The Toxicity of Universal Dental Adhesives: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Universal Dental Adhesives Used in the Study
2.2. Cell Line and Eluate Preparation
2.3. Cytotoxicity Analysis
2.4. Genotoxicity Assessment
2.5. Apoptosis Detection
2.6. Cell Cycle Analysis
2.7. Statistical Analysis
3. Results
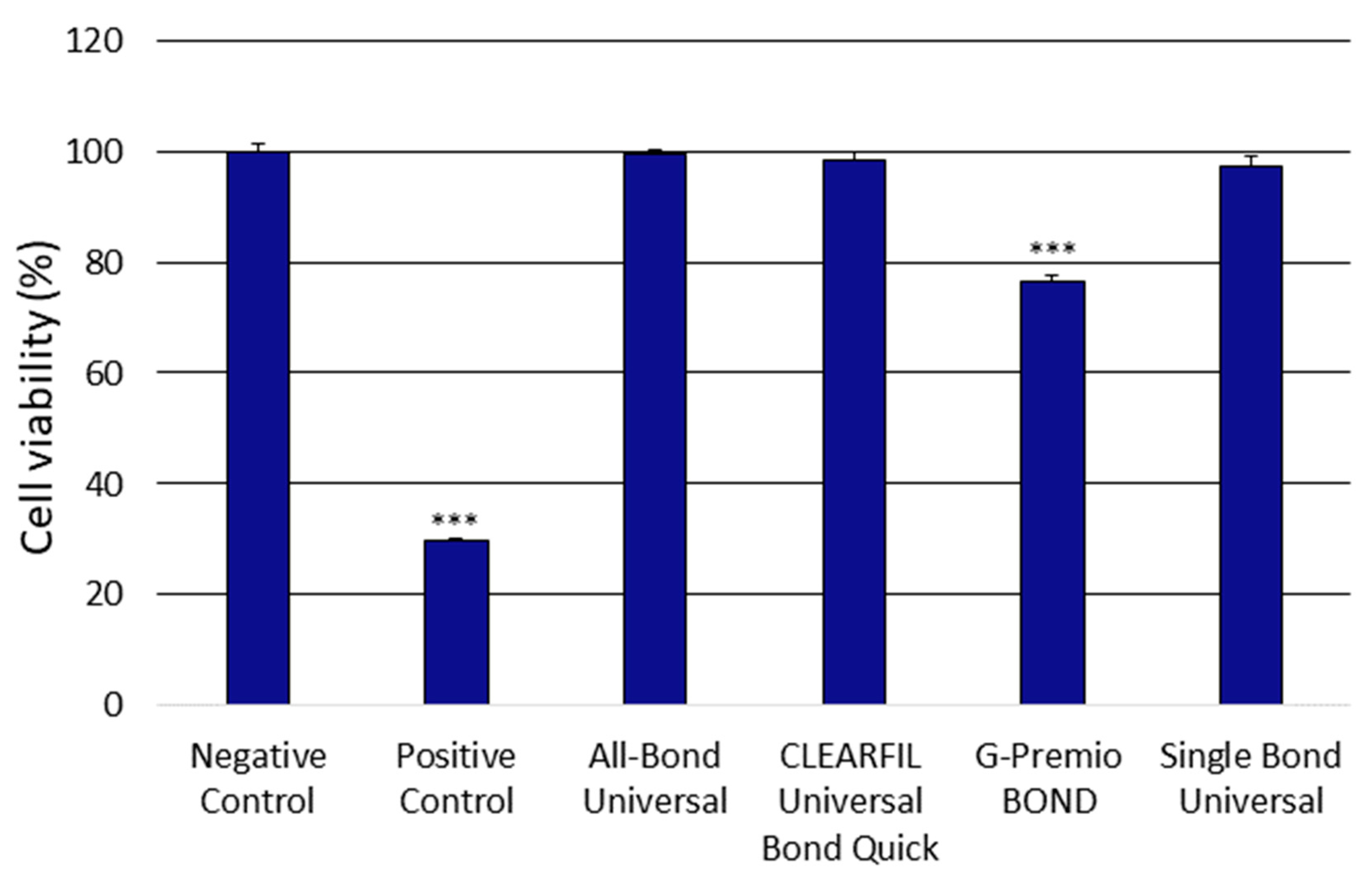
3.1. Analysis of the Cytotoxicity of the Universal Dental Adhesives
3.2. Analysis of the Genotoxicity of the Universal Dental Adhesives
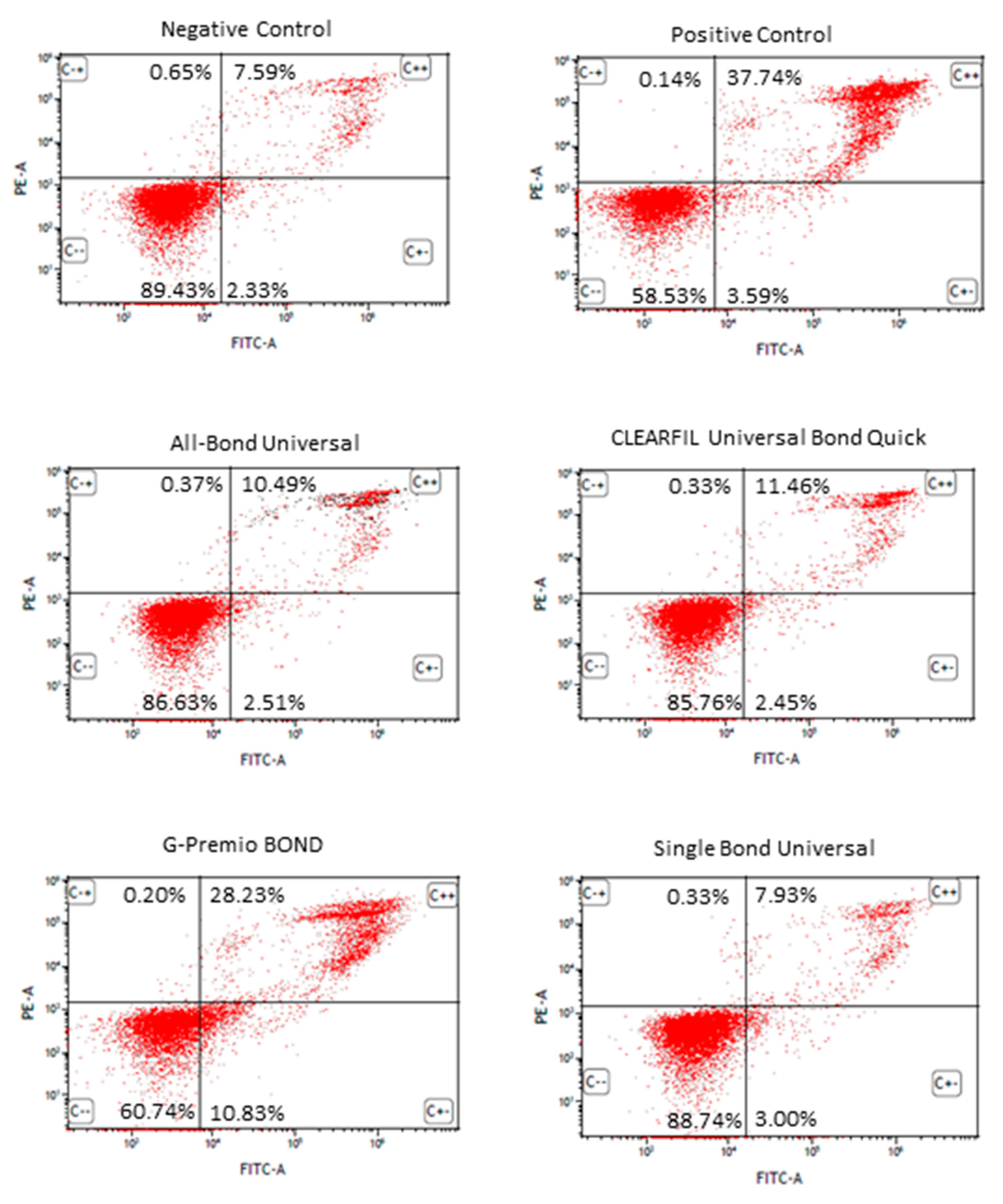
3.3. Apoptosis Detection by FITC Annexin V/PI Double Staining of the Universal Dental Adhesives
3.4. Analysis of the Cell Cycle Progression by PI Staining of the Universal Dental Adhesives
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoniazzi, B.F.; Nicoloso, G.F.; Lenzi, T.L.; Soares, F.Z.M.; de Oliveira Rocha, R. Selective acid etching improves the bond strength of universal adhesive to sound and demineralized enamel of primary teeth. J. Adhes. Dent. 2016, 18, 311–316. [Google Scholar] [CrossRef]
- Da Rosa, W.L.D.O.; Piva, E.; Da Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 9999B, 1–11. [Google Scholar] [CrossRef]
- Alex, G. Universal adhesives: The next evolution in adhesive dentistry? Compend. Contin. Educ. Dent. 2015, 36, 15–26; quiz 28, 40. [Google Scholar]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van Ende, A.; Van Meerbeek, B.; De Munck, J. Bonding effectiveness of a new “multi-mode” adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef]
