Aminated Graphene-Graft-Oligo(Glutamic Acid) /Poly(ε-Caprolactone) Composites: Preparation, Characterization and Biological Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
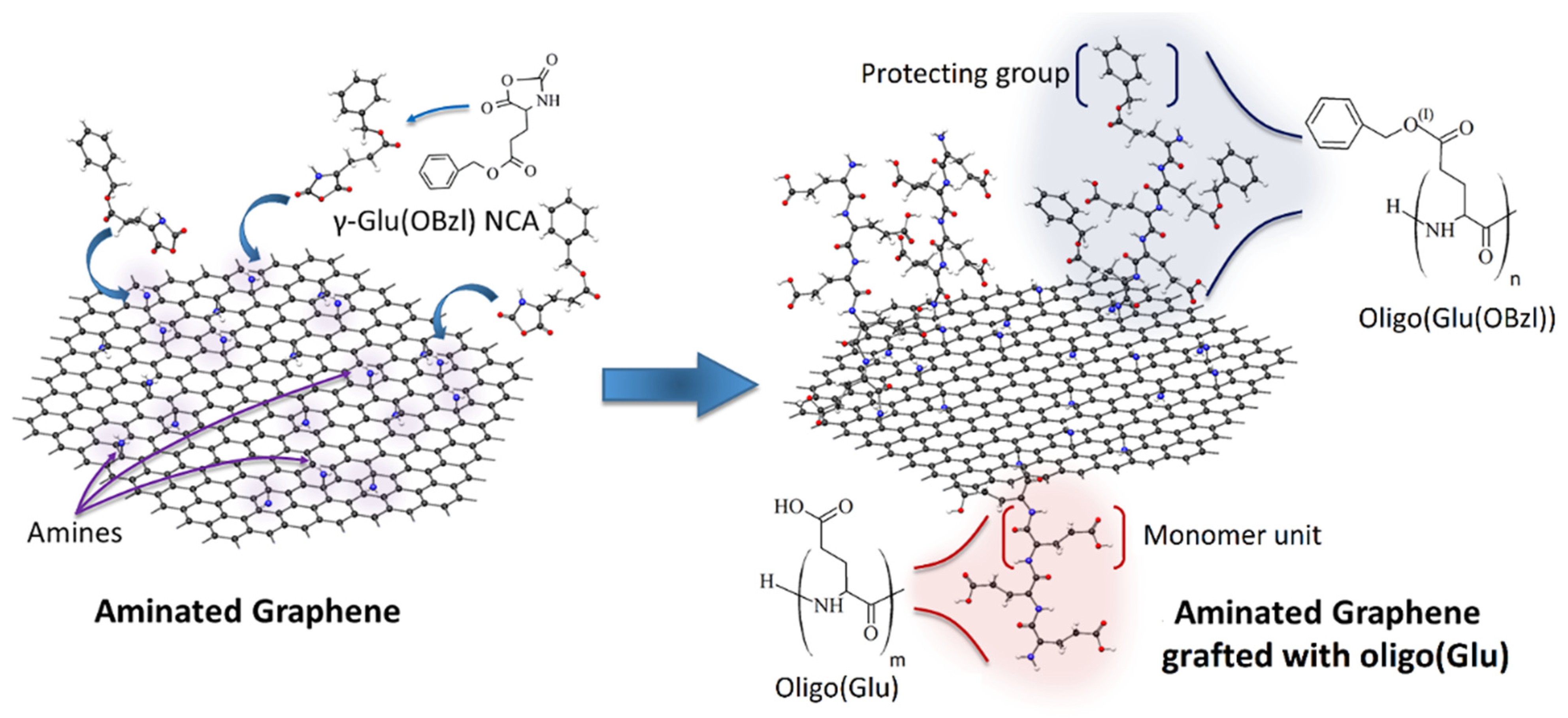
2.2. Synthesis of Aminated Graphene
2.3. Modification of Aminated Graphene with Oligomers of Glutamic Acid
2.4. Characterization of rGO-Am and Its Derivatives
2.5. Synthesis of PCL
2.6. Manufacturing of Composite Films
2.7. Mechanical Testing
2.8. Biological Evaluation
3. Results and Discussion
3.1. Synthesis of rGO-Am
3.2. Modification of rGO-Am with Oligomers of Glutamic Acid
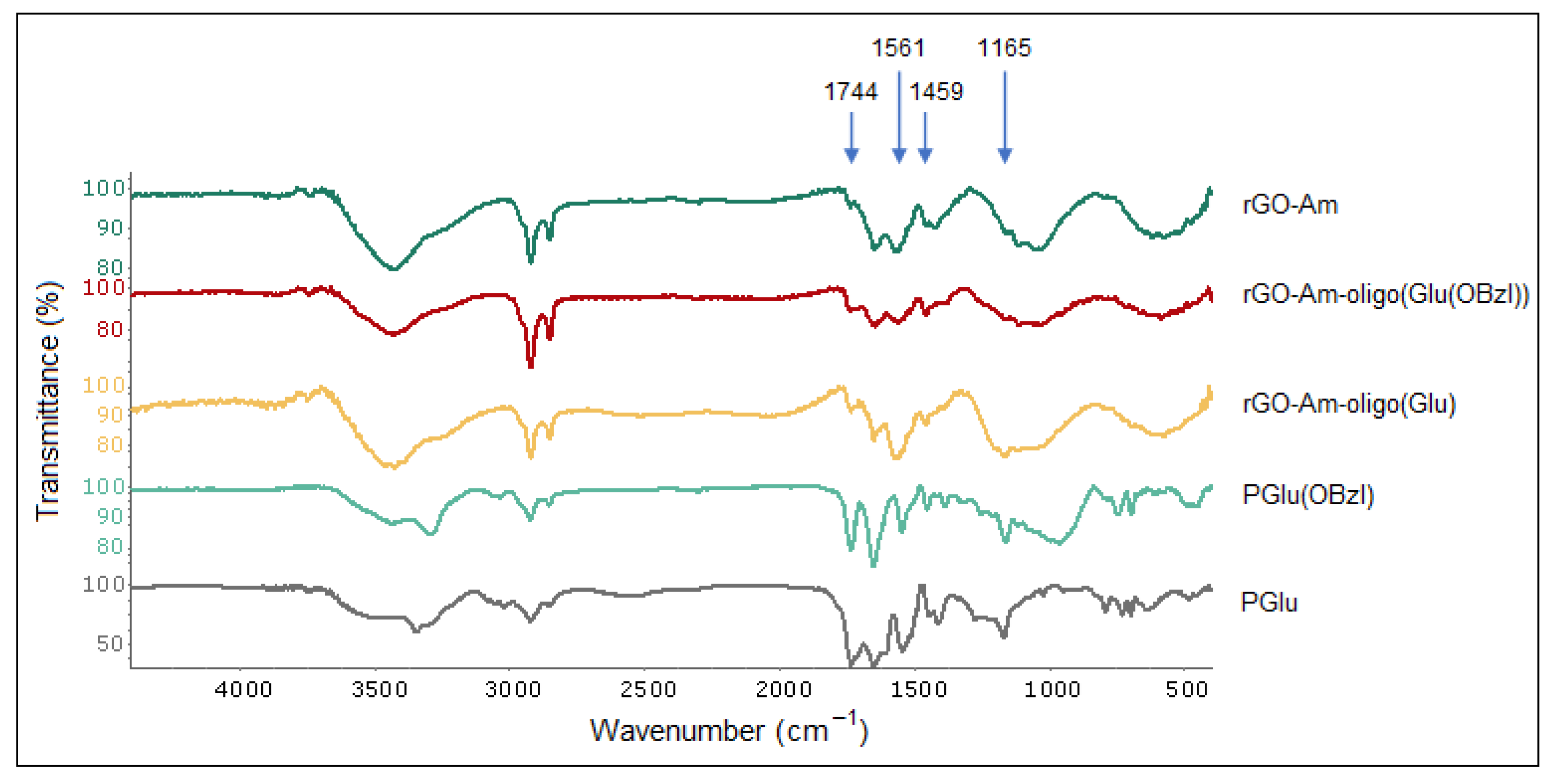
3.3. Characterization of Modified rGO-Am
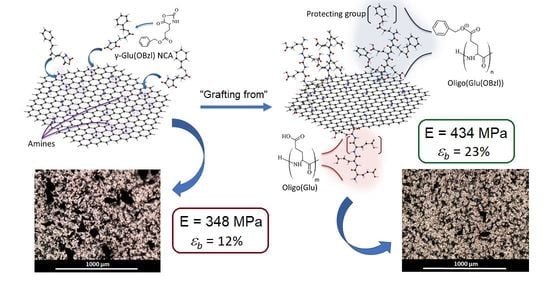
3.4. Manufacturing and Characterization of the Composite Films
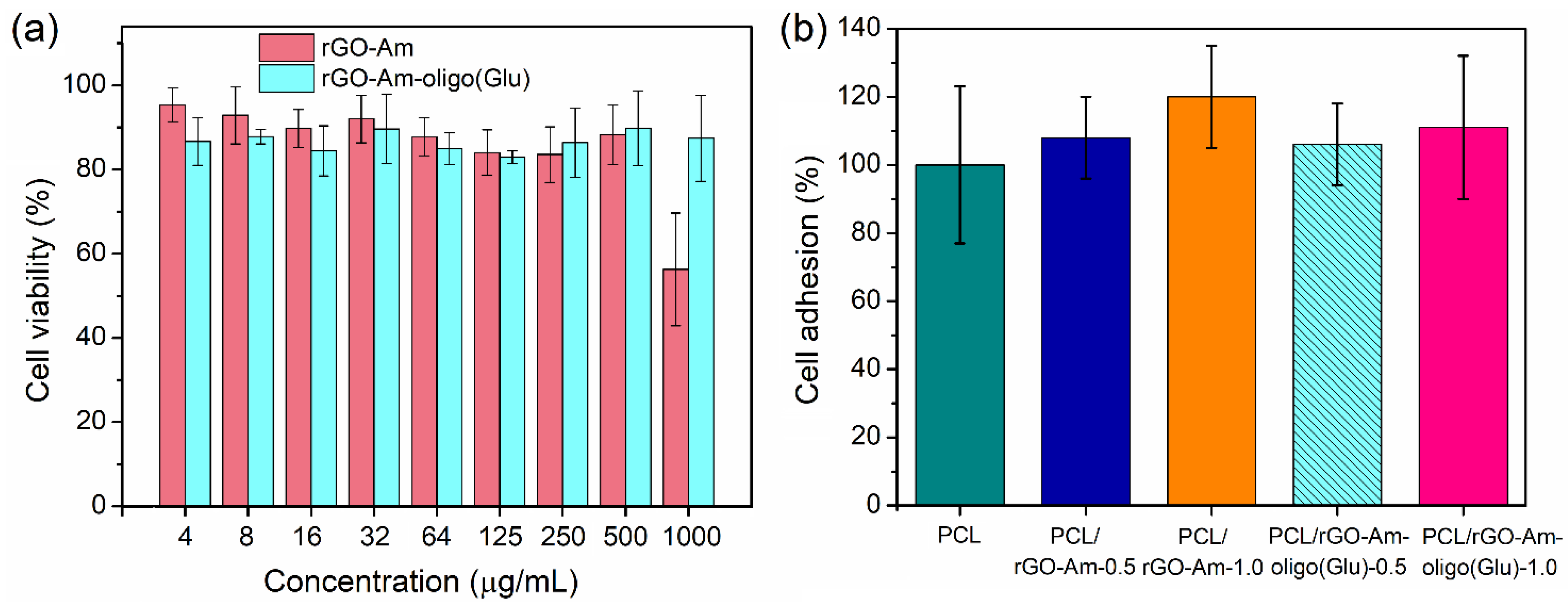
3.5. In Vitro Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, Y.; Zhao, L.; Jiang, X.; Wang, H.; Gao, W. Poly(lactic acid)-based biocomposites reinforced with modified cellulose nanocrystals. Cellulose 2017, 24, 4773–4784. [Google Scholar] [CrossRef]
- Gredes, T.; Schönitz, S.; Gedrange, T.; Stepien, L.; Kozak, K.; Kunert-Keil, C. In vivo analysis of covering materials composed of biodegradable polymers enriched with flax fibers. Biomater. Res. 2017, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Korzhikov, V.; Averianov, I.; Litvinchuk, E.; Tennikova, T.B. Polyester-based microparticles of different hydrophobicity: The patterns of lipophilic drug entrapment and release. J. Microencapsul. 2016, 33, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.W.; Yao, C.H.; Chen, Y.S.; Hsieh, T.C.; Lin, J.H.; Hsing, W.H. Manufacturing and Properties of PLA Absorbable Surgical Suture. Text. Res. J. 2008, 78, 958–965. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.P.; Chen, Y.Y.; Hsieh, M.F. Biofabrication and in vitro study of hydroxyapatite/mPEG–PCL–mPEG scaffolds for bone tissue engineering using air pressure-aided deposition technology. Mater. Sci. Eng. C 2013, 33, 680–690. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Dhert, W.J.A.