachment, proliferation, and differentiation of stem cells derived from buccal fat pad. J. Tissue Eng. Regen. Med. 2013, 10, 115–121. [Google Scholar] [CrossRef]
- Schupbach, P.; Glauser, R.; Bauer, S. Al2O3 particles on titanium dental implant systems following sandblasting and acid-etching process. Int. J. Biomater. 2019, 2019, 6318429. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Yin, X.; Xie, L.; Liu, Z.; Li, J.; Zou, S.; Chen, J. Enhancing osseointegration of titanium implants through large-grit sandblasting combined with micro-arc oxidation surface modification. J. Mater. Sci. Mater. Med. 2019, 30, 73. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; McLachlan, T.; Cai, Y.; Hyzy, S.L.; Schneider, J.M.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium-aluminum-vanadium alloy surfaces. Biomaterials 2012, 33, 8986–8994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.S.; Chang, J.H.; Huang, H.H. Enhancing the biological response of titanium surface through the immobilization of bone morphogenetic protein-2 using the natural cross-linker genipin. Surf. Coat. Technol. 2016, 303, 289–297. [Google Scholar] [CrossRef]
- Kim, S.E.; Song, S.H.; Yun, Y.P.; Choi, B.J.; Kwon, I.K.; Bae, M.S.; Moon, H.J.; Kwon, Y.D. The effect of immobilization of heparin and bone morphogenic protein-2 (BMP-2) to titanium surfaces on inflammation and osteoblast function. Biomaterials 2011, 32, 366–373. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.G.; Ferraz, E.P.; Abuna, R.P.F.; de Oliveira, P.T.; Morra, M.; Beloti, M.M.; Rosa, A.L. The effect of collagen coating on titanium with nanotopography on in vitro osteogenesis. J. Biomed. Mater. Res. A 2017, 105, 2783–2788. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kim, D.M.; Lee, C.; da Silva, J.; Nagai, S.; Nojiri, T.; Nagai, M. Biological efficacy of perpendicular type-I collagen protruded from TiO2-nanotubes. Int. J. Oral. Sci. 2020, 12, 36. [Google Scholar] [CrossRef]
- Ao, H.Y.; Xie, Y.T.; Yang, S.B.; Wu, X.D.; Li, K.; Zheng, X.B.; Tang, T.T. Covalently immobilised type I collagen facilitates osteoconduction and osseointegration of titanium coated implants. J. Orthop. Translat. 2016, 5, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Brum, I.S.; Elias, C.N.; de Carvalho, J.J.; Pires, J.L.S.; Pereira, M.J.S.; de Biasi, R.S. Properties of a bovine collagen type I membrane for guided bone regeneration applications. e-Polymers 2021, 21, 210–221. [Google Scholar] [CrossRef]
- Chen, W.C.; Chen, Y.S.; Chang, K.C.; Chen, C.H.; Lin, D.J. An in vitro assessment and comparative effectiveness of silanized-glutaraldehyde functionalized titanium surfaces with phosphatidylcholine and type I collagen grafts. Dent. Mater. 2020, 36, 320–328. [Google Scholar] [CrossRef]
- Yu, X.; Walsh, J.; Wei, M. Covalent immobilization of collagen on titanium through polydopamine coating to improve cellular performances of MC3T3-E1 cells. RSC Adv. 2013, 4, 7185–7192. [Google Scholar] [CrossRef] [Green Version]
