Methodological and Conceptual Progresses in Studies on the Latent Tracks in PADC
Abstract
:1. Introduction
1.1. Background and Applications
1.2. One Decade of Methodological and Conceptual Evolution
2. Materials and Methods
2.1. Preparation of PADC Films
2.2. FT-IR Spectra of PADC
3. Results and Discussion
3.1. Removal Cross-Section
3.2. Chemical Damage Parameters
3.3. Critical Dose for Loss of Carbonate Ester
3.4. Layered Structure of Ion Tracks
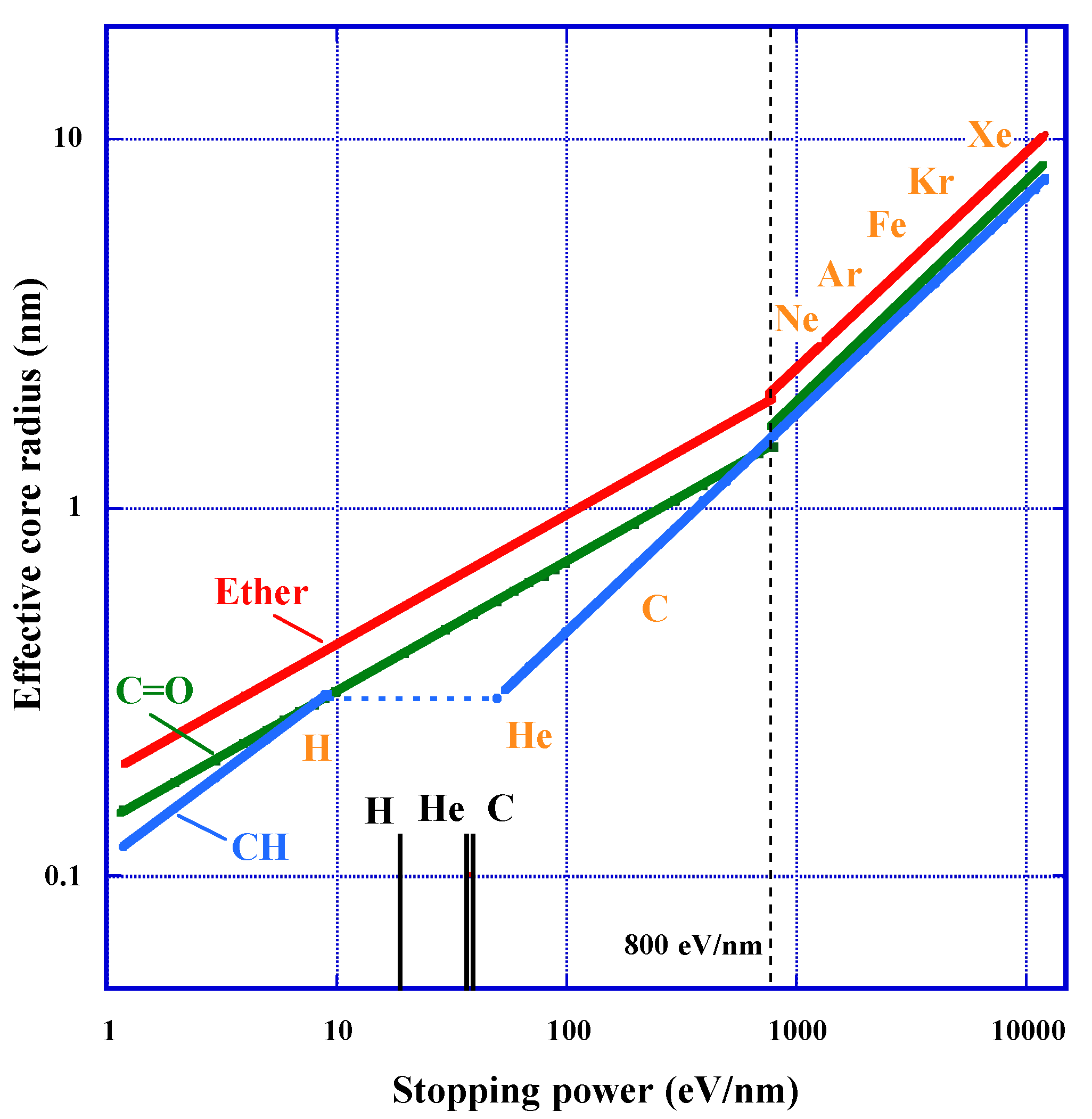
3.5. Effective Track Core Radius and Detection Threshold
3.6. Radial Electron Fluence around Ion Track
3.7. Detection Threshold for C Ion
3.8. Chemical Criterion for Track Registration Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cartwrigte, B.G.; Shirk, E.K.; Price, P.B. A nuclear-track-recording polymer of unique sensitivity and resolution. Nucl. Instrum. Meth. 1978, 41, 447–460. [Google Scholar]
- Guo, S.-L.; Chen, B.-L.; Durrani, S.A. Chapter 3 Solid-state nuclear track detectors. In Handbook of Radioactivity Analysis, 4th ed.; Michael, F.L., Ed.; Academic Press: London, UK, 2020; pp. 154–196. [Google Scholar]
- Zylstra, A.B.; Frenje, J.A.; Séguin, F.H.; Gatu Johnson, M.; Casey, D.T.; Rosenberg, M.J.; Waugh, C.; Sinenian, N.; Manuel, M.J.-E.; Li, C.K.; et al. A new model to account for track overlap in CR-39 data. Nucl. Instrum. Meth. 2012, 68, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Faenov, A.Y.; Tampo, M.; Pikuz, T.A.; Nakamura, T.; Kando, M.; Hayashi, Y.; Yogo, A.; Sakaki, H.; Kameshima, T.; et al. Energy increase in multi-MeV ion acceleration in the interaction of a short pulse laser with a Cluster-Gas target. Phy. Rev. Lett. 2009, 103, 165002. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, M.; Sakaki, H.; Maeda, S.; Sagisaka, A.; Pirozhkov, A.S.; Pikuz, T.; Faenov, A.; Ogura, K.; Kanasaki, M.; Matsukawa, K.; et al. Multi-charged heavy ion acceleration from the ultra-intense short pulse laser system interacting with the metal target. Rev. Sci. Instrum. 2014, 85, 02B904. [Google Scholar] [CrossRef]
- Nishiura, Y.; Inoue, S.; Kojima, S.; Teramoto, K.; Furukawa, Y.; Hashida, M.; Sakabe, S. Detection of alpha particles from 7Li(p,α)4He and 19F(p,α)16O reactions induced by laser-accelerated protons using CR-39 with potassium hydroxide-ethanol-water etching solution. Rev. Sci. Instrum. 2019, 90, 083307. [Google Scholar] [CrossRef]
- Kanasaki, M.; Sakamoto, K.; Asai, T.; JInno, S.; Kodaira, S.; Yamauchi, T.; Oda, K.; Fukuda, Y. Correction method for the energy spectrum of laser-accelerated protons measured by CR-39 track detectors with stepwise energy filters. High. Energy Density Phys. 2020, 37, 100852. [Google Scholar] [CrossRef]
- Benton, E.R.; Benton, E.V.; Frank, A.L. Passive dosimetry aboard the Mir Orbital Station: Internal measurements. Radiat. Meas. 2002, 35, 349–455. [Google Scholar] [CrossRef]