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater. 2011, 7, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of inorganic fillers on the thermal and mechanical properties of poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Serdobintsev, M.; Gofman, I.; Blum, N.; Semenova, N.; Nashchekina, Y.; Vinogradova, T.; Korzhikov-Vlakh, V.; Karttunen, M.; et al. PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites. Materials 2019, 12, 3435. [Google Scholar] [CrossRef]
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Yusof, N.M.; Idris, A. Review on nanocrystalline cellulose in bone tissue engineering applications. Polymers 2020, 12, 2818. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Gofman, I.; Solomakha, O.; Nashchekina, Y.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Poly(ϵ-caprolactone)-based biocomposites reinforced with nanocrystalline cellulose grafted with poly(L-lactic acid). IOP Conf. Ser. Mater. Sci. Eng. 2019, 500, 012021. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Averianov, I.; Sinitsyna, E.; Nashchekina, Y.; Polyakov, D.; Guryanov, I.; Lavrentieva, A.; Raddatz, L.; Korzhikova-Vlakh, E.; Scheper, T.; et al. Novel Pathway for Efficient Covalent Modification of Polyester Materials of Different Design to Prepare Biomimetic Surfaces. Polymers 2018, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Karaman, O.; Kumar, A.; Moeinzadeh, S.; He, X.; Cui, T.; Jabbari, E. Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J. Tissue Eng. Regen. Med. 2016, E132–E146. [Google Scholar] [CrossRef]
- Shi, X.; Wu, H.; Yan, H.; Wang, Y.; Wang, Z.; Zhang, P. Electroactive Nanocomposite Porous Scaffolds of PAPn/op-HA/PLGA Enhance Osteogenesis in Vivo. ACS Appl. Bio Mater. 2019, 2, 1464–1476. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Wang, Y.; Ito, Y.; Zhang, P.; Chen, X. Enhanced in Vitro Mineralization and in Vivo Osteogenesis of Composite Scaffolds through Controlled Surface Grafting of L-Lactic Acid Oligomer on Nanohydroxyapatite. Biomacromolecules 2016, 17, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Deng, M.; Ulery, B.; Nair, L.; Laurencin, C. Nano-ceramic composite scaffolds for bioreactor-based bone engineering. Clin. Orthop. Relat. Res. 2013, 471, 2422–2433. [Google Scholar] [CrossRef] [PubMed]