- Kim, J.H.; Chae, S.Y.; Lee, Y.; Han, G.J.; Cho, B.H. Effects of multipurpose, universal adhesives on resin bonding to zirconia ceramic. Oper. Dent. 2015, 40, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, K.; Kojima, K.; Endo, T.; Kadoma, Y. Effect of the combination of dithiooctanoate monomers and acidic adhesive monomers on adhesion to precious metals, precious metal alloys and non-precious metal alloys. Dent. Mater. J. 2011, 30, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Papadogiannis, D.; Dimitriadi, M.; Zafiropoulou, M.; Gaintantzopoulou, M.D.; Eliades, G. Universal adhesives: Setting characteristics and reactivity with dentin. Materials 2019, 12, 1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdigão, J.; Swift, E.J. Universal Adhesives. J. Esthet. Restor. Dent. 2015, 27, 331–334. [Google Scholar] [CrossRef]
- Sezinando, A.; Perdigão, J.; Ceballos, L. Long-term In Vitro Adhesion of Polyalkenoate-based Adhesives to Dentin. J. Adhes. Dent. 2017, 19, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Sezinando, A.; Serrano, M.L.; Pérez, V.M.; Muñoz, R.G.A.; Ceballos, L.; Perdigão, J. Chemical adhesion of polyalkenoate-based adhesives to hydroxyapatite. J. Adhes. Dent. 2016, 18, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ergün, G.; Eǧilmez, F.; Üçtaşli, M.B.; Yilmaz, Ş. Effect of light curing type on cytotoxicity of dentine-bonding agents. Int. Endod. J. 2007, 40, 216–223. [Google Scholar] [CrossRef]
