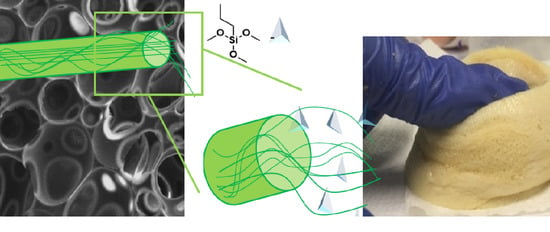
Chemically Functionalized Cellulose Nanocrystals as Reactive Filler in Bio-Based Polyurethane Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modified CNC Preparations
2.2.1. Dried Lyophilized CNC Preparation
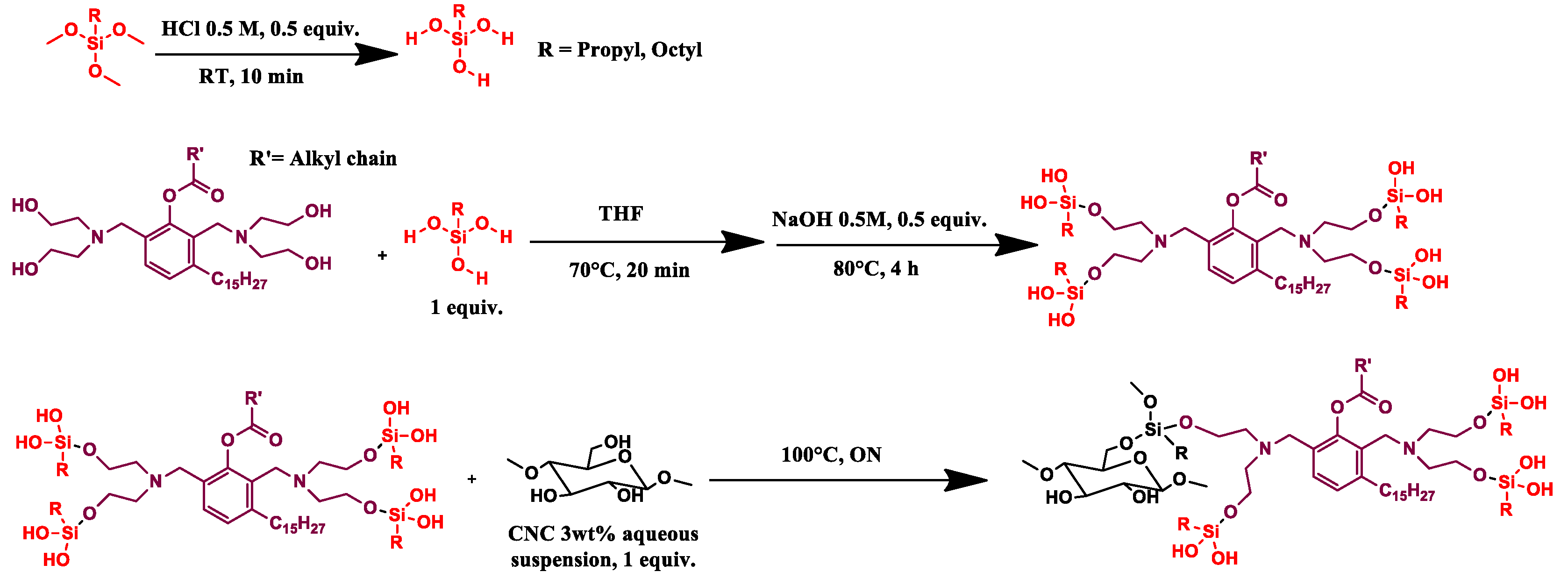
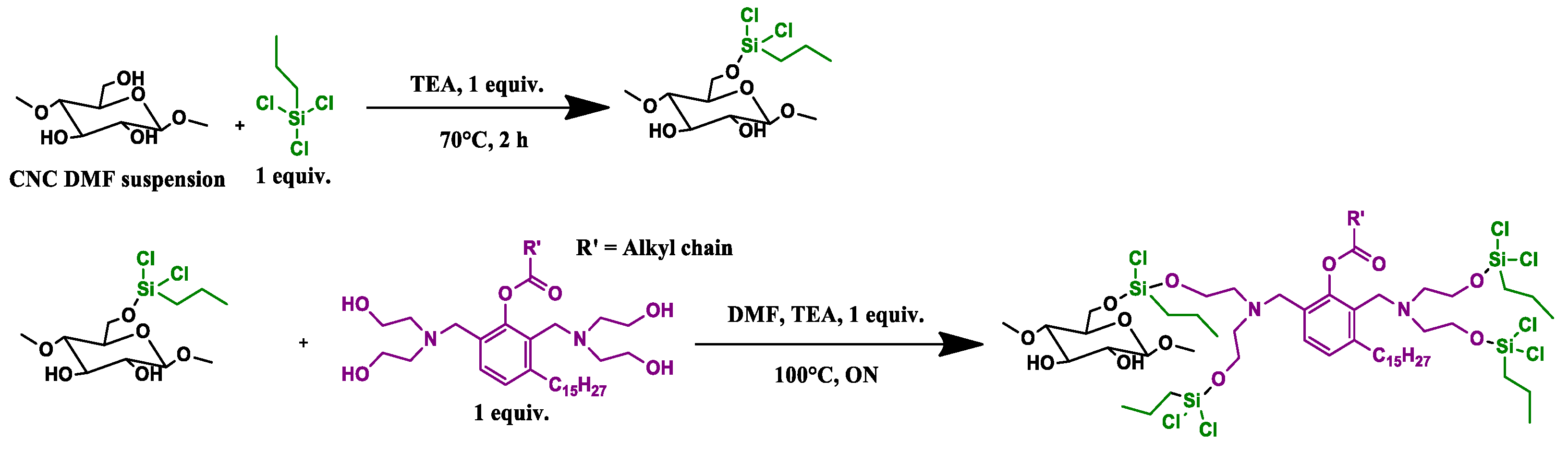
2.2.2. CNC_grafted_TMPS_polyol (CNC1) and CNC_grafted_TMOS_polyol (CNC2) Preparations
2.2.3. CNC_grafted_TCPS_polyol (CNC3) Preparations
2.2.4. CNC_grafted_APTES_polyol (CNC4) Preparations
2.2.5. CNC Purification
2.3. PU Foams Preparation
2.4. Characterization of Modified CNCs and PU Foams
2.4.1. Modified CNCs Characterization
2.4.2. PU Foams Characterization
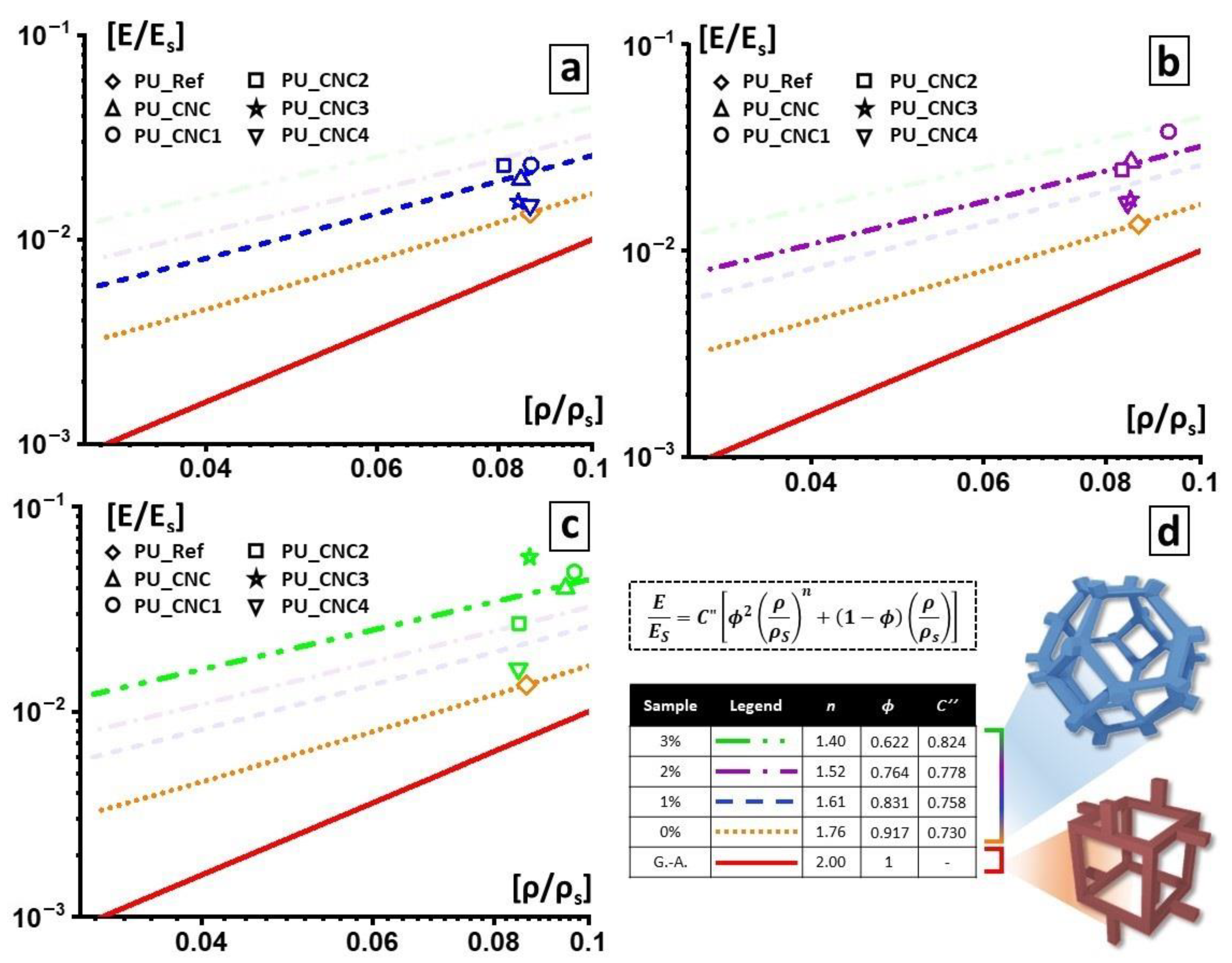
2.4.3. Modified Gibson–Ashby Model
3. Results and Discussion
3.1. Characterization of CNCs-Grafted-Silanes-Polyol
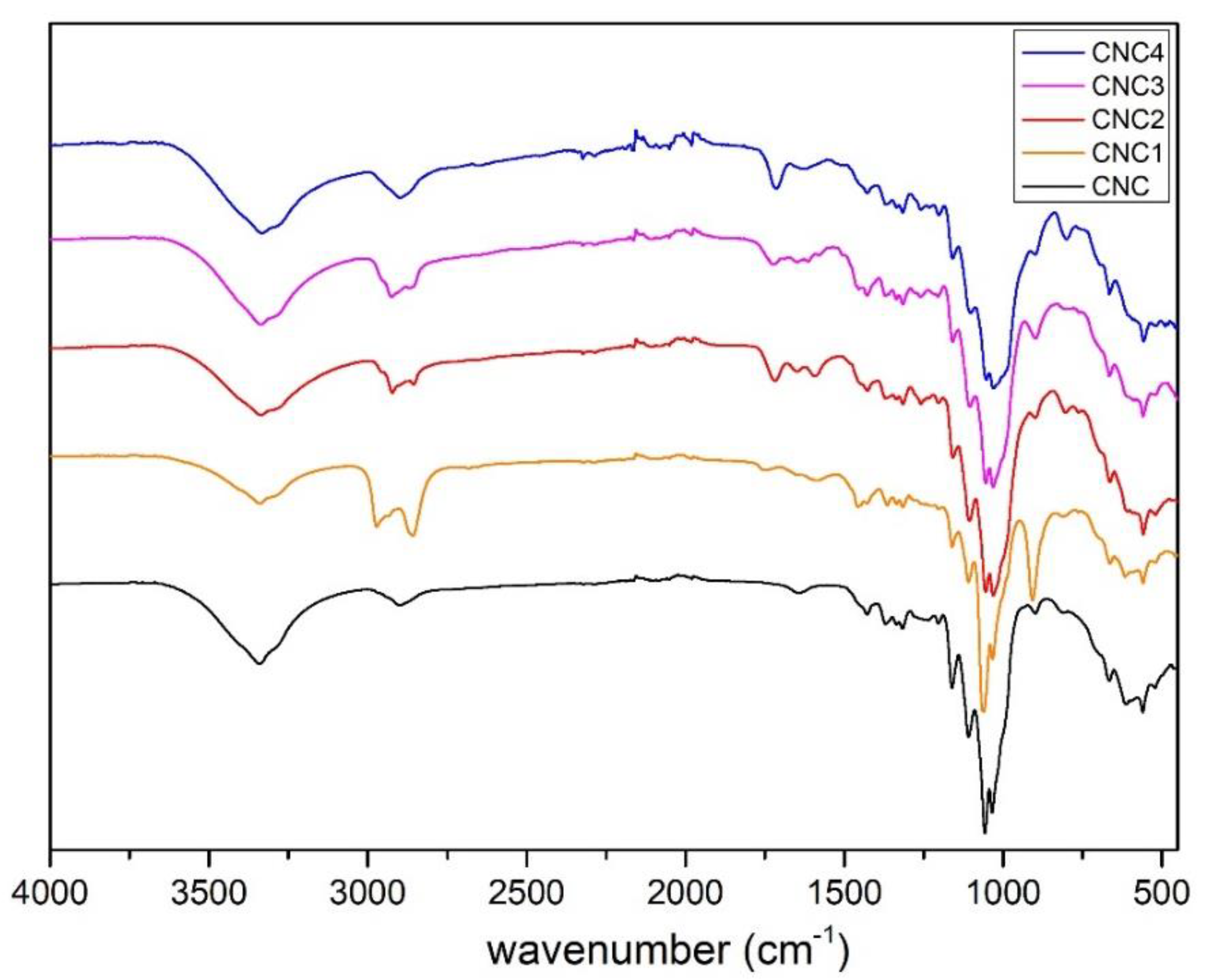
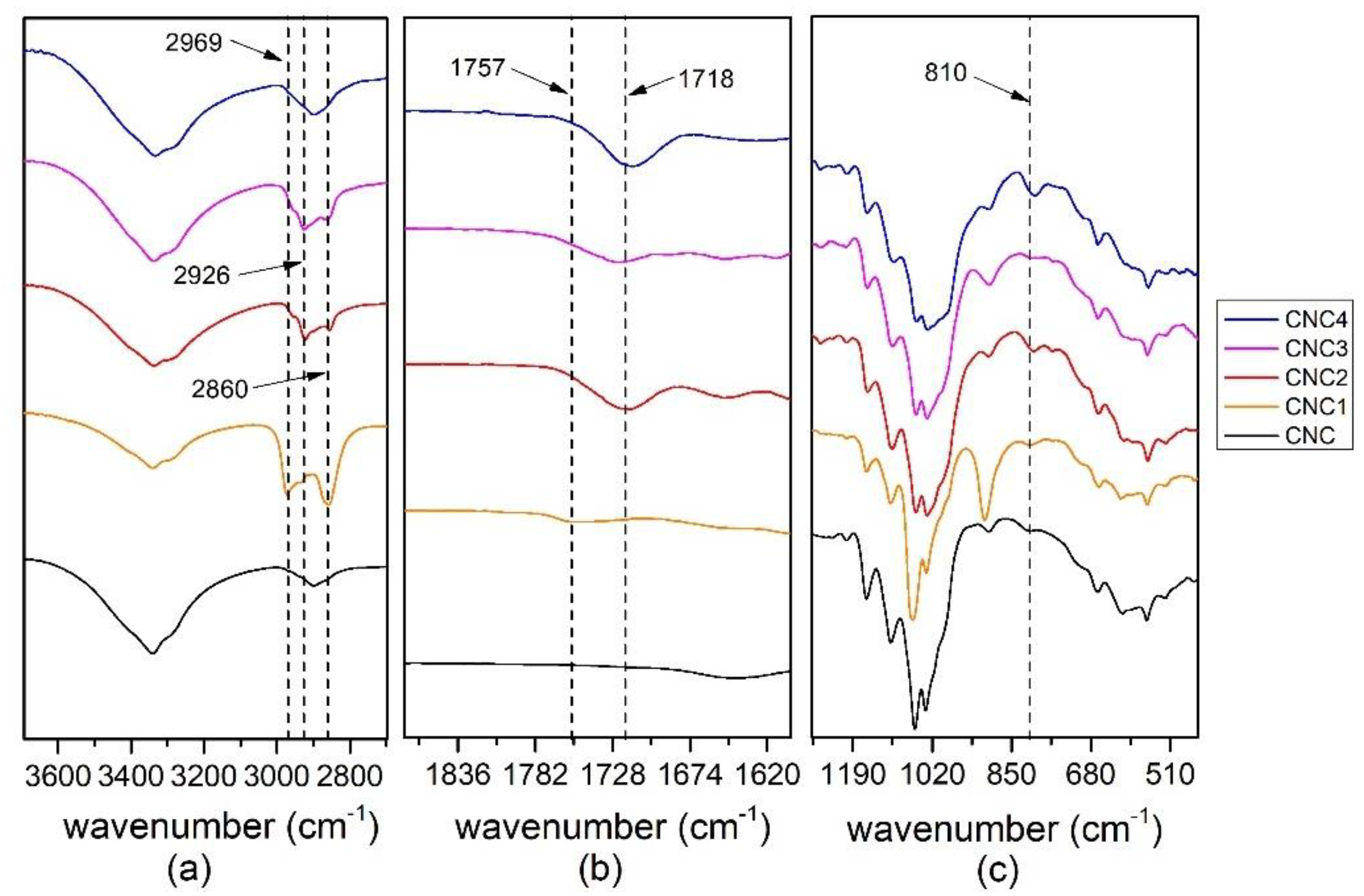
3.1.1. FTIR Characterization
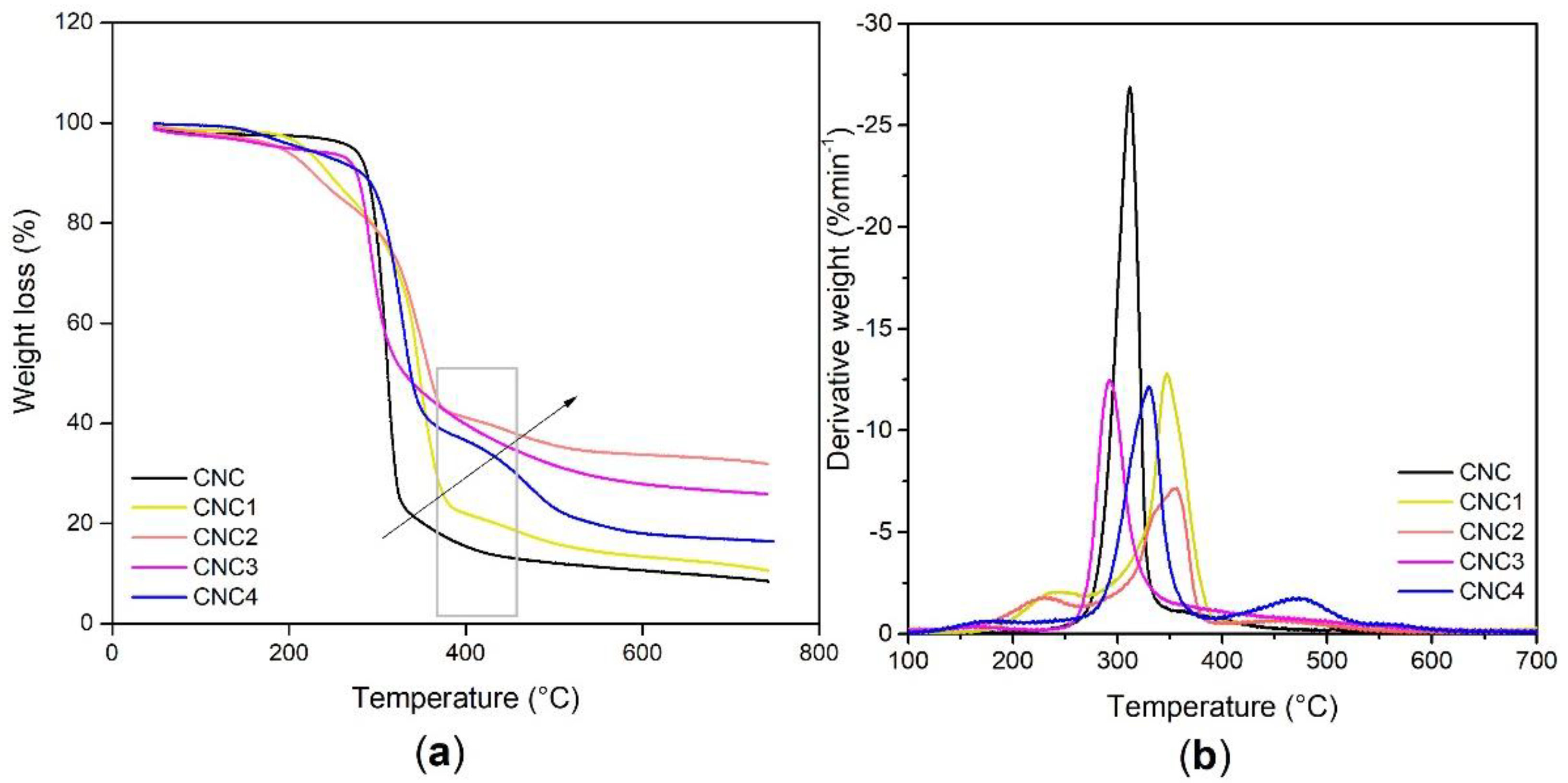
3.1.2. TGA Analysis
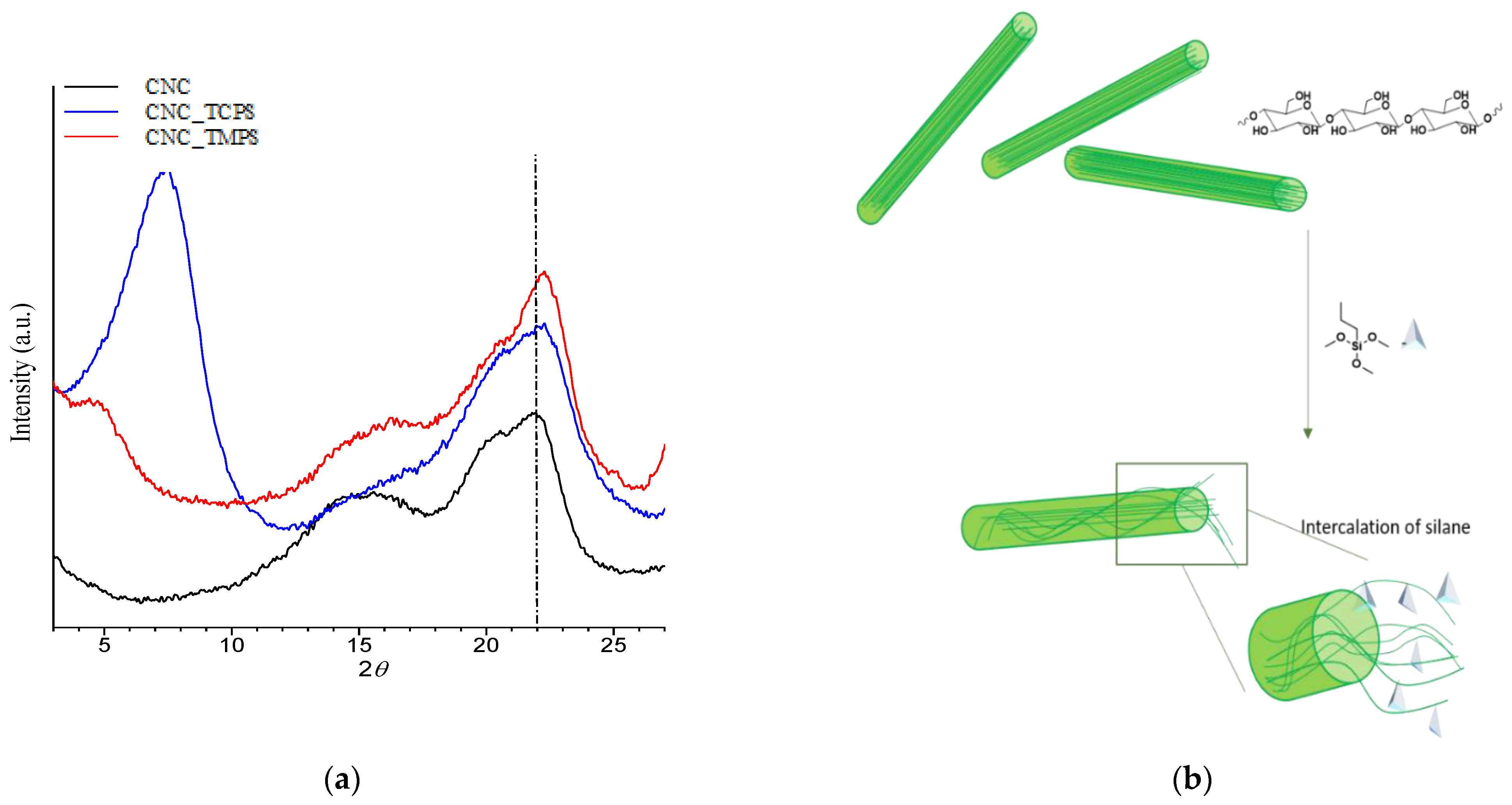
3.1.3. WAXS Characterization of CNCs-Grafted-Silanes
3.2. PUFs Characterization
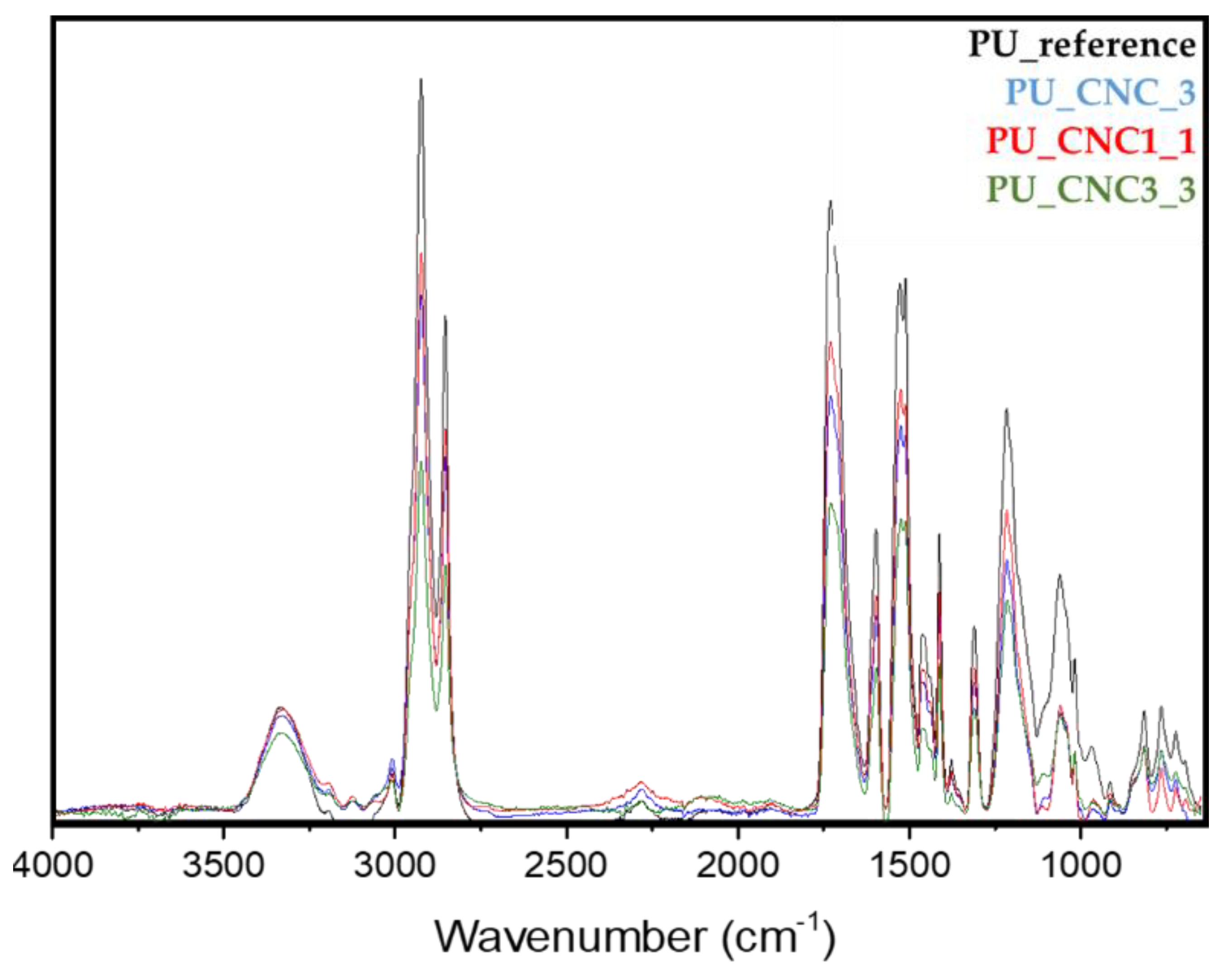
3.2.1. FTIR Characterization
3.2.2. SEM Observation
3.2.3. Contact Angle Measurements as PUFs Hydrophobicity Criterion
3.2.4. Thermal Stability of PUs Prepared with Developed Fillers
3.2.5. Density and Elastic Modulus of PUFs Prepared with the CNC Modified Fillers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, R. Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels: Chemistry, Extractives, Lignins, Hemicelluloses and Cellulose, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Xie, J.; Wu, Q.; Boldor, D.; Qi, J. High bio-content polyurethane (PU) foam made from bio-polyol and cellulose nanocrystals (CNCs) via microwave liquefaction. Mater. Des. 2018, 138, 11–20. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. 3D printed cork/polyurethane composite foams. Mater. Des. 2019, 179, 107905. [Google Scholar] [CrossRef]
- Szycher, M. Szycher’s Handbook of Polyurethanes; CRC Press LLC: New York, NY, USA, 1999. [Google Scholar]