- Naito, M.; Kodaira, S.; Ogawara, R.; Tobita, K.; Someya, Y.; Kusumoto, T.; Kusano, H.; Kitaramura, H.; Koike, M.; Uchihori, Y.; et al. Investigation of shielding material properties for effective space radiation protection. Life Sci. Space Res. 2020, 26, 69–76. [Google Scholar] [CrossRef]
- Inozemtsev, K.O.; Kodaira, S.; Kusumoto, T.; Kitamura, H.; Stradi, A.; Szabo, J.; Ambrozova, I.; Shurshakov, V.A. Etched track detector methods for the identification of target nuclear fragments in cosmic radiation and accelerator proton beams. Radiat. Meas. 2020, 140, 106505. [Google Scholar] [CrossRef]
- Ogawara, R.; Kusumoto, T.; Konishi, T.; Hamano, T.; Kodaira, S. Detection of alpha and 7Li particles from 10B(n,α)7Li reactions using a combination of CR-39 nuclear track detector and potassium hydroxide-ethanol-water solution in accelerator-based neutron fields. Nucl. Instrum. Meth. 2020, 467, 9–12. [Google Scholar] [CrossRef]
- Mori, Y. Structural Modification along Nuclear Tracks of Proton and Heavy Ions in Poly(Allyl Diglycol Carbonate) Detectors. Ph.D. Thesis, Kobe University, Kobe, Japan, 25 March 2014. [Google Scholar]
- Kusumoto, T. Radial Electron Fluence around Ion Tracks as a New Physical Concept for the Detection Threshold of PADC. Ph.D. Thesis, Kobe University, Kobe, Japan, 25 March 2018; The University of Strasbourg, Strasbourg, France, 22 March 2018. [Google Scholar]
- Yamauchi, T.; Barillon, R.; Balanzat, E.; Asuka, T.; Izumi, K.; Masutani, T.; Oda, K. Yields of CO2 formation and scissions at ether bonds along nuclear tracks in CR-39. Radiat. Meas. 2005, 40, 224–228. [Google Scholar] [CrossRef]
- Yamauchi, T.; Mori, Y.; Oda, K.; Yasuda, N.; Kitamura, H.; Barillon, R. Structural modification along heavy ion tracks in poly(ally diglycol carbonate) films. Jpn. J. Appl. Phys. 2008, 47, 3606–3609. [Google Scholar] [CrossRef]
- Yamauchi, T.; Watanabe, S.; Seto, A.; Oda, K.; Yasuda, N.; Barillon, R. Loss of carbonate ester bonds along Fe ion tracks in thin CR-39 films. Radiat. Meas. 2008, 43, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Mori, Y.; Oda, K.; Yasuda, N.; Barillon, R. On the tracks of proton and heavy ions in PC and PADC plastics detectors. In Radiation Detectors and Their Uses (KEK Proceedings), Proceedings of the 24th Workshop on Radiation Detectors and Their Uses, Tsukuba, Japan, 26–28 January 2010; High Energy Accelerator Research Organization (KEK): Tsukuba, Japan, 2010; pp. 1–6. [Google Scholar]
- Mori, Y.; Yamauchi, T.; Kanasaki, M.; Maeda, Y.; Oda, K.; Kodaira, S.; Konishi, T.; Yasuda, N.; Barillon, R. Radiation chemical yields for loss of ether and carbonate ester bonds in PADC films exposed to proton and heavy ion beams. Radiat. Meas. 2011, 46, 1147–1153. [Google Scholar] [CrossRef]