- Tambe, N.; Di, J.; Zhang, Z.; Bernacki, S.; El-Shafei, A.; King, M.W. Novel genipin-collagen immobilization of polylactic acid (PLA) fibers for use as tissue engineering scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Best, S.M.; Cameron, R.E. Crosslinking Collagen Constructs: Achieving Cellular Selectivity through Modifications of Physical and Chemical Properties. Appl. Sci. 2020, 10, 6911. [Google Scholar] [CrossRef]
- Makita, R.; Akasaka, T.; Tamagawa, S.; Yoshida, Y.; Miyata, S.; Miyaji, H.; Sugaya, T. Preparation of micro/nanopatterned gelatins crosslinked with genipin for biocompatible dental implants. Beilstein J. Nanotechnol. 2018, 9, 1735–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, P.; Pedraz, J.L.; Orive, G. Biologically active and biomimetic dual gelatin scaffolds for tissue engineering. Int. J. Biol. Macromol. 2017, 98, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Yang, W.E.; Huang, H.H. Multiform TiO2 nano-network enhances biological response to titanium surface for dental implant applications. Appl. Surf. Sci. 2019, 471, 1041–1052. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Peng, M.; Gama, M.; Yang, Z.; Deng, X.; Zhou, J.; Ouyang, C.; Luo, H. Controllable synthesis of biomimetic nano/submicro-fibrous tubes for potential small-diameter vascular grafts. J. Mater. Chem. B 2020, 8, 5694–5706. [Google Scholar] [CrossRef]