- Hiba, S.; Xu, X.; Shifa, W. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Verdolotti, L.; Di Maio, E.; Forte, G.; Lavorgna, M.; Iannace, S. Hydration-induced reinforcement of polyurethane–cement foams: Solvent resistance and mechanical properties. J. Mater. Sci. 2010, 45, 3388–3391. [Google Scholar] [CrossRef]
- Gandhi, T.S.; Patel, M.R.; Dholakiya, B.Z. Synthesis of cashew Mannich polyol via a three-step continuous route and development of PU rigid foams with mechanical, thermal and fire studies. J. Polym. Eng. 2015, 35, 533–544. [Google Scholar] [CrossRef]
- Shrestha, M.L.; Ionescu, M.; Wan, X.; Bili´c, N.; Petrovi´c, Z.S.; Upshaw, T. Biobased Aromatic-Aliphatic Polyols from Cardanol by Thermal Thiol-Ene Reaction. J. Renew. Mater. 2018, 6, 87–101. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A.J. Cellulose nano whiskers as a reinforcing filler in polyurethanes. Algae 2011, 75, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ragauskas, A.J. Ethanol organosolv lignin-based rigid polyurethane foam reinforced with cellulose nanowhiskers. RSC Adv. 2012, 2, 3347–3351. [Google Scholar] [CrossRef]
- Li, Y.; Ren, H.; Ragauskas, A.J. Rigid polyurethane foam reinforced with cellulose whiskers: Synthesis and characterization. Nano Micro Lett. 2010, 2, 89–94. [Google Scholar] [CrossRef]
- Li, Y.; Ren, H.; Ragauskas, A.J. Rigid polyurethane foam/cellulose whisker nanocomposites: Preparation, characterization, and properties. J. Nanosci. Nanotechnol. 2011, 11, 6904–6911. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.K.; Sabu, A.; Santosh, K.T. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ha, S.K.; Verma, K.; Tiwari, S.K. Recent progress in selected bio-nanomaterials and their engineering applications: An overview. J. Sci. Adv. Mater. Devices 2018, 3, 263–288. [Google Scholar] [CrossRef]
- Goffin, A.; Habibi, Y.; Raquez, J.; Dubois, P. Polyester-Grafted Cellulose Nanowhiskers: A New Approach for Tuning the Microstructure of Immiscible Polyester Blends. ACS Appl. Mater. Interfaces 2012, 4, 3364–3371. [Google Scholar] [CrossRef]
- Yu, H.Y.; Huang, J.; Chang, P.R. Surface Grafting of Cellulose Nanowhisker. In Cellulose-Based Graft Copolymers, 1st ed.; Thakur, V.K., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 497–518. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Vizireanu, S.; Stoian, S.A.; Nicolae, C.-A.; Gabor, A.R.; Damian, C.M.; Trusca, R.; Carpen, L.G.; Dinescu, G. Poly(3-hydroxybutyrate) Modified by Plasma and TEMPO-Oxidized Celluloses. Polymers 2020, 12, 1510. [Google Scholar] [CrossRef]