- Yamauchi, T.; Mori, Y.; Morimoto, A.; Kanasaki, M.; Oda, K.; Kodaira, S.; Konishi, T.; Yasuda, N.; Tojo, S.; Honda, Y.; et al. Thresholds of Etchable Track Formation and Chemical Damage Parameters in Poly(allyl diglycol carbonate) films at the stopping powers ranging from 10 to 12,000 keV/µm. Jpn. J. Appl. Phys. 2012, 51, 056301. [Google Scholar]
- Mori, Y.; Yamauchi, T.; Kanasaki, M.; Hattori, A.; Matai, Y.; Matsukawa, K.; Oda, K.; Kodaira, S.; Kitamura, H.; Konishi, T.; et al. Greater radiation chemical yields for losses of ether and carbonate ester bonds at lower stopping powers along heavy ion tracks in poly(allyl diglycol carbonate) films. Appl. Phys. Express 2012, 5, 086401. [Google Scholar] [CrossRef]
- Mori, Y.; Yamauchi, T.; Kanasaki, M.; Hattori, A.; Oda, K.; Kodaira, S.; Konishi, T.; Yasuda, N.; Tojo, S.; Honda, Y.; et al. Vacuum effects on the radiation chemical yields in PADC films exposed to gamma rays and heavy ion. Radiat. Meas. 2013, 50, 97–102. [Google Scholar] [CrossRef]
- Kusumoto, T.; Mori, Y.; Kanasaki, M.; Ueno, T.; Kameda, Y.; Oda, K.; Kodaira, S.; Kitamura, H.; Barillon, R.; Yamauchi, T. Yields on the formation of OH groups and the loss of CH groups along nuclear tracks in PADC films. Radiat. Meas. 2015, 83, 59–62. [Google Scholar] [CrossRef]
- Kusumoto, T.; Mori, Y.; Kanasaki, M.; Ikenaga, R.; Oda, K.; Kodaira, S.; Kitamura, H.; Barillon, R.; Yamauchi, T. Radiation chemical yields for the losses of typical functional groups in PADC films for high energy protons registered as unetchable tracks. Radiat. Meas. 2016, 87, 35–42. [Google Scholar] [CrossRef]
- Mori, Y.; Ikeda, T.; Yamauchi, T.; Sakamoto, A.; Chikada, H.; Honda, Y.; Oda, K. Radiation chemical yield for loss of carbonate ester bonds in PADC films exposed to gamma ray. Radiat. Meas. 2009, 44, 211–213. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, T.; Mori, Y.; Kanasaki, M.; Oda, K.; Kodaira, S.; Honda, Y.; Tojo, S.; Barillon, R.; Yamauchi, T. Sudden increase of the radiation chemical yield for loss of carbonate ester in PADC detector where the track overlapping of 28 MeV electrons becomes significant. In JPS Conference Proceedings, Proceedings of the International Symposium on Radiation Detectors and Their Uses (ISRD2016), Tsukuba, Japan, 18–21 January 2016; High Energy Accelerator Research Organization (KEK): Tsukuba, Japan, 2018; Volume 11, p. 010001. [Google Scholar]
- Kusumoto, T.; Okada, S.; Kurashige, H.; Kobayashi, K.; Fromm, M.; Raffy, Q.; Ludwig, N.; Kanasaki, M.; Oda, K.; Honda, Y.; et al. Evidence for a critical dose above which damage to carbonate ester bonds in PADC appear after gamma ray and ultra soft X-ray exposures. Radiat. Phys. Chem. 2020, 170, 108628. [Google Scholar] [CrossRef]
- Kusumoto, T.; Mori, Y.; Kanasaki, M.; Oda, K.; Kodaira, S.; Barillon, R.; Yamauchi, T. Drastic decrease of carbonyl group after the loss of ether in PADC exposed to 222 nm UV photons. Radiat. Phys. Chem. 2019, 157, 60–64. [Google Scholar] [CrossRef]