- Pagano, S.; Lombardo, G.; Balloni, S.; Bodo, M.; Cianetti, S.; Barbati, A.; Montaseri, A.; Marinucci, L. Cytotoxicity of universal dental adhesive systems: Assessment in vitro assays on human gingival fibroblasts. Toxicol. In Vitro 2019, 60, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Zarow, M.; Kharouf, N.; Mancino, D.; Villares, C.F.; Skaba, D.; Lukomska-Szymanska, M. The bond strength and antibacterial activity of the universal dentin bonding system: A systematic review and meta-analysis. Microorganisms 2021, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Eid, A.; Hardan, L.; Bourgi, R.; Arntz, Y.; Jmal, H.; Foschi, F.; Sauro, S.; Ball, V.; Haikel, Y.; et al. Antibacterial and bonding properties of universal adhesive dental polymers doped with pyrogallol. Polymers 2021, 13, 1538. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Brooki, I.M.; Van Noortr, R. Biocompatibility of resin-based dental materials. Materials 2009, 2, 514–548. [Google Scholar] [CrossRef] [Green Version]
- Stanford, J.W. Recommendations for determining biocompatibility and safety for the clinical use of metals in dentistry. Int. Dent. J. 1986, 36, 45–48. [Google Scholar]
- Schmalz, G. Materials science: Biological aspects. J. Dent. Res. 2002, 81, 660–663. [Google Scholar] [CrossRef] [PubMed]
- ISO/EN10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods; ISO: Geneva, Switzerland, 2009; Volume 3, p. 42. [Google Scholar]
- Review of the Threshold of Toxicological Concern (TTC) Approach and Development of New TTC Decision Tree; Wiley: Hoboken, NJ, USA, 2016; Volume 13.