- Valapa, R.B.; Pugazhenthi, G.; Katiyar, V. Effect of graphene content on the properties of poly(lactic acid) nanocomposites. RSC Adv. 2015, 5, 28410–28423. [Google Scholar] [CrossRef]
- Camargo, J.C.; Machado, Á.R.; Almeida, E.C.; Silva, E.F.M.S. Mechanical properties of PLA-graphene filament for FDM 3D printing. Int. J. Adv. Manuf. Technol. 2019, 103, 2423–2443. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Barajaa, M.; Tahmasbi Rad, A.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Healthc. Mater. 2021, 10, 2001414. [Google Scholar] [CrossRef]
- Pang, W.; Ni, Z.; Chen, G.; Huang, G.; Huang, H.; Zhao, Y. Mechanical and thermal properties of graphene oxide/ultrahigh molecular weight polyethylene nanocomposites. RSC Adv. 2015, 5, 63063–63072. [Google Scholar] [CrossRef]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of polylactic acid/hydroxyapatite/graphene oxide composite and their thermal stability, hydrophobic and mechanical properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M. Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef]
- Mohamad, S.N.K.; Ramli, I.; Abdullah, L.C.; Mohamed, N.H.; Islam, M.S.; Ibrahim, N.A.; Ishak, N.S. Evaluation on structural properties and performances of graphene oxide incorporated into chitosan/poly-lactic acid composites: Cs/pla versus cs/pla-go. Polymers 2021, 13, 1839. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells on Graphene Oxide-Incorporated Electrospun Poly(lactic-co-glycolic acid) Nanofibrous Mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Kaushik, A.; Arruda, E.; Waas, A.; Shim, B.; Xu, J.; Nandivada, H.; Pumplin, B.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Bonderer, L.J.; Studart, A.R.; Gauckler, L.J. Bioinspired Design and Assembly of Platelet Reinforced Polymer Films. Science 2008, 319, 1069–1073. [Google Scholar] [CrossRef]