- El-Shahawy, M.; Hussein, A.; Tawansi, A. CR-39 as a gamma dosimeter: Dielectric and infrared studies. J. Mater. Sci. 1992, 27, 6605–6608. [Google Scholar] [CrossRef]
- Chong, C.S.; Ishak, I.; Mahat, R.H.; Amin, Y.M. UV-vis and FT-IR spectral studies of CR-39 plastics irradiated with X-rays. Radiat. Meas. 1997, 28, 119–122. [Google Scholar] [CrossRef]
- Saad, A.F.; Atwa, S.T.; Yasuda, N.; Fujii, M. FT-IR spectroscopy of carbon dioxide in CR-39 and SR-90 track detectors irradiated with ions and gamma-rays at different energies and fluences. Radiat. Meas. 2001, 34, 51–54. [Google Scholar] [CrossRef]
- Malek, M.A.; Chong, C.S. Generation of CO2 in γ-ray-irradiated CR-39 plastic. Radiat. Meas. 2002, 35, 109–112. [Google Scholar] [CrossRef]
- Rickards, J.; Zironi, E.P.; Andrade, E. Gas ejection from CR-39 under ion bombardment. Radiat. Eff. Defects Solids 1992, 124, 383–389. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nakai, H.; Somaki, Y.; Oda, K. Formation of CO2 gas and OH groups in CR-39 plastics due to gamma-ray and ions irradiation. Radiat. Meas. 2003, 36, 119–122. [Google Scholar] [CrossRef]
- Hassan, N.M.; Matai, Y.; Kusumoto, T.; Mori, Y.; Kanasaki, M.; Oda, K.; Kitamura, H.; Konishi, K.; Kodaira, S.; Yasuda, N.; et al. On the mechanism of the sensitization of PADC (Poly(allyl diglycol carbonate)) track detectors by carbon dioxide treatment. Radiat. Meas. 2013, 59, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Waligórski, M.P.R.; Hamm, R.N.; Katz, R. The radial distribution of dose around the path of a heavy ion in liquid water. R Nucl. Tracks Radiat. Meas. 1986, 11, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Barillon, R.; Mori, Y.; Kanasaki, M.; Yamauchi, T.; Yasuda, N. Chemical cross sections induced by ions in solid organic detectors: Experimentation and simulation. Radiat. Meas. 2013, 50, 38–42. [Google Scholar] [CrossRef]
- Barillon, R.; Yamauchi, T.; Mori, Y.; Raffy, Q. A first attempt to simulate oxidization effects on latent track structure in PADC combining the radial dose theory and a radio-oxidation kinetic model. Radiat. Meas. 2015, 83, 1–4. [Google Scholar] [CrossRef]
- Incerti, S.; Psaltaki, M.; Gillet, P.; Barberet, P.; Bardiés, M.; Bernal, M.A.; Bordage, M.-C.; Breton, V.; Davidkova, M.; Delage, E.; et al. The Geant4-DNA Collaboration. Simulating radial dose of ion tracks in liquid water simulated with Geant4-DNA: A comparative study. Nucl. Instrum. Meth. 2014, 333, 92–98. [Google Scholar] [CrossRef]
- Kusumoto, T.; EL Bitar, Z.; Okada, S.; Gillet, P.; Arbor, N.; Kanasaki, M.; Mori, Y.; Oda, K.; Nourreddine, A.-M.; Kurashige, H.; et al. Radial electron fluence around ion tracks as a new physical parameter for the detection threshold of PADC using Geant4-DNA toolkit. Radiat. Meas. 2018, 118, 50–53. [Google Scholar] [CrossRef]