- Schmalz, G.; Galler, K.M. Biocompatibility of biomaterials—Lessons learned and considerations for the design of novel materials. Dent. Mater. 2017, 33, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Ausiello, P.; Cassese, A.; Miele, C.; Beguinot, F.; Garcia-Godoy, F.; Jeso, B.D.; Ulianich, L. Cytotoxicity of dental resin composites: An in vitro evaluation. J. Appl. Toxicol. 2013, 33, 451–457. [Google Scholar] [CrossRef]
- Willershausen, I.; Callaway, A.; Briseño, B.; Willershausen, B. In vitro analysis of the cytotoxicity and the antimicrobial effect of four endodontic sealers. Head Face Med. 2011, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlowska, E.; Poplawski, T.; Ksiazek, D.; Szczepanska, J.; Blasiak, J. Genotoxicity and cytotoxicity of 2-hydroxyethyl methacrylate. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 696, 122–129. [Google Scholar] [CrossRef]
- Lee, Y.; An, S.Y.; Park, Y.J.; Yu, F.H.; Park, J.C.; Seo, D.G. Cytotoxic effects of one-step self-etching adhesives on an odontoblast cell line. Scanning 2016, 38, 36–42. [Google Scholar] [CrossRef]
- Lukomska-Szymanska, M.; Konieczka, M.; Zarzycka, B.; Lapinska, B.; Grzegorczyk, J.; Sokolowski, J. Antibacterial activity of commercial dentine bonding systems against E. faecalis-flow cytometry study. Materials 2017, 10, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapinska, B.; Konieczka, M.; Zarzycka, B.; Sokolowski, K.; Grzegorczyk, J.; Lukomska-Szymanska, M. Flow Cytometry Analysis of Antibacterial Effects of Universal Dentin Bonding Agents on Streptococcus mutans. Molecules 2019, 24, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuncer, S.; Demirci, M.; Schweikl, H.; Erguven, M.; Bilir, A.; Kara Tuncer, A. Inhibition of cell survival, viability and proliferation by dentin adhesives after direct and indirect exposure in vitro. Clin. Oral Investig. 2012, 16, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Ribeiro, A.P.D.; De Oliveira Carrilho, M.R.; Pashley, D.H.; De Souza Costa, C.A.; Hebling, J. Cytotoxicity of adhesive systems of different hydrophilicities on cultured odontoblast-like cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1498–1507. [Google Scholar] [CrossRef]
- Bianchi, L.; Ribeiro, A.P.D.; De Oliveira Carrilho, M.R.; Pashley, D.H.; De Souza Costa, C.A.; Hebling, J. Transdentinal cytotoxicity of experimental adhesive systems of different hydrophilicity applied to ethanol-saturated dentin. Dent. Mater. 2013, 29, 980–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawrzynkiewicz, A.; Rozpedek-Kaminska, W.; Galita, G.; Lukomska-Szymanska, M.; Lapinska, B.; Sokolowski, J.; Majsterek, I. The cytotoxicity and genotoxicity of three dental universal adhesives—An in vitro study. Int. J. Mol. Sci. 2020, 21, 3950. [Google Scholar] [CrossRef]
- Zecin-Deren, A.; Lukomska-Szymanska, M.; Szczesio-Wlodarczyk, A.; Piwonski, I.; Sokolowski, J.; Lapinska, B. The Influence of Application Protocol of Simplified and Universal Adhesives on the Dentin Bonding Performance. Appl. Sci. 2020, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Sideridou, I.D.; Achilias, D.S. Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Volk, J.; Ziemann, C.; Leyhausen, G.; Geurtsen, W. Non-irradiated campherquinone induces DNA damage in human gingival fibroblasts. Dent. Mater. 2009, 25, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef] [PubMed]