- Liu, C.F.; Li, S.J.; Hou, W.T.; Hao, Y.L.; Huang, H.H. Enhancing corrosion resistance and biocompatibility of interconnected porous β-type Ti-24Nb-4Zr-8Sn alloy scaffold through alkaline treatment and type I collagen immobilization. Appl. Surf. Sci. 2019, 476, 325–334. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, C.; Bai, B. Molecular dynamics study on the effect of surface hydroxyl groups on three-phase wettability in oil-water-graphite systems. Polymers 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.P.; Hackenhaar, C.R.; Lorenzoni, A.S.G.; Rodrigues, R.C.; Costa, T.M.H.; Ninow, J.L.; Hertz, P.F. Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and beta-D-galactosidase immobilization. Carbohydr. Polym. 2016, 137, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Kahoush, M.; Behary, N.; Guan, J.; Cayla, A.; Mutel, B.; Nierstrasz, V. Genipin-mediated immobilization of glucose oxidase enzyme on carbon felt for use as heterogeneous catalyst in sustainable wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 105633. [Google Scholar] [CrossRef]
- Ao, H.; Xie, Y.; Tan, H.; Wu, X.; Liu, G.; Qin, A.; Zheng, X.; Tang, T. Improved hMSC functions on titanium coatings by type I collagen immobilization. J. Biomed. Mater. Res. A 2014, 102, 204–214. [Google Scholar] [CrossRef]