- Kusumoto, T.; Barillon, R.; Okada, S.; Yamauchi, T.; Kodaira, S. Improved criterion of the mechanism for forming latent tracks in poly(allyl diglycol carbonate) based on the number of interactions induced by secondary electrons. Radiat. Meas. 2020, 138, 106445. [Google Scholar] [CrossRef]
- Benton, E.V.; Nix, W.D. The restricted energy loss criterion for registration of charged particle in plastics. Nucl. Instrum. Meth. 1969, 67, 343–347. [Google Scholar] [CrossRef]
- Yamauchi, T. Studies on the nuclear tracks in CR-39 plastics. Radiat. Meas. 2003, 36, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Fromm, M.; Kodaira, S.; Kusumoto, T.; Barillon, R.; Yamauchi, T. Role of intermediate species in the formation of ion tracks in PADC: A review. Polym. Degrad. Stabil. 2019, 161, 213–224. [Google Scholar] [CrossRef]
- Stejny, J. The polymer physics of CR-39—The state of understanding. Radiat. Prot. Dosim. 1987, 20, 31–36. [Google Scholar] [CrossRef]
- Darraud, C.; Bennamane, B.; Gagnadre, C.; Decossas, J.L.; Vareille, J.C. Optical modifications of polymers by ion beam irradiation. Polymer 1994, 35, 2447–2451. [Google Scholar] [CrossRef]
- Lounis-Mokrani, Z.; Fromm, M.; Barillon, R.; Chambaudet, A.; Allab, M. Characterization of chemical and optical modifications induced by 22.5 MeV proton beams in CR-39 detectors. Radiat. Meas. 2003, 36, 615–620. [Google Scholar] [CrossRef]
- Malek, M.A.; Chong, C.S. FTIR study of H2O in polyallyl diglycol carbonate. Vib. Spectrosc. 2000, 24, 181–184. [Google Scholar] [CrossRef]
- Ziegler, J.F. SRIM-2003. Nucl. Instrum. Meth. 2004, 219–220, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kusumoto., T.; Ueno, T.; Mori, Y.; Kanasaki, M.; Oda, K.; Kodaira, S.; Barillon, R. Distinct step-like changes in G values for the losses of typical functional groups in poly(ethylene terephthalate) along boron ion tracks around the detection threshold. Radiat. Meas. 2018, 116, 51–54. [Google Scholar]
- Salehpour, M.; Hakansson, P.; Sundqvist, B. Damage cross sections for fast heavy ion induced desorption of biomolecules. Nucl. Instrum. Meth. 1984, 219–220, 752–756. [Google Scholar] [CrossRef]
- Seki, S.; Tsukuda, S.; Maeda, K.; Matsui, Y.; Saeki, A.; Tagawa, S. Inhomogeneous distribution of crosslinks in ion tracks in polystyrene and polysilanes. Phys. Rev. 2004, 70, 144203. [Google Scholar] [CrossRef]
- Yamauchi, T.; Ichijo, H.; Oda, K. Gamma-ray and ion irradiation effects on the optical property of CR-39 detector and their latent track size. In Proceedings of the First International Symposium on Supercritical Water-cooled Reactor, Designed Technology (SCR-2000), Tokyo, Japan, 6–9 November 2000; The University of Tokyo: Tokyo, Japan, 2010; Volume 407, pp. 274–287. [Google Scholar]
- Kusumoto, T.; Ngono-Ravache, Y.; Balanzat, B.; Galindo, C.; Ludwig, N.; Raffy, Q.; Yamauchi, T.; Kodaira, S.; Barillon, R. The role of molecular and radical mobility in the creation of CO2 molecules and OH groups in PADC irradiated with C and O ions. Polym. Degrad. Stabil. 2019, 164, 102–108. [Google Scholar] [CrossRef]