- Kusdemir, M.; Gunal, S.; Ozer, F.; Imazato, S.; Izutani, N.; Ebisu, S.; Blatz, M.B. Evaluation of cytotoxic effects of six self-etching adhesives with direct and indirect contact tests. Dent. Mater. J. 2011, 30, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidou, E.A.; Helvatjoglu-Antoniades, M.; Palaghias, G.; Karanika-Kouma, A.; Antoniades, D. Cytotoxicity evaluation of an antibacterial dentin adhesive system on established cell lines. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Chang, Y.C. Cytotoxicity of dentine-bonding agents on human pulp cells in vitro. Int. Endod. J. 2002, 35, 905–909. [Google Scholar] [CrossRef]
- Kostoryz, E.; Eick, J.D.; Chappelow, C.; Glaros, A.; Wetmore, L.; Yourtee, D. In vitro effect of light-cure dental adhesive on IL-6 release from LPS-stimulated and unstimulated macrophages. J. Biomed. Mater. Res. 2003, 65A, 89–94. [Google Scholar] [CrossRef]
- Chang, M.C.; Lin, L.D.; Chuang, F.H.; Chan, C.P.; Wang, T.M.; Lee, J.J.; Jeng, P.Y.; Tseng, W.Y.; Lin, H.J.; Jeng, J.H. Carboxylesterase expression in human dental pulp cells: Role in regulation of BisGMA-induced prostanoid production and cytotoxicity. Acta Biomater. 2012, 8, 1380–1387. [Google Scholar] [CrossRef]
- Engelmann, J.; Janke, V.; Volk, J.; Leyhausen, G.; Von Neuhoff, N.; Schlegelberger, B.; Geurtsen, W. Effects of BisGMA on glutathione metabolism and apoptosis in human gingival fibroblasts in vitro. Biomaterials 2004, 25, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chang, M.C.; Lin, L.D.; Lee, J.J.; Wang, T.M.; Huang, C.H.; Yang, T.T.; Lin, H.J.; Jeng, J.H. The mechanisms of cytotoxicity of urethane dimethacrylate to Chinese hamster ovary cells. Biomaterials 2010, 31, 6917–6925. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, M.C.; Wang, H.H.; Huang, G.F.; Lee, Y.L.; Wang, Y.L.; Chan, C.P.; Yeung, S.Y.; Tseng, S.K.; Jeng, J.H. Urethane dimethacrylate induces cytotoxicity and regulates cyclooxygenase-2, hemeoxygenase and carboxylesterase expression in human dental pulp cells. Acta Biomater. 2014, 10, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Wataha, J.C.; Hanks, C.T.; Ciucchi, B.; Holz, J. In vitro cytotoxicity and dentin permeability of HEMA. J. Endod. 1996, 22, 244–248. [Google Scholar] [CrossRef]
- Chang, H.H.; Guo, M.K.; Kasten, F.H.; Chang, M.C.; Huang, G.F.; Wang, Y.L.; Wang, R.S.; Jeng, J.H. Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials 2005, 26, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Cataldi, A.; di Giacomo, V. HEMA-induced cytotoxicity: Oxidative stress, genotoxicity and apoptosis. Int. Endod. J. 2014, 47, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Park, H.; Lee, S.I.; Kim, S.Y. Effect of the Acidic Dental Resin Monomer 10-methacryloyloxydecyl Dihydrogen Phosphate on Odontoblastic Differentiation of Human Dental Pulp Cells. Basic Clin. Pharmacol. Toxicol. 2015, 117, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Nishida, M.; Imazato, S.; Takahashi, Y.; Ebisu, S.; Ishimoto, T.; Nakano, T.; Yasuda, Y.; Saito, T. The influence of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide on the proliferation, differentiation and mineralization of odontoblast-like cells. Biomaterials 2010, 31, 1518–1532. [Google Scholar] [CrossRef]
- Nakagawa, K.; Saita, M.; Ikeda, T.; Hirota, M.; Park, W.; Chang-Il Lee, M.; Ogawa, T. Biocompatibility of 4-META/MMA-TBB resin used as a dental luting agent. J. Prosthet. Dent. 2015, 114, 114–121. [Google Scholar] [CrossRef]
- Ogawa, T. Biological and biochemical characterization of 4-META/ MMA-TBB resin. J. Dent. Oral Disord. Ther. 2015, 3, 01–07. [Google Scholar] [CrossRef]
