Influence of Evaporation Drying on the Porous Properties of Carbon/Carbon Composite Xerogels
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of C/C Composites
2.2. Characterization
3. Results and Discussion
3.1. Morphology of C/C Composite Xerogel
3.2. Porous Properties of C/C Composite Xerogels Dried by Evaporation Drying
4. Summary and Conclusions
- (a)
- The preparation of C/C composite xerogels does not result in a change in their carbon structure compared to the carbon xerogels and the carbon cryogels at a high R/C ratio.
- (b)
- Not only does the CF addition develop the mesopores of C/C composite xerogels, but the CF addition also maintains the mesopore size and the micropore size to a level nearly equivalent to the carbon cryogels under the same RF synthesis conditions by evaporation drying with solvent exchange using TBA.
- (c)
- The mesopore shrinkage of C/C composite xerogels during evaporation drying can be alleviated by CF addition.
- (d)
- Evaporation drying with solvent exchange using TBA can prevent the decrease in micropore volume in the preparation of C/C composite xerogels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Pekala, R.W.; Alviso, C.T.; Kong, F.M.; Hulsey, S.S. Aerogels derived from multifunctional organic monomers. J. Non-Cryst. Solids 1992, 145, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Pekala, R.W.; Alviso, C.T. Carbon aerogels and xerogels. Mater. Res. Soc. Symp. Proc. 1992, 270, 3–14. [Google Scholar] [CrossRef]
- Pekala, R.W.; Schaefer, D.W. Structure of organic aerogels. 1. Morphology and scaling. Macromolecules 1993, 26, 5487–5493. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Mikami, M.; Okazaki, M. Porous structure of organic and carbon aerogels synthesized by sol-gel polycondensation of resorcinol with formaldehyde. Carbon 1997, 35, 791–796. