Enhancement of Antioxidant and Hydrophobic Properties of Alginate via Aromatic Derivatization: Preparation, Characterization, and Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
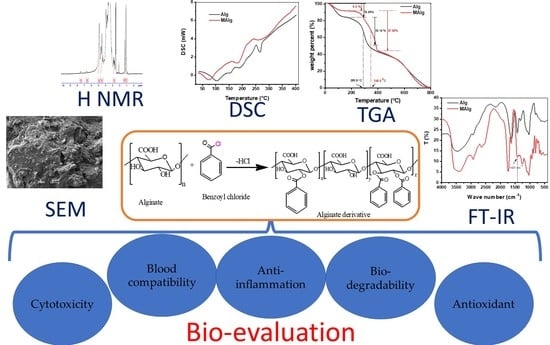
2.2.1. Preparation of Phenolic Alginate Derivative
2.2.2. Fourier Transfer Infrared Spectroscopy (FT-IR)
2.2.3. Elemental Analysis
2.2.4. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.2.5. Thermogravimetric Analysis (TGA)
2.2.6. Differential Scanning Calorimeter (DSC)
2.2.7. Scanning Electron Microscopy (SEM)
2.2.8. Biodegradability
2.2.9. Antioxidant Activity
DPPH Radical Scavenging Activity
In Vitro Anti-Inflammation Activity
2.2.10. Hemolysis Test
2.2.11. Investigating the Cytotoxicity
3. Results
3.1. FT-IR
3.2. Thermal Gravimetric Analysis
3.3. Differential Scanning Calorimetry
3.4. Morphological Analysis
3.5. Nuclear Magnetic Resonance Spectroscopy (NMR)
3.6. Biodegradability
3.7. Antioxidant Experimental
3.7.1. Antioxidant Capacity Assay (ABTS Assay)
3.7.2. Free Radical Scavenging Activity of DPPH (2,2-Diphenyll-Picrylhydrazyl)
3.8. In Vitro Anti-Inflammation Assays
3.9. Invitro Blood Compatibility
3.10. Investigating the Cytotoxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Law, B.M.H.; Waye, M.M.Y.; So, W.K.W.; Chair, S.Y. Hypotheses on the Potential of Rice Bran Intake to Prevent Gastrointestinal Cancer through the Modulation of Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 1352. [Google Scholar] [CrossRef]
- Aziz, M.A.; Majeed, G.H.; Diab, K.S.; Al-Tamimi, R.J. The association of oxidant–antioxidant status in patients with chronic renal failure. Ren. Fail. 2015, 38, 20–26. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2009, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial Nanoparticle Antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Publications: Washington, DC, USA, 2011; pp. 235–253. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Seidi, F.; Jenjob, R.; Phakkeeree, T.; Crespy, D. Saccharides, oligosaccharides, and polysaccharides nanoparticles for biomedical applications. J. Control. Release 2018, 284, 188–212. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Zahraee, Z. Controlled release of metformin from chitosan–based nanocomposite films containing mesoporous MCM-41 nanoparticles as novel drug delivery systems. J. Colloid Interface Sci. 2017, 501, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Kohsari, I.; Shariatinia, Z.; Pourmortazavi, S.M. Antibacterial electrospun chitosan-polyethylene oxide nanocomposite mats containing ZIF-8 nanoparticles. Int. J. Biol. Macromol. 2016, 91, 778–788. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer forcell encapsulation. Carbohydr Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kohsari, I.; Shariatinia, Z.; Pourmortazavi, S.M. Antibacterial electrospun chitosanepolyethylene oxidenanocomposite mats containing bioactive silver nanoparticles. Carbohydr Polym. 2016, 140, 287–298. [Google Scholar] [CrossRef]
- Fazli, Y.; Shariatinia, Z.; Kohsari, I.; Azadmehr, A.; Pourmortazavi, S.M. A novel chitosan-polyethylene oxide nanofibrous mat designed for controlled co-release of hydrocortisone and imipenem/cilastatin drugs. Int. J. Pharm. 2016, 513, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Germershaus, O.; Lühmann, T.; Ritzer, J.; Meinel, L. Application of natural and semi-synthetic polymers for the delivery of sensitive drugs. Int. Mater. Rev. 2015, 60, 101–130. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Nikfar, Z. Synthesis and antibacterial activities of novel nanocomposite films of chitosan/phosphoramide/Fe3O4 NPs. Int. J. Biol. Macromol. 2013, 60, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Nikfar, Z.; Gholivand, K.; Tarei, S.A. Antibacterial activities of novel nanocomposite biofilms of chitosan/phosphoramide/Ag NPs. Polym. Compos. 2014, 36, 454–466. [Google Scholar] [CrossRef]