- Yasuda, Y.; Inuyama, H.; Maeda, H.; Akamine, A.; NÖr, J.E.; Saito, T. Cytotoxicity of one-step dentin-bonding agents toward dental pulp and odontoblast-like cells. J. Oral Rehabil. 2008, 35, 940–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Landuyt, K.L.; Krifka, S.; Hiller, K.A.; Bolay, C.; Waha, C.; Van Meerbeek, B.; Schmalz, G.; Schweikl, H. Evaluation of cell responses toward adhesives with different photoinitiating systems. Dent. Mater. 2015, 31, 916–927. [Google Scholar] [CrossRef]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, e1–e22. [Google Scholar] [CrossRef] [PubMed]
- Rueggeberg, F.A.; Giannini, M.; Arrais, C.A.G.; Price, R.B.T. Light curing in dentistry and clinical implications: A literature review. Braz. Oral Res. 2017, 31, 64–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruschel, V.C.; Stolf, S.C.; Shibata, S.; Chung, Y.; Boushell, L.W.; Baratieri, L.N.; Walter, R. Three-year clinical evaluation of universal adhesives in non-carious cervical lesions. Am. J. Dent. 2019, 32, 223–228. [Google Scholar]
- Wang, R.; Shi, Y.; Li, T.; Pan, Y.; Cui, Y.; Xia, W. Adhesive interfacial characteristics and the related bonding performance of four self-etching adhesives with different functional monomers applied to dentin. J. Dent. 2017, 62, 72–80. [Google Scholar] [CrossRef] [PubMed]


| Name | Manufacturer | Lot Number | Composition |
|---|---|---|---|
| All-Bond Universal | Bisco, Inc. Schaumburg, IL 60193, USA | 2000000048 | bis-GMA (20–50%), Ethanol (30–50%), 10-MDP (5–25%), HEMA (5–25%) [14] |
| CLEARFIL Universal Bond Quick | Kuraray Europe GmbH, 65795 Hattersheim am Main, Germany | CL0201 | bis-GMA (10–25%), ethanol (10–25%), HEMA (2.5–10%), Other ingredients: 10-MDP, Hydrophilic amide monomers, Colloidal silica, Silane coupling agent, Sodium fluoride, dl-Camphorquinone, Water [27] |
| G-Premio BOND | GC EUROPE, 3001 Leuven, Belgium | 1910251 | ethyl alcohol (35–50%), 2,2’-[(4-methylphenyl)imino]bisethanol (5–10%) Other ingredients: 4-MET, 10-MDP, MDTP [8] |
| Single Bond Universal | 3M ESPE Dental Products, 3M Center, St. Paul, MN 55144-1000, USA | 00305A | bis-GMA (15–25%), HEMA (15–25%), D3MA (5–15%), silane treated silica (5–15%), ethanol (10–15%), water (10–15%), 2-propenoic acid, 2-methyl-, reaction products with 1,10-decanediol and phosphorous oxide (P2O5) (1–10%), copolymer of acrylic and itaconic acid (1–5%), dimethylaminobenzoat(−4) (<2%), (dimethylamino)ethyl methacrylate (<2%), camphorquinone (<2%), methyl ethyl ketone (<2%) [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzynkiewicz, A.; Rozpedek-Kaminska, W.; Galita, G.; Lukomska-Szymanska, M.; Lapinska, B.; Sokolowski, J.; Majsterek, I. The Toxicity of Universal Dental Adhesives: An In Vitro Study. Polymers 2021, 13, 2653. https://doi.org/10.3390/polym13162653
Wawrzynkiewicz A, Rozpedek-Kaminska W, Galita G, Lukomska-Szymanska M, Lapinska B, Sokolowski J, Majsterek I. The Toxicity of Universal Dental Adhesives: An In Vitro Study. Polymers. 2021; 13(16):2653. https://doi.org/10.3390/polym13162653
Chicago/Turabian StyleWawrzynkiewicz, Adam, Wioletta Rozpedek-Kaminska, Grzegorz Galita, Monika Lukomska-Szymanska, Barbara Lapinska, Jerzy Sokolowski, and Ireneusz Majsterek. 2021. "The Toxicity of Universal Dental Adhesives: An In Vitro Study" Polymers 13, no. 16: 2653. https://doi.org/10.3390/polym13162653
APA StyleWawrzynkiewicz, A., Rozpedek-Kaminska, W., Galita, G., Lukomska-Szymanska, M., Lapinska, B., Sokolowski, J., & Majsterek, I. (2021). The Toxicity of Universal Dental Adhesives: An In Vitro Study. Polymers, 13(16), 2653. https://doi.org/10.3390/polym13162653