- Spinella, S.; Samuel, C.; Raquez, J.; McCallum, S.A.; Gross, R.; Dubois, P. Green and Efficient Synthesis of Dispersible Cellulose Nanocrystals in Biobased Polyesters for Engineering Applications. ACS Sustain. Chem. Eng. 2016, 4, 2517–2527. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Pinheiro, I.F.; Gouveia, R.F.; Thim, G.P.; Lonal, L.M.F. Functionalized cellulose nanocrystals as reinforcement in biodegradable polymer nanocomposites. Polym. Compos. 2018, 39, 9–29. [Google Scholar] [CrossRef] [Green Version]
- Mokhena, T.C.; Sefadi, J.S.; Sadiku, E.R.; John, M.J.; Mochane, M.J.; Mtibe, A. Thermoplastic Processing of PLA/Cellulose Nanomaterials Composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Xu, J.; Gong, J.; Li, J.; Mo, L. Preparation, characterization and acetylation of cellulose nanocrystal allomorphs. Cellulose 2018, 25, 4905–4918. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.H.; Wu, Q.Q.; Kuang, Y.S. Acetylated cellulose nanocrystals with high-crystallinity obtained by one-step reaction from the traditional acetylation of cellulose. Carbohydr. Polym. 2020, 229, 1155532–1155538. [Google Scholar] [CrossRef]
- Xu, X.; Dong, F.; Yang, X.; Liu, H.; Guo, L.; Qian, Y.; Wang, A.; Wang, S.; Luo, J. Preparation and Characterization of Cellulose Grafted with Epoxidized Soybean Oil Aerogels for Oil-Absorbing Materials. J. Agric. Food Chem. 2019, 67, 637–643. [Google Scholar] [CrossRef]
- Gorade, V.G.; Kotwal, A.; Chaudhary, B.U.; Kale, R.D. Surface modification of microcrystalline cellulose using rice bran oil: A bio-based approach to achieve water repellency. J. Polym. Res. 2019, 26, 217–228. [Google Scholar] [CrossRef]
- Alzate-Arbeláez, A.F.; Dorta, E.; López-Alarcón, C.; Cortés, F.B.; Rojano, B.A. Immobilization of Andean berry (Vaccinium meridionale) polyphenols on nanocellulose isolated from banana residues: A natural food additive with antioxidant properties. Food Chem. 2019, 294, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Preparation, Characterization and Mechanical Properties of Bio-Based Polyurethane Adhesives from Isocyanate-Functionalized Cellulose Acetate and Castor Oil for Bonding Wood. Polymers 2017, 9, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hettegger, H.; Beaumont, M.; Potthast, A.; Rosenau, T. Aqueous Modification of Nano- and Microfibrillar, Cellulose with a Click Synthon. ChemSusChem 2016, 9, 75–79. [Google Scholar] [CrossRef]