- Barillon, R.; Yamauchi, T. Chemical bond scission induced by 1H+, 16O+8 and γ-rays in a cellulose nitrate detector. Nucl. Instrum. Meth. 2003, 208, 336–339. [Google Scholar] [CrossRef]
- Barillon, R. Track etch velocity and chemical damages induced by ions in a cellulose nitrate detector. Radiat. Meas. 2005, 40, 214–217. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yasuda, N.; Asuka, T.; Izumi, K.; Masutani, T.; Oda, K.; Barillon, R. Track core size estimation for heavy ions in CR-39 by AFM and UV methods. Nucl. Instrum. Meth. 2005, 236, 318–322. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Matsukawa, K.; Mori, Y.; Kanasaki, M.; Hattori, A.; Matai, Y.; Kusumoto, T.; Tao, A.; Oda, K.; Kodaira, S.; et al. Applicability of polyimide films as etched-track detectors for ultra-heavy cosmic ray components. Appl. Phys. Express 2013, 6, 04601. [Google Scholar] [CrossRef]
- Kusumoto, T.; Sakai, M.; Yoshida, A.; Kambara, T.; Yanagisawa, Y.; Kodaira, S.; Oda, K.; Kanasaki, M.; Kuraoka, K.; Barillon, R.; et al. An update of radial dose distribution theory for the detection threshold of Kapton as a nuclear track detector irradiated with 345 MeV/u U and other heavy ions. Nucl. Instrum. Meth. 2019, 460, 240–243. [Google Scholar] [CrossRef]
- Kusumoto, T.; Barillon, R.; Yamauchi, T. Application of Radial Electron Fluence around ion tracks for the description of track response data of polyethylene terephthalate as a polymeric nuclear track detector. Nucl. Instrum. Meth. 2019, 461, 260–266. [Google Scholar] [CrossRef]
- Ludwig, N.; Kusumoto, T.; Galindo, C.; Peaupardin, P.; Pin, S.; Renault, J.-P.; Muller, D.; Yamauchi, T.; Kodaira, S.; Barillon, R.; et al. Radiolysis of phenylalanine in solution with Bragg-Peak energy protons. Radiat. Meas. 2018, 116, 55–59. [Google Scholar] [CrossRef]
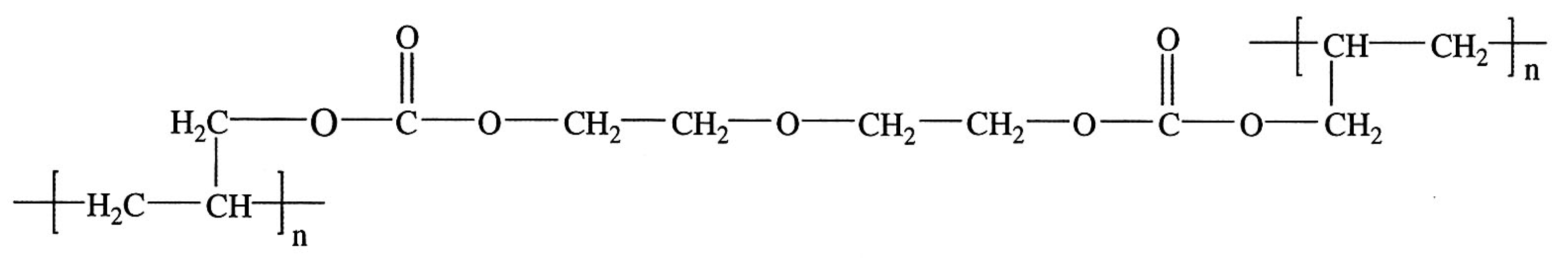







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, T.; Kanasaki, M.; Barillon, R. Methodological and Conceptual Progresses in Studies on the Latent Tracks in PADC. Polymers 2021, 13, 2665. https://doi.org/10.3390/polym13162665
Yamauchi T, Kanasaki M, Barillon R. Methodological and Conceptual Progresses in Studies on the Latent Tracks in PADC. Polymers. 2021; 13(16):2665. https://doi.org/10.3390/polym13162665
Chicago/Turabian StyleYamauchi, Tomoya, Masato Kanasaki, and Rémi Barillon. 2021. "Methodological and Conceptual Progresses in Studies on the Latent Tracks in PADC" Polymers 13, no. 16: 2665. https://doi.org/10.3390/polym13162665
APA StyleYamauchi, T., Kanasaki, M., & Barillon, R. (2021). Methodological and Conceptual Progresses in Studies on the Latent Tracks in PADC. Polymers, 13(16), 2665. https://doi.org/10.3390/polym13162665