- Blatt, S.; Pabst, A.M.; Schiegnitz, E.; Hosang, M.; Ziebart, T.; Walter, C.; Al-Nawas, B.; Klein, M.O. Early cell response of osteogenic cells on differently modified implant surfaces: Sequences of cell proliferation, adherence and differentiation. J. Craniomaxillofac. Surg. 2018, 46, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.E.; Huang, H.H. TiO2 Nanonetwork on rough Ti enhanced osteogenesis in vitro and in vivo. J. Dent. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tugulu, S.; Löwe, K.; Scharnweber, D.; Schlottig, F. Preparation of superhydrophilic microrough titanium implant surfaces by alkali treatment. J. Mater. Sci. Mater. Med. 2010, 21, 2751–2763. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Valderrama, P.; Jones, A.A.; Wilson, T.G.; Seibl, R.; Cochran, D.L. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in canine mandibles. Clin. Oral Implant. Res. 2008, 19, 233–241. [Google Scholar] [CrossRef]
- Rausch-fan, X.; Qu, Z.; Wieland, M.; Matejka, M.; Schedle, A. Differentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells (MG63) in response to titanium surfaces. Dent. Mater. 2008, 24, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; von der Mark, K.; Schmuki, P. Improved attachment of mesenchymal stem cells on super-hydrophobic TiO2 nanotubes. Acta Biomater. 2008, 4, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, K.; Miura, T.; Kou, I.; Muramatsu, T.; Furusawa, M.; Yoshinari, M. Influence of various superhydrophilic treatments of titanium on the initial attachment, proliferation, and differentiation of osteoblast-like cells. Dent. Mater. J. 2015, 34, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.S.; Liu, J.F.; Wu, C.P.; Huang, H.H. Nanoporous surface topography enhances bone cell differentiation on Ti-6Al-7Nb alloy in bone implant applications. J. Alloys Compd. 