- Wei, J.; Liu, A.; Zhang, P.; Chen, L.; Chen, X.; Jing, X. The surface modification of hydroxyapatite nanoparticles by the ring opening polymerization of gamma-benzyl-l-glutamate N-carboxyanhydride. Macromol. Biosci. 2009, 9, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Nikolaeva, A.l.; Korzhikov-Vlakh, V.A.; Karttunen, M.; Korzhikova-Vlakh, E.G. Chemical modification of nanocrystalline cellulose for enchanced interfacial compatibility with poly(lactic acid). Mendeleev Commun. 2019, 29, 220–222. [Google Scholar] [CrossRef]
- Qu, P.; Zhou, Y.; Zhang, X.; Yao, S.; Zhang, L. Surface modification of cellulose nanofibrils for poly(lactic acid) composite application. J. Appl. Polym. Sci. 2012, 125, 3084–3091. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Solomakha, O.; Zabolotnykh, N.; Gofman, I.; Serdobintsev, M.; Vinogradova, T.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Composite biomaterials based on poly(L-lactic acid) and functionalized cellulose nanocrystals. J. Renew. Mater. 2020, 8, 383–395. [Google Scholar] [CrossRef]
- Gu, A.; Wu, J.; Shen, L.; Zhang, X.; Bao, N. High-Strength GO / PA66 Nanocomposite Fibers via In Situ. Polymers 2021, 13, 1688. [Google Scholar] [CrossRef]
- Wang, L.-N.; Wang, P.-Y.G.; Wei, J.-C. Graphene Oxide-Graft-Poly(l-lactide)/Poly(l-lactide) Nanocomposites: Mechanical and Thermal Properties. Polymers 2017, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Kirilenko, D.A.; Ulin, N.V.; Baidakova, M.V.; Shnitov, V.V.; Pavlov, S.I.; Chumakov, R.G.; Stolyarova, D.Y.; Besedina, N.A.; et al. From graphene oxide towards aminated graphene: Facile synthesis, its structure and electronic properties. Sci. Rep. 2020, 10, 6902. [Google Scholar] [CrossRef] [PubMed]
- Vlakh, E.; Ananyan, A.; Zashikhina, N.; Hubina, A.; Pogodaev, A.; Volokitina, M.; Sharoyko, V.; Tennikova, T. Preparation, characterization, and biological evaluation of poly(glutamic acid)-b-polyphenylalanine polymersomes. Polymers 2016, 8, 212. [Google Scholar] [CrossRef]