- Tingaut, P.; Hauert, R.; Zimmermann, T. Highly efficient and straightforward functionalization of cellulose films with thiol-ene click chemistry. J. Mater. Chem. 2011, 21, 16066. [Google Scholar] [CrossRef]
- Hettegger, H.; Sumerskii, I.; Sortino, S.; Potthast, A.; Rosenau, T. Silane Meets Click Chemistry: Towards the Functionalization of Wet Bacterial Cellulose Sheets. ChemSusChem 2015, 8, 680–687. [Google Scholar] [CrossRef]
- Beaumont, M.; Bacher, M.; Opietnik, M.; Gindl-Altmutter, W.; Potthast, A.; Rosenau, T. A General Aqueous Silanization Protocol to Introduce Vinyl, Mercapto or Azido Functionalities onto Cellulose Fibers and Nanocelluloses. Molecules 2018, 23, 1427. [Google Scholar] [CrossRef] [Green Version]
- Hajlane, A.; Kaddami, H.; Joffe, R. Chemical modification of regenerated cellulose fibres by cellulose nano-crystals: Towards hierarchical structure for structural composites reinforcement. Ind. Crops Prod. 2017, 100, 41–50. [Google Scholar] [CrossRef]
- Robles, E.; Csóka, L.; Labidi, J. Effect of Reaction Conditions on the Surface Modification of Cellulose Nanofibrils with Aminopropyl Triethoxysilane. Coatings 2018, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Fathi, B.; Harirforoush, M.J.; Foruzanmehr, M.R.; Elkoun, S.; Robert, M. Effect of TEMPO oxidation of flax fibers on the grafting efficiency of silane coupling agents. J. Mater. Sci. 2017, 52, 10624–10636. [Google Scholar] [CrossRef]
- Zimmermann, M.V.G.; de Macedo, V.; Zattera, A.J.; Santana, R.M.C. Influence of chemical treatments on cellulose fibers for use as reinforcements in poly(ethylene-co-vinyl acetate) composites. Polym. Compos. 2016, 37, 1991–2000. [Google Scholar] [CrossRef]
- Rachini, A.; Le Troedec, M.; Peyratout, C.; Smith, A. Comparison of the thermal degradation of natural, alkali-treated and silane-treated hemp fibers under air and an inert atmosphere. J. Appl. Polym. Sci. 2009, 112, 226–234. [Google Scholar] [CrossRef]