2015, 643, S124–S132. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Hsu, C.M.; Sun, Y.S.; Huang, H.H. Enhanced cell response to zirconia surface immobilized with type I collagen. J. Dent. Res. 2019, 98, 556–563. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, L.; Wu, Z.; Zhang, Y.; Chu, P.K. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials 2012, 33, 2629–2641. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-Ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarano, A.; Lorusso, F.; Staiti, G.; Sinjari, B.; Tampieri, A.; Mortellaro, C. Sinus Augmentation with Biomimetic Nanostructured Matrix: Tomographic, Radiological, Histological and Histomorphometrical Results after 6 Months in Humans. Front. Physiol. 2017, 8, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.S.; Lin, Y.A.; Huang, H.H. Using genipin to immobilize bone morphogenetic protein-2 on zirconia surface for enhancing cell adhesion and mineralization in dental implant applications. Polymers 2021, 12, 2639. [Google Scholar] [CrossRef] [PubMed]
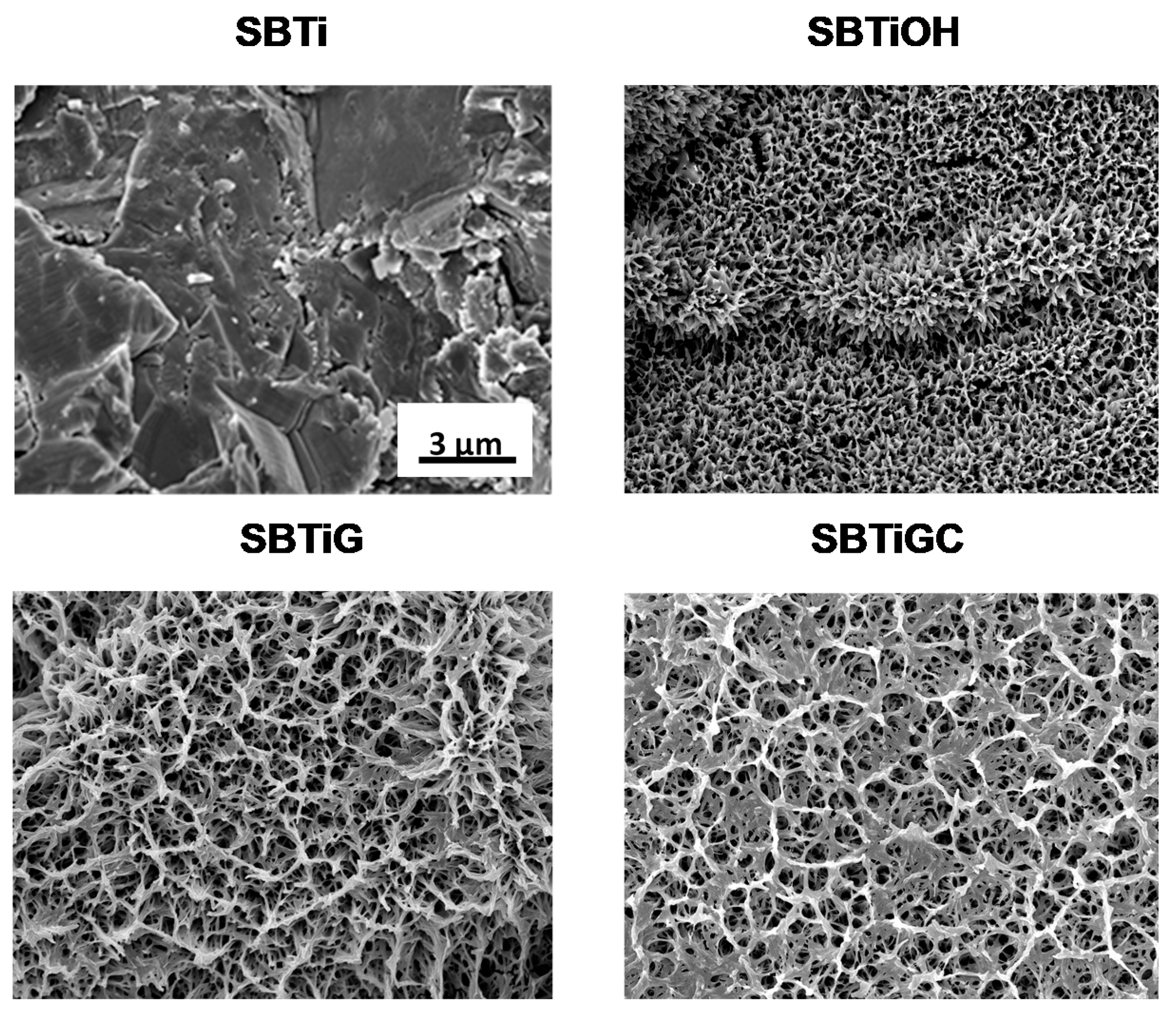
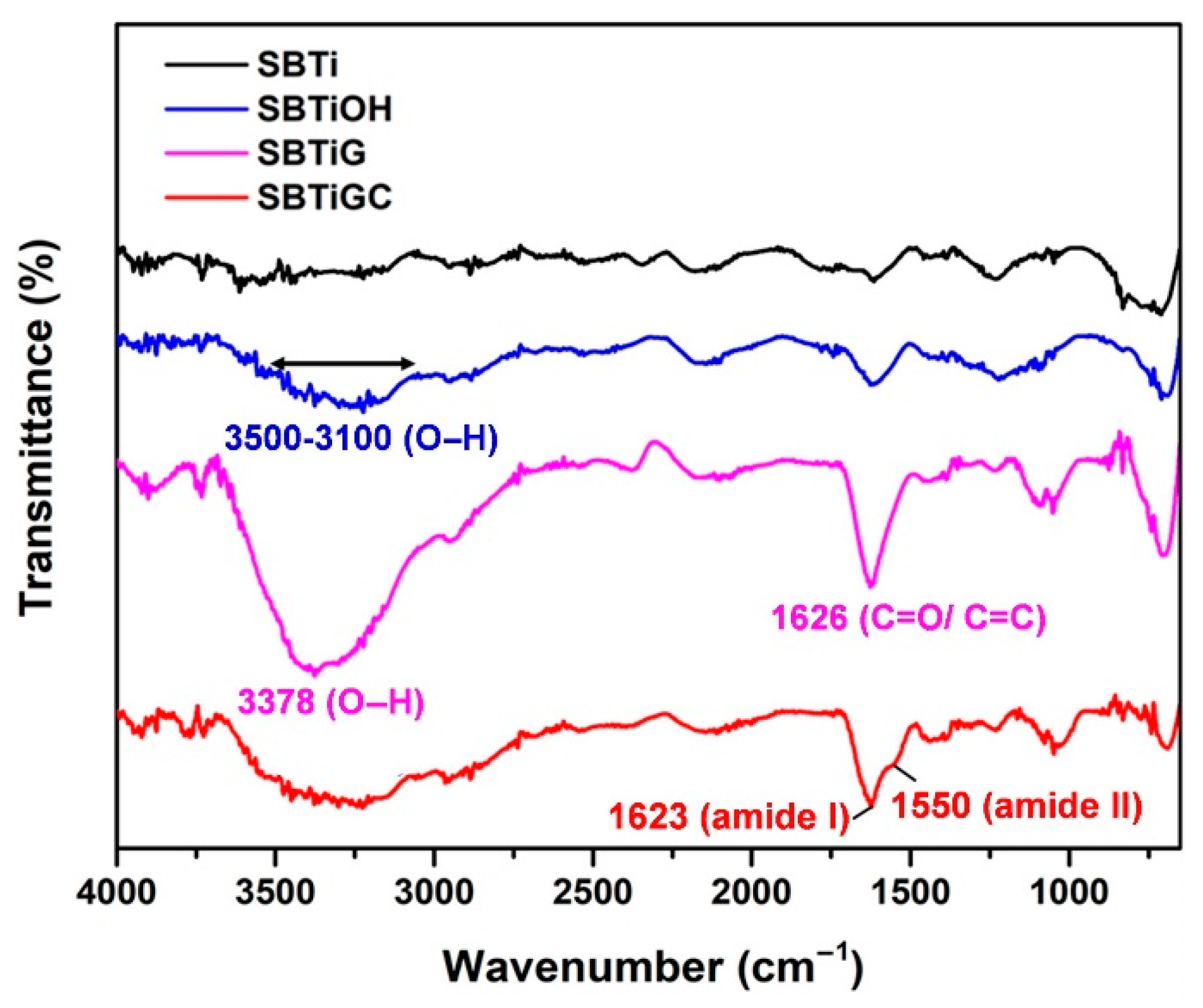
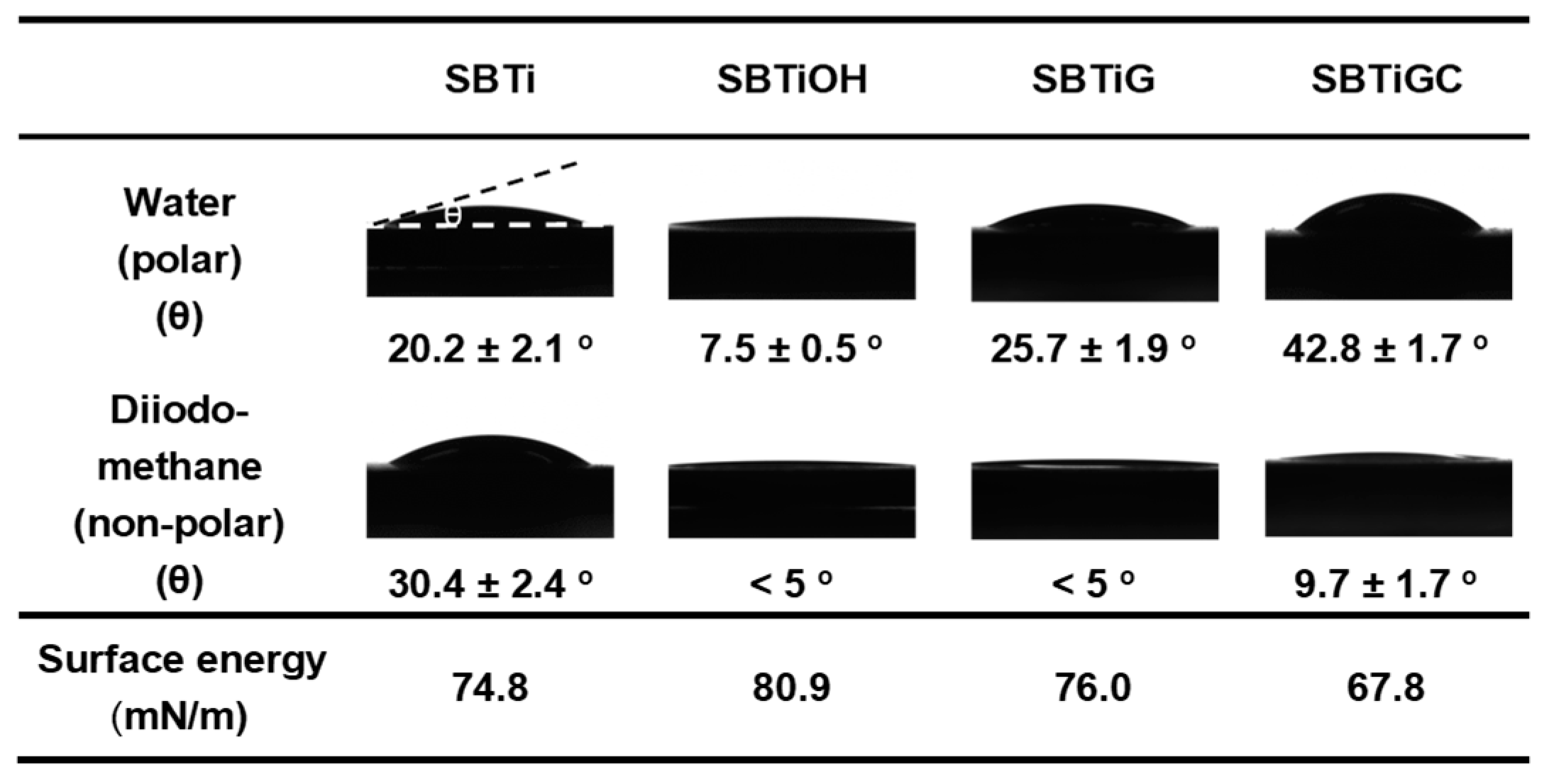
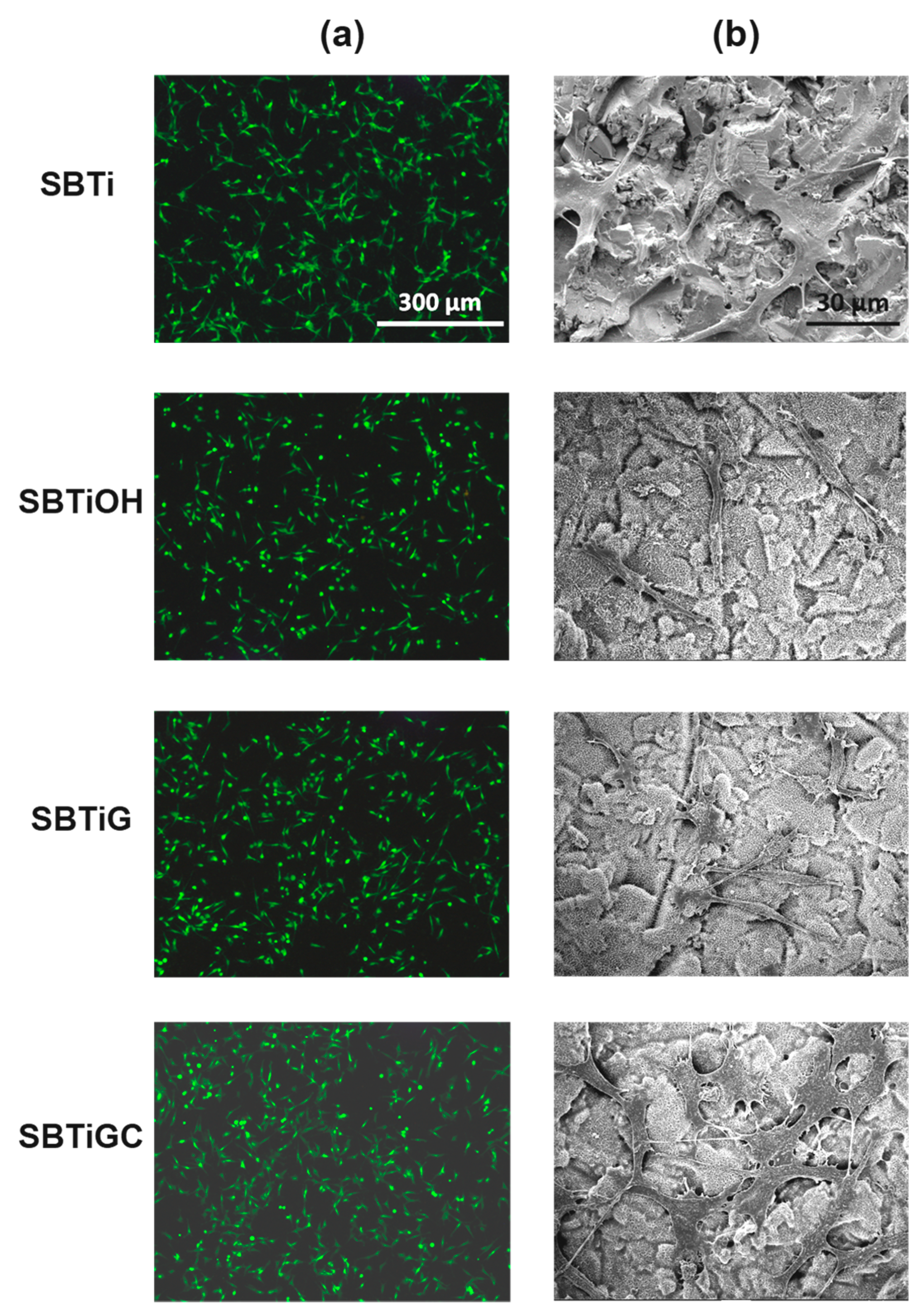


| Sa | Sq | |
|---|---|---|
| SBTi | 1.10 A ± 0.05 | 1.27 A ± 0.06 |