- Zashikhina, N.; Sharoyko, V.; Antipchik, M.; Tarasenko, I.; Anufrikov, Y.; Lavrentieva, A.; Tennikova, T.; Korzhikova-Vlakh, E. Novel Formulations of C-Peptide with Long-Acting Therapeutic Potential for Treatment of Diabetic Complications. Pharmaceutics 2019, 11, 27. [Google Scholar] [CrossRef]
- Schultz, B.J.; Dennis, R.V.; Aldinger, J.P.; Jaye, C.; Wang, X.; Fischer, D.A.; Cartwright, A.N.; Banerjee, S. X-ray absorption spectroscopy studies of electronic structure recovery and nitrogen local structure upon thermal reduction of graphene oxide in an ammonia environment. RSC Adv. 2014, 4, 634–644. [Google Scholar] [CrossRef]
- Lazar, P.; Mach, R.; Otyepka, M. Spectroscopic Fingerprints of Graphitic, Pyrrolic, Pyridinic, and Chemisorbed Nitrogen in N-Doped Graphene. J. Phys. Chem. C 2019, 123, 10695–10702. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Varezhnikov, A.S.; Sysoev, V.V.; Solomatin, M.A.; Ryzhkov, S.A.; Baidakova, M.V.; Stolyarova, D.Y.; Shnitov, V.V.; Pavlov, S.S.; Kirilenko, D.A.; et al. Hole-matrixed carbonylated graphene: Synthesis, properties, and highly-selective ammonia gas sensing. Carbon 2021, 172, 236–247. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Saveliev, S.D.; Stolyarova, D.Y.; Brzhezinskaya, M.; Kirilenko, D.A.; Baidakova, M.V.; Ryzhkov, S.A.; Shnitov, V.V.; Sysoev, V.V.; Brunkov, P.N. Modulating nitrogen species via N-doping and post annealing of graphene derivatives: XPS and XAS examination. Carbon 2021, 182, 593–604. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Vargas-Astudillo, D.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Fuentealba, P.; Contreras-Cid, A.; Verdejo, R.; López-Manchado, M.A. Facile and Scalable One-Step Method for Amination of Graphene Using Leuckart Reaction. Chem. Mater. 2017, 29, 6698–6705. [Google Scholar] [CrossRef]
- Cheng, J.; Deming, T.J. Synthesis of Polypeptides by Ring-Opening Polymerization of α-Amino Acid N-Carboxyanhydrides. Top. Curr. Chem. 2012, 310, 1–26. [Google Scholar]
- Yu, M.; Zhang, S.; Chen, Y.; Jin, H.; Zhang, Y.; Lu, L.; Shu, Z.; Hou, S.; Xie, B.; Cui, H. A green method to reduce graphene oxide with carbonyl groups residual for enhanced electrochemical performance. Carbon 2018, 133, 101–108. [Google Scholar] [CrossRef]