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Araki, T.; Okazaki, M. Control of mesoporous structure of organic and carbon aerogels. Carbon 1998, 36, 1257–1262. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4265. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and Properties of Resorcinol–Formaldehyde Organic and Carbon Gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Preparation of mesoporous carbon by freeze drying. Carbon 1999, 37, 2049–2055. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Influence of freeze-drying conditions on the mesoporosity of organic gels as carbon precursors. Carbon 2000, 38, 1099–1105. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Freeze drying for preparation of aerogel-like carbon. Drying Technol. 2001, 19, 313–324. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishimura, T.; Suzuki, T.; Tamon, H. Control of mesoporosity of carbon gels prepared by sol-gel polycondensation and freeze drying. J. Non-Cryst. Solids 2001, 288, 46–55. [Google Scholar] [CrossRef]
- Mayer, S.T.; Kaschmitter, J.; Pekala, R.W. Method of low pressure and/or evaporative drying of aerogels. U.S. Patent 5420168, 30 May 1995. [Google Scholar]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.-P. Porous carbon xerogels with texture tailored by pH control during sol–gel process. Carbon 2004, 42, 619–628. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A. Effect of synthesis pH on the structure of carbon xerogels. Carbon 1997, 35, 1271–1278. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishimura, T.; Suzuki, T.; Tamon, H. Effect of drying method on mesoporosity of resorcinol-formaldehyde drygel and carbon gel. Dry. Technol. 2001, 19, 1319–1333. [Google Scholar] [CrossRef]