- Ashby, M.F.; Gibson, L.J. Cellular Solids: Structure and Properties; Press Syndicate of the University of Cambridge: Cambridge, UK, 1997; pp. 1–510. [Google Scholar] [CrossRef]
- Gibson, L.J. Modelling the mechanical behavior of cellular materials. Mater. Sci. Eng. A 1989, 110, 1–36. [Google Scholar] [CrossRef]
- Ashby, M.F.; Medalist, R.M. The Mechanical Properties of Cellular Solids. Metall. Trans. A 1983, 14, 1755–1769. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar]
- D’auria, M.; Davino, D.; Pantani, R.; Sorrentino, L. Magnetic field-structuring as versatile approach to shape the anisotropic mechanical response of composite foams. Compos. Part B Eng. 2021, 212, 108659–108672. [Google Scholar] [CrossRef]
- Davino, D.; D’auria, M.; Pantani, R.; Sorrentino, L. Reinforced smart foams produced with time-profiled magnetic fields. Polymers 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Sèbe, G.; Ham-Pichavant, F.; Ibarboure, E.; Koffi, A.L.C.; Tingaut, P. Supramolecular Structure Characterization of Cellulose II Nanowhiskers Produced by Acid Hydrolysis of Cellulose I Substrates. Biomacromolecules 2012, 13, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Posocco, P.; Toth, R.; Fermeglia, M.; Pricl, S.; Mensitieri, G.; Lavorgna, M. Sodium montmorillonite silylation: Unexpected effect of the aminosilane chain length. J. Colloid Interface Sci. 2010, 351, 108–115. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Di Maio, E.; Iannace, S. Hydration-induced reinforcement of rigid polyurethane–cement foams: The effect of the co-continuous morphology on the thermal-oxidative stability. Polym. Degrad. Stab. 2013, 98, 64–72. [Google Scholar] [CrossRef]
- Stanzione, M.; Russo, V.; Oliviero, M.; Verdolotti, L.; Sorrentino, A.; Di Serio, M.; Tesser, R.; Iannace, S.; Lavorgna, M. Characterization of sustainable polyhydroxyls, produced from bio-based feedstock, and polyurethane and copolymer urethane-amide foams. Polymer 2018, 21, 269–275. [Google Scholar] [CrossRef]
- Sittinun, A.; Pisitsak, P.; Ummartyotin, S. Improving the oil sorption capability of porous polyurethane composites by the incorporation of cellulose fibers extracted from water hyacinth. Compos. Commun. 2020, 20, 100351–100357. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, J.M.; Kim, M.S.; Kim, Y.H.; Kim, W.N. Effects of Nucleating Agents on the Morphological, Mechanical and Thermal Insulating Properties of Rigid Polyurethane Foams. Macromol. Res. 2009, 17, 856–862. [Google Scholar] [CrossRef]