- Ngwuluka, N.C.; Ochekpe, N.A.; Aruoma, O.I. Naturapolyceutics: The Science of Utilizing Natural Polymers for Drug Delivery. Polymers 2014, 6, 1312–1332. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Alonso, M.J.; de la Fuente, M. Nanoengineering of vaccines using natural polysaccharides. Biotechnol. Adv. 2015, 33, 1279–1293. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Fazli, M. Mechanical properties and antibacterial activities of novel nanobiocomposite films of chitosan and starch. Food Hydrocoll. 2015, 46, 112–124. [Google Scholar] [CrossRef]
- Fazli, Y.; Shariatinia, Z. Controlled release of cefazolin sodium antibiotic drug from electrospun chitosan-polyethylene oxide nanofibrous Mats. Mater. Sci. Eng. C 2017, 71, 641–652. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.; Mohammadi, A.; Khosravi-Darani, K.; Alibadi, S.S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.; Sagis, L.M. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Andriamanantoanina, H.; Rinaudo, M. Characterization of the alginates from five madagascan brown algae. Carbohydr. Polym. 2010, 82, 555–560. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Freile-Pelegrin, Y.; Hernández-Garibay, E. Conventional and alternative technologies for the extraction of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Herminia, D., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 475–516. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.-Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Xue, C.; Sun, L. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. Environ. Boil. Fishes 2011, 24, 295–300. [Google Scholar] [CrossRef]
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). Environ. Boil. Fishes 2008, 20, 1033–1043. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Effects of extraction methods on molecular characteristics, antioxidant properties and immunomodulation of alginates from Sargassum angustifolium. Int. J. Biol. Macromol. 2017, 101, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, M.; Kumar, M.S. Extraction of alginate from brown seaweeds and evolution of bioactive alginate film coated textile fabrics for wound healing application. J. Ind. Text. 2018, 49, 328–351. [Google Scholar] [CrossRef]
- Sidiropoulos, P.I.; Hatemi, G.; Song, I.-H.; Avouac, J.; Collantes, E.; Hamuryudan, V.; Herold, M.; Kvien, T.K.; Mielants, H.; Mendoza, J.M.; et al. Evidence-based recommendations for the management of ankylosing spondylitis: Systematic literature search of the 3E Initiative in Rheumatology involving a broad panel of experts and practising rheumatologists. Rheumatology 2008, 47, 355–361. [Google Scholar] [CrossRef][Green Version]
- Fattahi, M.J.; Abdollahi, M.; Agha Mohammadi, A.; Rastkari, N.; Khorasani, R.; Ahmadi, H.; Tofighi Zavareh, F.; Sedaghat, R.; Tabrizian, N.; Mirshafiey, A. Preclinical assessment of beta-d-mannuronic acid (M2000) as a non-steroidal anti-inflammatory drug. Immunopharmacol. Immunotoxicol. 2015, 37, 535–540. [Google Scholar] [CrossRef]
- Mirshafiey, A.; Rehm, B.; Sotoude, M.; Razavi, A.; Abhari, R.S.; Borzooy, Z. Therapeutic Approach by a Novel Designed Anti-Inflammatory Drug, M2000, in Experimental Immune Complex Glomerulonephritis. Immunopharmacol. Immunotoxicol. 2007, 29, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Vauchel, P.; Arhaliass, A.; Legrand, J.; Kaas, R.; Baron, R. Decrease in dynamic viscosity and average molecular weight of alginate from Laminaria digitata during alkaline extraction. J. Phycol. 2008, 44, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Vauchel, P.; Kaas, R.; Arhaliass, A.; Baron, R.; Legrand, J. A New Process for Extracting Alginates from Laminaria digitata: Reactive Extrusion. Food Bioprocess Technol. 2008, 1, 297–300. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Peteiro, C. Alginate production from marine macroalgae, with emphasis on kelp farming. In Alginates and Their Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 27–66. [Google Scholar]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Meyer, A.S. Characterization of alginates from Ghanaian brown seaweeds: Sargassum spp. and Padina spp. Food Hydrocoll. 2017, 71, 236–244. [Google Scholar] [CrossRef]