- Hebalkar, N.; Arabale, G.; Sainkar, S.R.; Pradhan, S.D.; Mulla, I.S.; Vijayamohanan, K.; Ayyub, P.; Kulkarni, S.K. Study of correlation of structural and surface properties with electrochemical behaviour in carbon aerogels. J. Mater. Sci. 2005, 40, 3777–3782. [Google Scholar] [CrossRef]
- Matos, I.; Fernandes, S.; Guerreiro, L.; Barata, S.; Ramos, A.M.; Vital, J.; Fonseca, I.M. The effect of surfactants on the porosity of carbon xerogels. Microporous Mesoporous Mater. 2006, 92, 38–46. [Google Scholar] [CrossRef]
- Job, N.; Sabatier, F.; Pirard, J.-P.; Crine, M.; Leonard, A. Towards the production of carbon xerogel monoliths by optimizing convective drying conditions. Carbon 2006, 44, 2534–2542. [Google Scholar] [CrossRef] [Green Version]
- Job, N.; Thery, A.; Pirard, R.; Marien, J.; Kocon, L.; Rouzaud, J.-N.; Beguin, F.; Pirard, J.-P. Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 2005, 43, 2481–2492. [Google Scholar] [CrossRef]
- Wu, D.C.; Fu, R.W.; Zhang, S.T.; Dresselhaus, M.S.; Dresselhaus, G. Preparation of low-density carbon aerogels by ambient pressure drying. Carbon 2004, 42, 2033–2039. [Google Scholar] [CrossRef]
- Wiener, M.; Reichenauer, G.; Scherb, T.; Fricke, J. Accelerating the synthesis of carbon aerogel precursors. J. Non-Cryst. Solids 2004, 350, 126–130. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Arenillas, A.; Dominguez, A.; Menendez, J.A.; Pis, J.J. Development of microporous carbon xerogels by controlling synthesis conditions. J. Non-Cryst. Solids 2008, 354, 817–825. [Google Scholar] [CrossRef]
- Czakkel, O.; Marthi, K.; Geissler, E.; Laszlo, K. Influence of drying on the morphology of resorcinol–formaldehyde-based carbon gels. Microporous Mesoporous Mater. 2005, 86, 124–133. [Google Scholar] [CrossRef]