- Kolosov, A.E.; Sivetskii, V.I.; Kolosova, E.P.; Vanin, V.V.; Gondlyakh, A.V.; Sidorov, D.E.; Ivitskiy, I.I.; Symoniuk, V.P. Use of physicochemical modification methods for producing traditional and nanomodified polymeric composites with improved operational properties. Int. J. Polym. Sci. 2020, 2019, 1258727. [Google Scholar] [CrossRef]
- Daukiya, L.; Seibel, J.; Feyter, S. De Chemical modification of 2D materials using molecules and assemblies of molecules. Adv. Phys. X 2019, 4, 1625723. [Google Scholar]
- Rive, C.; Reina, G.; Wagle, P.; Treossi, E.; Palermo, V.; Bianco, A.; Delogu, L.G.; Rieckher, M.; Schumacher, B. Improved Biocompatibility of Amino-Functionalized Graphene Oxide in Caenorhabditis elegans. Small 2019, 15, 1902699. [Google Scholar] [CrossRef]
- Iudin, D.; Zashikhina, N.; Demyanova, E.; Korzhikov-Vlakh, V.; Shcherbakova, E.; Boroznjak, R.; Tarasenko, I.; Zakharova, N.; Lavrentieva, A.; Skorik, Y.; et al. Polypeptide self-assembled nanoparticles as delivery systems for polymyxins B and E. Pharmaceutics 2020, 12, 868. [Google Scholar] [CrossRef] [PubMed]







| Component | C–V | C=C | C–C | C–OH and C–O–C/C–OH(p) | C=O | COOH | C–N | C/O Ratio |
|---|---|---|---|---|---|---|---|---|
| Binding Energy (eV) | 283.7 | 284.6 | 285.1 | 286.8 / 286.4 | 288.2 | 289.0 | 285.9 | |
| GO | 5.31 | 52.94 | 4.49 | 28.03 | 6.68 | 2.55 | - | 2.51 |
| rGO-Am | <0.1 | 89.05 | 4.93 | 1.5 | <0.6 | <0.9 | 3.02 | 32.51 |
| Component | C–V | C=C | C–C | C–OH and C–O–C/C–OH(p) | C=O | COOH | C–N | C/O Ratio |
|---|---|---|---|---|---|---|---|---|
| Binding Energy (eV) | 283.7 | 284.6 | 285.1 | 286.8 / 286.4 | 288.2 | 289.0 | 285.9 | |
| rGO-Am-oligo(Glu(OBzl)) | 1.93 | 69.55 | 12.12 | 8.01 | 3.39 | 1.48 | 3.52 | 6.87 |
| rGO-Am-oligo(Glu) | <0.1 | 78.79 | 6.77 | 6.48 | 1.65 | 2.84 | 3.47 | 7.38 |
| Sample | DH (nm) | PDI | ζ-potential (mV) |
|---|---|---|---|
| rGO-Am | 465 ± 71 | 0.56 | −38.4 ± 7.1 |
| rGO-Am-oligo(Glu(OBzl)) | 369 ± 66 | 0.42 | −32.1 ± 5.6 |