- Zia, K.M.; Zuber, M.; Ali, M. Algae Based Polymers, Blends, and Composites: Chemistry, Biotechnology and Materials Science; Elsevier: Amsterdam, The Netherlands, 2017; p. 738. [Google Scholar]
- Rinaudo, M. Biomaterials based on a natural polysaccharide: Alginate. TIP 2014, 17, 92–96. [Google Scholar] [CrossRef]
- Viswanathan, S.; Nallamuthu, T. Extraction of sodium alginate from selected seaweeds and their physiochemical and biochemical properties. J. Innov. Res. 2014, 3, 10998–11003. [Google Scholar]
- Silva, T.H.; Alves, A.; Ferreira, B.M.; Oliveira, J.M.; Reys, L.; Ferreira, R.J.F.; Sousa, R.; Silva, S.; Mano, J.; Reis, R.L. Materials of marine origin: A review on polymers and ceramics of biomedical interest. Int. Mater. Rev. 2012, 57, 276–306. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Koenig, J.L. Vibrational Spectra of Carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987, 44, 7–89. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Tul’chinsky, V.M.; Zurabyan, Z.E.; Asankoshoev, K.A.; Kogan, G.A.; Khorlin, A.Y. Facile synthesis of new sodium alginate–anthracene-based photosensitizers. Carbohydr. Res. 1976, 51, 1–8. [Google Scholar] [PubMed]
- Chandıa, N.P.; Matsuhiro, B.; Vásquez, A.E. Alginic acids in Lessonia trabeculata: Characterization by formic acid hydrolysis and FT-IR spectroscopy. Carbohydr. Polym. 2001, 46, 81–87. [Google Scholar] [CrossRef]
- Chandı´a, N.P.; Matsuhiro, B.; Mejı´as, E.; Moenne, A. Alginic acids in Lessonia vadosa: Partial hydrolysis and elicitor properties of the polymannuronic acid fraction. J. Appl. Phycol. 2004, 16, 127–133. [Google Scholar] [CrossRef]
- Guarino, V.; Caputo, T.; Altobelli, R.; Ambrosio, L. Review: Degradation properties and metabolic activity of alginate and chitosan polyelectrolytes for drug delivery and tissue engineering applications. AIMS Mater. Sci. 2015, 2, 497–502. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Analysis of Botanicals and Dietary Supplements for Antioxidant Capacity: A Review. J. AOAC Int. 2000, 83, 950–956. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical review and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Sa´nchez-Moreno, C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Hernandez-Marin, E.; Martinez, A. Carbohydrates and Their Free Radical Scavenging Capability: A Theoretical Study. J. Phys. Chem. B 2012, 116, 9668–9675. [Google Scholar] [CrossRef]
- Peshev, D.; Vergauwen, R.; Moglia, A.; Hideg, E.; Van den Ende, W. Towards understanding vacuolar antioxidant mechanisms: A role for fructans? J. Exp. Bot. 2013, 64, 1025–1038. [Google Scholar] [CrossRef]
- Hrabarova, E.; Valachova, K.; Rapta, P.; Soltes, L. An alternative standardfor trolox-equivalent antioxidant-capacity estimation based on thiolantioxidants. Comparative 2, 2’-azinobis[3-ethylbenzothiazoline-6-sulfonicacid] decolorization and rotational viscometry study regarding hyaluronandegradation. Chem. Biodivers. 2010, 7, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Valachova, K.; Banasova, M.; Topolska, D.; Sasinkova, V.; Juranek, I.; Collins, M.N. Influence of tiopronin: Captopril and levamisole therapeutics onthe oxidative degradation of hyaluronan. Carbohydr. Polym. 2015, 134, 516–523. [Google Scholar] [CrossRef]