- Kraiwattanawong, K.; Tamon, H.; Praserthdam, P. Influence of solvent species used in solvent exchange for preparation of mesoporous carbon xerogels from resorcinol and formaldehyde via subcritical drying. Microporous Mesoporous Mater. 2011, 138, 8–16. [Google Scholar] [CrossRef]
- Kraiwattanawong, K.; Sano, N.; Tamon, H. Low-cost production of mesoporous carbon/carbon composite cryogels. Carbon 2011, 49, 3404–3411. [Google Scholar] [CrossRef]
- Kraiwattanawong, K.; Sano, N.; Tamon, H. Carbon tunnels formed in carbon/carbon composite cryogels. Microporous Mesoporous Mater. 2012, 153, 47–54. [Google Scholar] [CrossRef]
- Kraiwattanawong, K.; Sano, N.; Tamon, H. Capacitive performance of binder-free carbon/carbon composite cryogels. Microporous Mesoporous Mater. 2013, 165, 228–233. [Google Scholar] [CrossRef]
- Wang, J.; Glora, M.; Petricevic, R.; Saliger, R.; Proebstle, H.; Fricke, J. Carbon cloth reinforced carbon aerogel films derived from resorcinol formaldehyde. J. Porous Mater. 2001, 8, 159–165. [Google Scholar] [CrossRef]
- Schmitt, C.; Proebstle, H.; Fricke, J. Carbon cloth-reinforced and activated aerogel films for supercapacitors. J. Non-Cryst. Solids 2001, 285, 277–282. [Google Scholar] [CrossRef]
- Fu, R.W.; Zhang, B.; Liu, J.; Weiss, S.; Ying, J.Y.; Dresselhaus, M.S.; Dresselhaus, G.; Satcher, J.H.; Baumann, T.F. Fabrication of activated carbon fibers/carbon aerogels composites by gelation and supercritical drying in isopropanol. J. Mater. Res. 2003, 18, 2765–2773. [Google Scholar] [CrossRef] [Green Version]
- Petricevic, R.; Glora, M.; Fricke, J. Planar fibre reinforced carbon aerogels for application in PEM fuel cells. Carbon 2001, 39, 857–867. [Google Scholar] [CrossRef]