- Pau, D.S.W.; Fleischmann, C.M.; Delichatsios, M.A. Thermal decomposition of flexible polyurethane foams in air. Fire Saf. J. 2020, 111, 102925. [Google Scholar] [CrossRef]
- Oprea, S. Effect of structure on the thermal stability of crosslinked poly(ester-urethane). Polimery 2009, 54, 120–125. [Google Scholar] [CrossRef]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2014. [Google Scholar]
- Zieleniewska, M.; Leszczynski, M.K.; Szczepkowski, L.; Bryskiewicz, A.; Bien, K.; Ryszkowska, J. Development and applicational evaluation of the rigid polyurethane foam composites with egg shellwaste. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Stanzione, M.; Oliviero, M.; Cocca, M.; Errico, M.E.; Gentile, G.; Avella, M.; Lavorgna, M.; Buonocore, G.G.; Verdolotti, L. Tuning of polyurethane foam mechanical and thermal properties using ball-milled cellulose. Carbohydr. Polym. 2020, 231, 15772. [Google Scholar] [CrossRef]
- Bossa, F.D.; Santillo, C.; Verdolotti, L.; Campaner, P.; Minigher, A.; Boggioni, L.; Losio, S.; Coccia, F.; Iannace, S.; Lama, G.C. Greener Nanocomposite Polyurethane Foam Based on Sustainable Polyol and Natural Fillers: Investigation of Chemico-Physical and Mechanical Properties. Materials 2020, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Goods, S.H.; Neuschwanger, C.L.; Whinnery, L.L.; Nix, W.D. Mechanical properties of a particle-strengthened polyurethane foam. J. Appl. Polym. Sci. 1999, 74, 2724–2736. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Ghosn, L.J.; Lerch, B.A. A general tetrakaidecahedron model for open-celled foams. Int. J. Solids Struct. 2008, 45, 1754–1765. [Google Scholar] [CrossRef] [Green Version]




| Chemical Formula | (C6H9O5)n(SO3Na)x |
|---|---|
| CNC, wt% | 0.5–3.5 |
| Deionized water, wt% | 99.5–96.5 |
| Product form | Transparent gel |
| Density, g/cm3 | 1.00–1.50 |
| Particle size by TEM | Width: 2–20 nm (mean 6.8 nm) Length: 20–500 nm (mean 170 nm) |
| pH | 2–7 |
| Viscosity (cP, 25 °C, 3.0% CNC) | 100–7500 |
| Sulfur content, mmol/kg | 10–160 |
| Zeta-potential, mV | (−40)–(−20) |
| Reagent | Amount (g) |
|---|---|