- Abdelrazik, T.M.; Valachová, K.; Mohyeldin, M.S.; Soltes, L. Free radical scavenger activity of cinnamyl chitosan schiff base. J. Appl. Pharm. Sci. 2016, 6, 130–136. [Google Scholar] [CrossRef][Green Version]
- Kelishomi, Z.H.; Goliaei, B.; Mahdavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Mamashli, F.; Bigdeli, B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC Method for Evaluation of the Free Radical-scavenging Activity of Foods by Using 1,1-Diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef]
- Zhao, H.; Harding, S.; Marinangeli, C.; Kim, Y.; Jones, P. Hypocholesterolemic and anti-obesity effects of Saponins from Platycodon grandiflorum in hamsters fed Atherogenic diets. J. Food Sci. 2008, 73, 195–200. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chandra, S.; Chatterjee, P.; Dey, P. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Saso, L.; Valentini, G.; Casini, M.L.; Grippa, E.; Gatto, M.T.; Leone, M.G.; Silvestrini, B. Inhibition of heat-induced denaturation of albumin by nonsteroidal anti-inflammatory drugs (NSAIDs): Pharmacological implications. Arch. Pharm. Res. 2001, 24, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Fried, J. Polymer Science and Technology; Pearson Education: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Zielhuis, S.W.; Nijsen, J.F.W.; Seppenwoolde, J.-H.; Bakker, C.J.; Krijger, G.C.; Dullens, H.F.; Zonnenberg, B.A.; Van Rijk, P.P.; Hennink, W.E.; Schip, A.D.V.H. Long-term toxicity of holmium-loaded poly(l-lactic acid) microspheres in rats. Biomaterials 2007, 28, 4591–4599. [Google Scholar] [CrossRef] [PubMed]
- Sovadinova, I.; Palermo, E.F.; Huang, R.; Thoma, L.; Kuroda, K. Mechanism of Polymer-Induced Hemolysis: Nanosized Pore Formation and Osmotic Lysis. Biomacromolecules 2011, 12, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Caputo, G.A.; DeGrado, W.F. The Role of Hydrophobicity in the Antimicrobial and Hemolytic Activities of Polymethacrylate Derivatives. Chem. Eur. J. 2008, 15, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]










| C% | H% | C/H% | |
|---|---|---|---|
| Alginic acid | 36.81 | 4.984 | 7.385 |
| Modified Alginate | 35.56 | 5.335 | 6.665 |
| Concentration mg/mL | Alginic Acid | Modified Alginate |
|---|---|---|
| 0.5 | 66.711 ± 0.026 | 62.868 ± 0.026 |
| 0.25 | 79.211 ± 1.316 | 71.921 ± 0.237 |
| 0.125 | 87.026 ± 1.711 | 83.079 ± 0.184 |
| 0.0625 | 90.895 ± 0.263 | 89.079 ± 0.237 |
| 0.03125 | 96.263 ± 0.263 | 95.158 ± 0.421 |
| 0.01563 | 100.632 ± 0.737 | 99.237 ± 0.395 |
| Concentration mg/mL | Alginic Acid | Modified Alginate |
|---|---|---|
| EC50 | 0.732 ± 0.013 | 0.630 ± 0.001 |
| EC100 | 0.065 ± 0.004 | 0.062 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbayomi, S.M.; Wang, H.; Tamer, T.M.; You, Y. Enhancement of Antioxidant and Hydrophobic Properties of Alginate via Aromatic Derivatization: Preparation, Characterization, and Evaluation. Polymers 2021, 13, 2575. https://doi.org/10.3390/polym13152575
Elbayomi SM, Wang H, Tamer TM, You Y. Enhancement of Antioxidant and Hydrophobic Properties of Alginate via Aromatic Derivatization: Preparation, Characterization, and Evaluation. Polymers. 2021; 13(15):2575. https://doi.org/10.3390/polym13152575
Chicago/Turabian StyleElbayomi, Smaher M., Haili Wang, Tamer M. Tamer, and Yezi You. 2021. "Enhancement of Antioxidant and Hydrophobic Properties of Alginate via Aromatic Derivatization: Preparation, Characterization, and Evaluation" Polymers 13, no. 15: 2575. https://doi.org/10.3390/polym13152575
APA StyleElbayomi, S. M., Wang, H., Tamer, T. M., & You, Y. (2021). Enhancement of Antioxidant and Hydrophobic Properties of Alginate via Aromatic Derivatization: Preparation, Characterization, and Evaluation. Polymers, 13(15), 2575. https://doi.org/10.3390/polym13152575