- Rasines, G.; Lavela, P.; Macías, C.; Zafra, M.C.; Tirado, J.L.; Ania, C.O. Mesoporous carbon black-aerogel composites with optimized properties for the electro-assisted removal of sodium chloride from brackish water. J. Electroanal. Chem. 2015, 741, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhang, C.; Feng, J. Carbon fiber reinforced carbon aerogel composites for thermal insulation prepared by soft reinforcement. Mater. Lett. 2012, 67, 266–268. [Google Scholar] [CrossRef]
- Dollimore, D.; Heal, G.R. An improved method for the calculation of pore-size distribution from adsorption data. J. Appl. Chem. 1964, 14, 109–114. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR 1947, 55, 327–329. [Google Scholar]
- Lippens, B.C.; de Boer, J.H. Pore system in catalysts. V: The t-method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Horvath, G.; Kawazoe, K. Method for the calculation of effective pore size distribution in molecular sieve carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef] [Green Version]
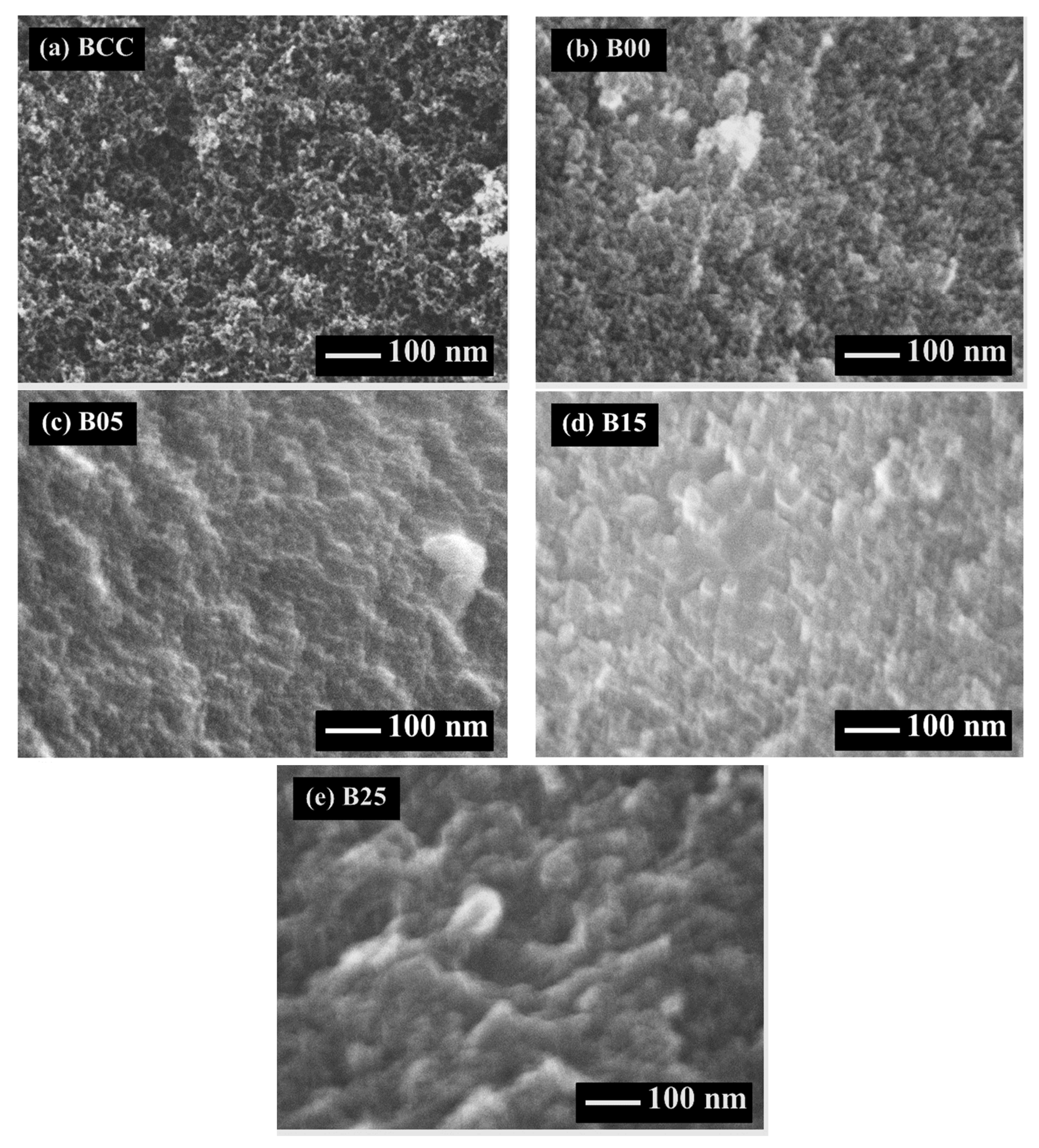

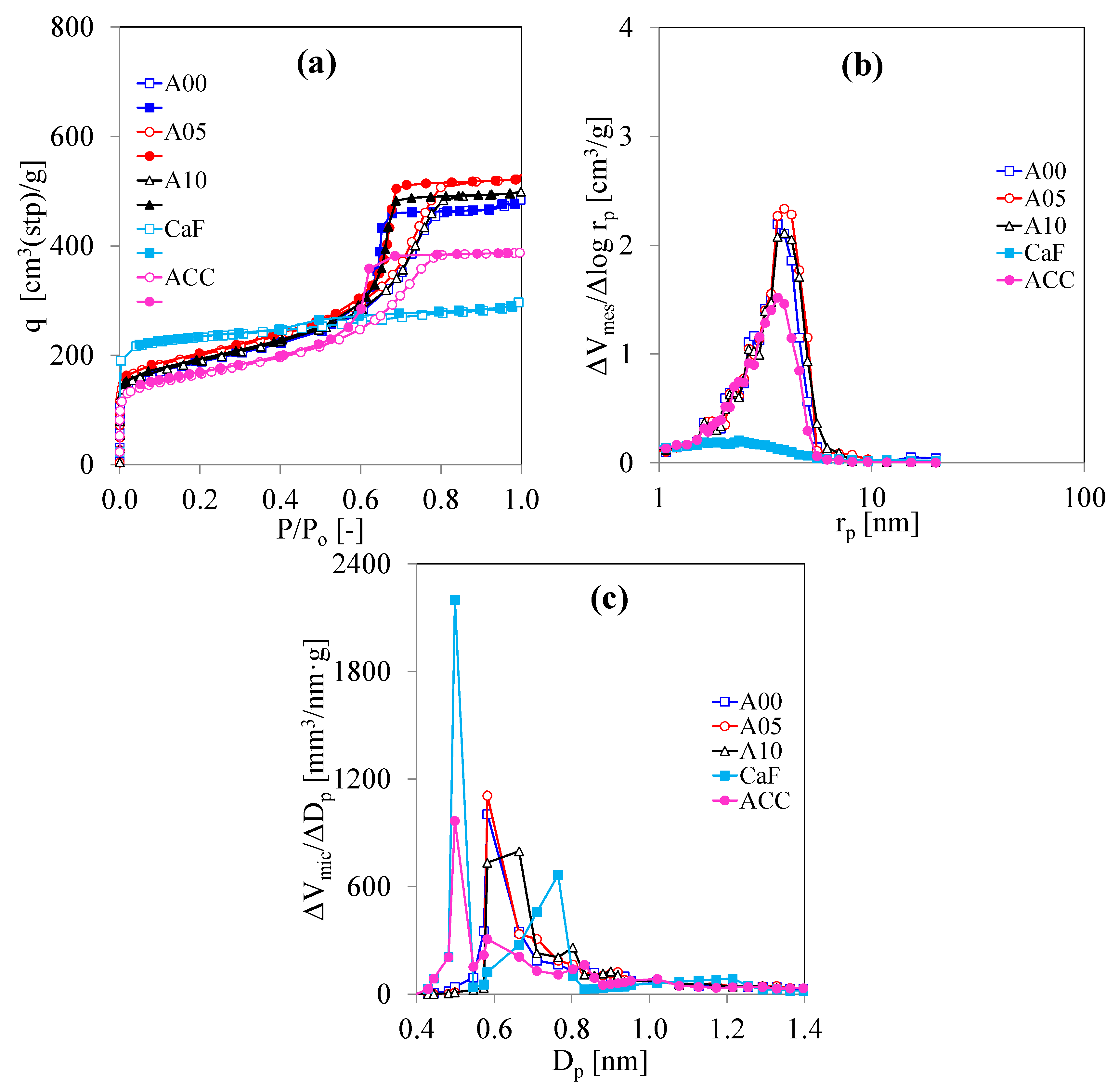
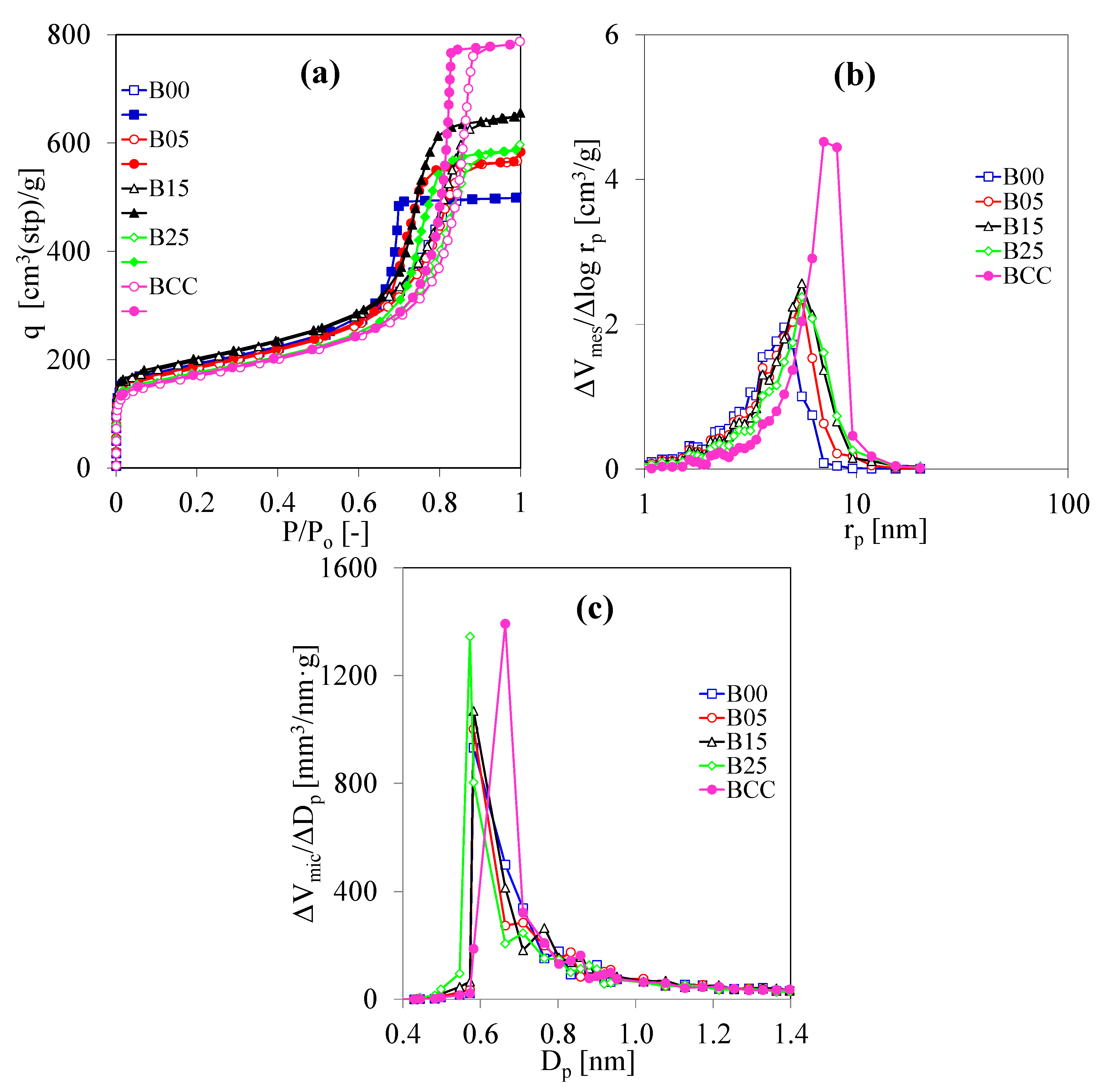

| Symbol | CF/RF (g/g) | R/C (mol/mol) | R/W (g/cm3) | C/W (× 105 mol/cm3) | Drying Method |
|---|---|---|---|---|---|
| A00 | 0.00 | 200 | 0.50 | 250 | Evaporation |
| A05 | 0.05 | 200 | 0.50 | 250 | Evaporation |
| A10 | 0.10 | 200 | 0.50 | 250 | Evaporation |
| ACC | 0.00 | 200 | 0.50 | 250 | Freeze drying |
| B00 | 0.00 | 200 | 0.25 | 125 | Evaporation |
| B05 | 0.05 | 200 | 0.25 | 125 | Evaporation |
| B15 | 0.15 | 200 | 0.25 | 125 | Evaporation |
| B25 | 0.25 | 200 | 0.25 | 125 | Evaporation |
| BCC | 0.00 | 200 | 0.25 | 125 | Freeze drying |
| C00 | 0.00 | 500 | 0.25 | 50 | Evaporation |
| C05 | 0.05 | 500 | 0.25 | 50 | Evaporation |
| C15 | 0.15 | 500 | 0.25 | 50 | Evaporation |
| C25 | 0.25 | 500 | 0.25 | 50 | Evaporation |
| CCC | 0.00 | 500 | 0.25 | 50 | Freeze drying |