| RV33 | 19.38 |
| Cardiolite NX-9201 | 23.25 |
| Sovermol® 750 | 7.76 |
| Sovermol® 815 | 7.76 |
| Glycerol | 0.97 |
| Blowing agent (water) | 0.78 |
| Exolit® OP 560 | 3.88 |
| Dabco® 33-LV | 0.39 |
| Dabco® NE 300 | 0.39 |
| TEGOSTAB® B 8747 LF2 | 0.78 |
| Lupranate M20S | 34.66 |
| Sample | T5% [°C] | T10% [°C] | T50% [°C] | DTG [°C] Peak | Residue at 750 °C [%] |
|---|---|---|---|---|---|
| CNC | 271 | 288 | 312 | 312 | 8.4 |
| CNC1 | 218 | 245 | 348 | 347 | 10.6 |
| CNC2 | 189 | 228 | 359 | 356 | 31.8 |
| CNC3 | 195 | 277 | 330 | 292 | 25.9 |
| CNC4 | 214 | 282 | 338 | 330 | 16.4 |
| Run | Silane (mmol)/1 g of CNC |
| CNC1 | 0.054 |
| CNC2 | 0.134 |
| CNC3 | 0.301 |
| CNC4 | 0.150 |
| Entry | Contact Angle, Average (°) |
|---|---|
| PU-Reference | 125.090 |
| PU-CNC_1 | 127.065 |
| PU-CNC_2 | 127.599 |
| PU-CNC_3 | 127.744 |
| PU-CNC1_1 | 126.371 |
| PU-CNC1_2 | 127.620 |
| PU-CNC1_3 | 133.200 |
| PU-CNC2_1 | 127.444 |
| PU-CNC2_2 | 128.889 |
| PU-CNC2_3 | 129.273 |
| PU-CNC3_1 | 130.441 |
| PU-CNC3_2 | 132.158 |
| PU-CNC3_3 | 137.344 |
| PU-CNC4_1 | 127.189 |
| PU-CNC4_2 | 128.803 |
| PU-CNC4_3 | 130.748 |
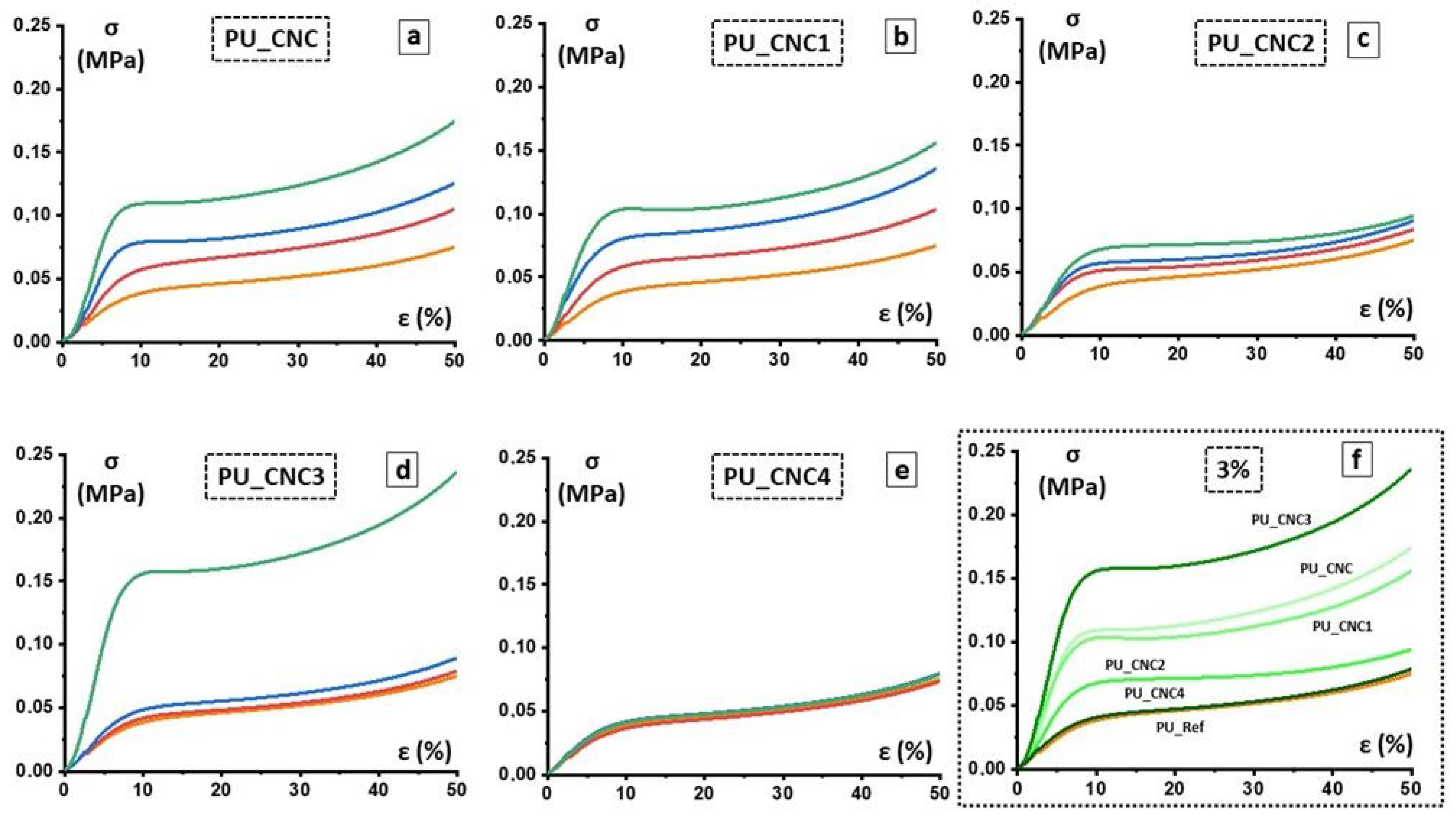
| Entry | ρ, kg/m3 | σ 10%(ǁ), kPa | σ 40%(ǁ), kPa | E (ǁ), MPa |
|---|---|---|---|---|
| PU_Ref | 103.5 ± 0.93 | 38.56 ± 1.75 | 60.21 ± 3.26 | 0.604 ± 0.11 |
| PU_CNC_1 | 101.3 ± 0.34 | 59.04 ± 8.98 | 86.13 ± 4.57 | 0.883 ± 0.25 |
| PU_CNC_2 | 101.9 ± 0.61 | 78.70 ± 3.30 | 102.49 ± 2.65 | 1.210 ± 0.48 |
| PU_CNC_3 | 113.8 ± 0.83 | 109.33 ± 2.68 | 142.12 ± 2.75 | 1.798 ± 0.67 |
| PU_CNC1_1 | 103.8 ± 0.46 | 58.33 ± 0.88 | 83.68 ± 1.45 | 1.043 ± 0.08 |
| PU_CNC1_2 | 111.3 ± 0.74 | 80.29 ± 2.62 | 109.36 ± 3.73 | 1.697 ± 0.12 |
| PU_CNC1_3 | 116.1 ± 0.39 | 103.58 ± 1.54 | 127.692 ± 1.86 | 2.154 ± 0.23 |
| PU_CNC2_1 | 97.3 ± 0.90 | 51.02 ± 7.17 | 68.018 ± 4.95 | 1.035 ± 0.19 |
| PU_CNC2_2 | 99.9 ± 0.57 | 56.85 ± 8.19 | 73.688 ± 5.34 | 1.106 ± 0.22 |
| PU_CNC2_3 | 101.9 ± 0.77 | 67.82 ± 9.24 | 80.406 ± 3.87 | 1.209 ± 0.15 |
| PU_CNC3_1 | 100.7 ± 0.35 | 41.57 ± 1.68 | 62.772 ± 1.05 | 0.688 ± 0.07 |
| PU_CNC3_2 | 101.7 ± 0.28 | 48.30 ± 1.04 | 71.31 ± 1.54 | 0.797 ± 0.05 |
| PU_CNC3_3 | 104.4 ± 0.44 | 156.07 ± 0.88 | 194.33 ± 5.98 | 2.546 ± 0.38 |
| PU_CNC4_1 | 103.6 ± 0.49 | 36.31 ± 3.05 | 58.23 ± 0.66 | 0.661 ± 0.09 |