| SBTiOH | 0.91 B ± 0.02 | 1.13 A ± 0.02 |
| SBTiG | 0.93 B ± 0.04 | 1.21 A ± 0.05 |
| SBTiGC | 0.87 B ± 0.09 | 1.14 A ± 0.08 |
| SBTi | SBTiOH | SBTiG | SBTiGC | |
|---|---|---|---|---|
| 7 d | 70.89 a ± 13.10 | 25.43 b ± 14.81 | 14.97 b ± 5.23 | 99.37 a ± 12.85 |
| 14 d | 123.80 a ± 22.73 | 39.82 b ± 13.31 | 32.23 b ± 9.96 | 241.31 c ± 41.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-F.; Chang, K.-C.; Sun, Y.-S.; Nguyen, D.T.; Huang, H.-H. Combining Sandblasting, Alkaline Etching, and Collagen Immobilization to Promote Cell Growth on Biomedical Titanium Implants. Polymers 2021, 13, 2550. https://doi.org/10.3390/polym13152550
Liu C-F, Chang K-C, Sun Y-S, Nguyen DT, Huang H-H. Combining Sandblasting, Alkaline Etching, and Collagen Immobilization to Promote Cell Growth on Biomedical Titanium Implants. Polymers. 2021; 13(15):2550. https://doi.org/10.3390/polym13152550
Chicago/Turabian StyleLiu, Chia-Fei, Kai-Chun Chang, Ying-Sui Sun, Diem Thuy Nguyen, and Her-Hsiung Huang. 2021. "Combining Sandblasting, Alkaline Etching, and Collagen Immobilization to Promote Cell Growth on Biomedical Titanium Implants" Polymers 13, no. 15: 2550. https://doi.org/10.3390/polym13152550
APA StyleLiu, C.-F., Chang, K.-C., Sun, Y.-S., Nguyen, D. T., & Huang, H.-H. (2021). Combining Sandblasting, Alkaline Etching, and Collagen Immobilization to Promote Cell Growth on Biomedical Titanium Implants. Polymers, 13(15), 2550. https://doi.org/10.3390/polym13152550