| Symbol | SBET (m2/g) | Smic (m2/g) | Vmes (cm3/g) | Vmic (cm3/g) | rmes,peak (nm) | Dmic,peak (nm) |
|---|---|---|---|---|---|---|
| ACC | 590 | 310 | 0.48 | 0.19 | 3.6 | 0.582 |
| A00 | 680 | 360 | 0.60 | 0.22 | 3.6 | 0.582 |
| A05 | 730 | 390 | 0.66 | 0.23 | 3.9 | 0.582 |
| A10 | 680 | 340 | 0.63 | 0.22 | 3.9 | 0.664 |
| BCC | 700 | 350 | 1.04 | 0.22 | 6.2 | 0.664 |
| B00 | 680 | 380 | 0.63 | 0.21 | 4.6 | 0.582 |
| B05 | 660 | 360 | 0.74 | 0.21 | 5.5 | 0.582 |
| B15 | 720 | 390 | 0.85 | 0.23 | 5.5 | 0.582 |
| B25 | 620 | 340 | 0.78 | 0.21 | 5.5 | 0.573 |
| CCC | 590 | 440 | 0.58 | 0.19 | nm | 0.582 |
| C00 | 630 | 500 | 0.87 | 0.21 | >20.02 | 0.582 |
| C05 | 720 | 560 | 0.88 | 0.23 | >20.02 | 0.664 |
| C15 | 590 | 450 | 0.62 | 0.19 | >20.02 | 0.582 |
| C25 | 620 | 490 | 0.57 | 0.20 | >20.02 | 0.498 |
| CaF | 910 | 890 | 0.13 | 0.28 | 2.5 | 0.498 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraiwattanawong, K.; Sano, N.; Tamon, H. Influence of Evaporation Drying on the Porous Properties of Carbon/Carbon Composite Xerogels. Polymers 2021, 13, 2631. https://doi.org/10.3390/polym13162631
Kraiwattanawong K, Sano N, Tamon H. Influence of Evaporation Drying on the Porous Properties of Carbon/Carbon Composite Xerogels. Polymers. 2021; 13(16):2631. https://doi.org/10.3390/polym13162631
Chicago/Turabian StyleKraiwattanawong, Kriangsak, Noriaki Sano, and Hajime Tamon. 2021. "Influence of Evaporation Drying on the Porous Properties of Carbon/Carbon Composite Xerogels" Polymers 13, no. 16: 2631. https://doi.org/10.3390/polym13162631
APA StyleKraiwattanawong, K., Sano, N., & Tamon, H. (2021). Influence of Evaporation Drying on the Porous Properties of Carbon/Carbon Composite Xerogels. Polymers, 13(16), 2631. https://doi.org/10.3390/polym13162631