| PU_CNC4_2 | 101.1 ± 0.40 | 41.54 ± 3.06 | 63.08 ± 0.93 | 0.766 ± 0.10 |
| PU_CNC4_3 | 101.7 ± 0.62 | 40.81 ± 2.66 | 62.43 ± 1.90 | 0.727 ± 0.09 |
| Entry | ρ/ρs | E/Es | na | ϕ b |
|---|---|---|---|---|
| PU_Ref | 0.0863 | 0.0134 | 1.759 | 0.917 |
| PU_CNC_1 | 0.0844 | 0.0196 | 1.590 | 0.825 |
| PU_CNC1_1 | 0.0865 | 0.0232 | 1.538 | 0.785 |
| PU_CNC2_1 | 0.0810 | 0.0230 | 1.501 | 0.763 |
| PU_CNC3_1 | 0.0863 | 0.0147 | 1.723 | 0.900 |
| PU_CNC4_1 | 0.0839 | 0.0153 | 1.687 | 0.883 |
| PU_CNC_2 | 0.0849 | 0.0269 | 1.466 | 0.728 |
| PU_CNC1_2 | 0.0927 | 0.0377 | 1.378 | 0.630 |
| PU_CNC2_2 | 0.0831 | 0.0246 | 1.490 | 0.751 |
| PU_CNC3_2 | 0.0842 | 0.0170 | 1.646 | 0.860 |
| PU_CNC4_2 | 0.0847 | 0.0177 | 1.634 | 0.853 |
| PU_CNC_3 | 0.0947 | 0.0400 | 1.366 | 0.614 |
| PU_CNC1_3 | 0.0967 | 0.0479 | 1.301 | 0.532 |
| PU_CNC2_3 | 0.0848 | 0.0269 | 1.466 | 0.728 |
| PU_CNC3_3 | 0.0869 | 0.0566 | 1.176 | 0.360 |
| PU_CNC4_3 | 0.0847 | 0.0162 | 1.671 | 0.874 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coccia, F.; Gryshchuk, L.; Moimare, P.; Bossa, F.d.L.; Santillo, C.; Barak-Kulbak, E.; Verdolotti, L.; Boggioni, L.; Lama, G.C. Chemically Functionalized Cellulose Nanocrystals as Reactive Filler in Bio-Based Polyurethane Foams. Polymers 2021, 13, 2556. https://doi.org/10.3390/polym13152556
Coccia F, Gryshchuk L, Moimare P, Bossa FdL, Santillo C, Barak-Kulbak E, Verdolotti L, Boggioni L, Lama GC. Chemically Functionalized Cellulose Nanocrystals as Reactive Filler in Bio-Based Polyurethane Foams. Polymers. 2021; 13(15):2556. https://doi.org/10.3390/polym13152556
Chicago/Turabian StyleCoccia, Francesca, Liudmyla Gryshchuk, Pierluigi Moimare, Ferdinando de Luca Bossa, Chiara Santillo, Einav Barak-Kulbak, Letizia Verdolotti, Laura Boggioni, and Giuseppe Cesare Lama. 2021. "Chemically Functionalized Cellulose Nanocrystals as Reactive Filler in Bio-Based Polyurethane Foams" Polymers 13, no. 15: 2556. https://doi.org/10.3390/polym13152556
APA StyleCoccia, F., Gryshchuk, L., Moimare, P., Bossa, F. d. L., Santillo, C., Barak-Kulbak, E., Verdolotti, L., Boggioni, L., & Lama, G. C. (2021). Chemically Functionalized Cellulose Nanocrystals as Reactive Filler in Bio-Based Polyurethane Foams. Polymers, 13(15), 2556. https://doi.org/10.3390/polym13152556