| rGO-Am-oligo(Glu) | 302 ± 49 | 0.52 | −40.0 ± 5.1 |
| Specimen | Filler Content (%) | E (MPa) | σ b (MPa) | ε b (%) |
|---|---|---|---|---|
| PCL | - | 396 ± 13 | 13.9 ± 0.5 | 25 ± 2 |
| PCL/rGO-Am | 0.5 | 369 ± 43 | 13.6 ± 0.3 | 25 ± 3 |
| 1.0 | 348 ± 32 | 11.4 ± 0.5 | 12 ± 1 | |
| 3.0 | 270 ± 28 | 7.5 ± 0.6 | 10 ± 1 | |
| PCL/rGO-Am-oligo(Glu(OBzl) | 0.5 | 415 ± 20 | 13.5 ± 0.5 | 21 ± 2 |
| 1.0 | 396 ± 32 | 13.1 ± 0.4 | 15 ± 2 | |
| PCL/rGO-Am-oligo(Glu) | 0.5 | 434 ± 39 | 14.8 ± 0.4 | 27 ± 2 |
| 1.0 | 444 ± 17 | 14.6 ± 0.4 | 23 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, M.; Solomakha, O.; Rabchinskii, M.; Averianov, I.; Gofman, I.; Nashchekina, Y.; Antonov, G.; Smirnov, A.; Ber, B.; Nashchekin, A.; et al. Aminated Graphene-Graft-Oligo(Glutamic Acid) /Poly(ε-Caprolactone) Composites: Preparation, Characterization and Biological Evaluation. Polymers 2021, 13, 2628. https://doi.org/10.3390/polym13162628
Stepanova M, Solomakha O, Rabchinskii M, Averianov I, Gofman I, Nashchekina Y, Antonov G, Smirnov A, Ber B, Nashchekin A, et al. Aminated Graphene-Graft-Oligo(Glutamic Acid) /Poly(ε-Caprolactone) Composites: Preparation, Characterization and Biological Evaluation. Polymers. 2021; 13(16):2628. https://doi.org/10.3390/polym13162628
Chicago/Turabian StyleStepanova, Mariia, Olga Solomakha, Maxim Rabchinskii, Ilia Averianov, Iosif Gofman, Yuliya Nashchekina, Grigorii Antonov, Aleksey Smirnov, Boris Ber, Aleksey Nashchekin, and et al. 2021. "Aminated Graphene-Graft-Oligo(Glutamic Acid) /Poly(ε-Caprolactone) Composites: Preparation, Characterization and Biological Evaluation" Polymers 13, no. 16: 2628. https://doi.org/10.3390/polym13162628
APA StyleStepanova, M., Solomakha, O., Rabchinskii, M., Averianov, I., Gofman, I., Nashchekina, Y., Antonov, G., Smirnov, A., Ber, B., Nashchekin, A., & Korzhikova-Vlakh, E. (2021). Aminated Graphene-Graft-Oligo(Glutamic Acid) /Poly(ε-Caprolactone) Composites: Preparation, Characterization and Biological Evaluation. Polymers, 13(16), 2628. https://doi.org/10.3390/polym13162628