Targeted Gold Nanohybrids Functionalized with Folate-Hydrophobic-Quaternized Pullulan Delivering Camptothecin for Enhancing Hydrophobic Anticancer Drug Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CPT-GNHs@FHQ-PUL
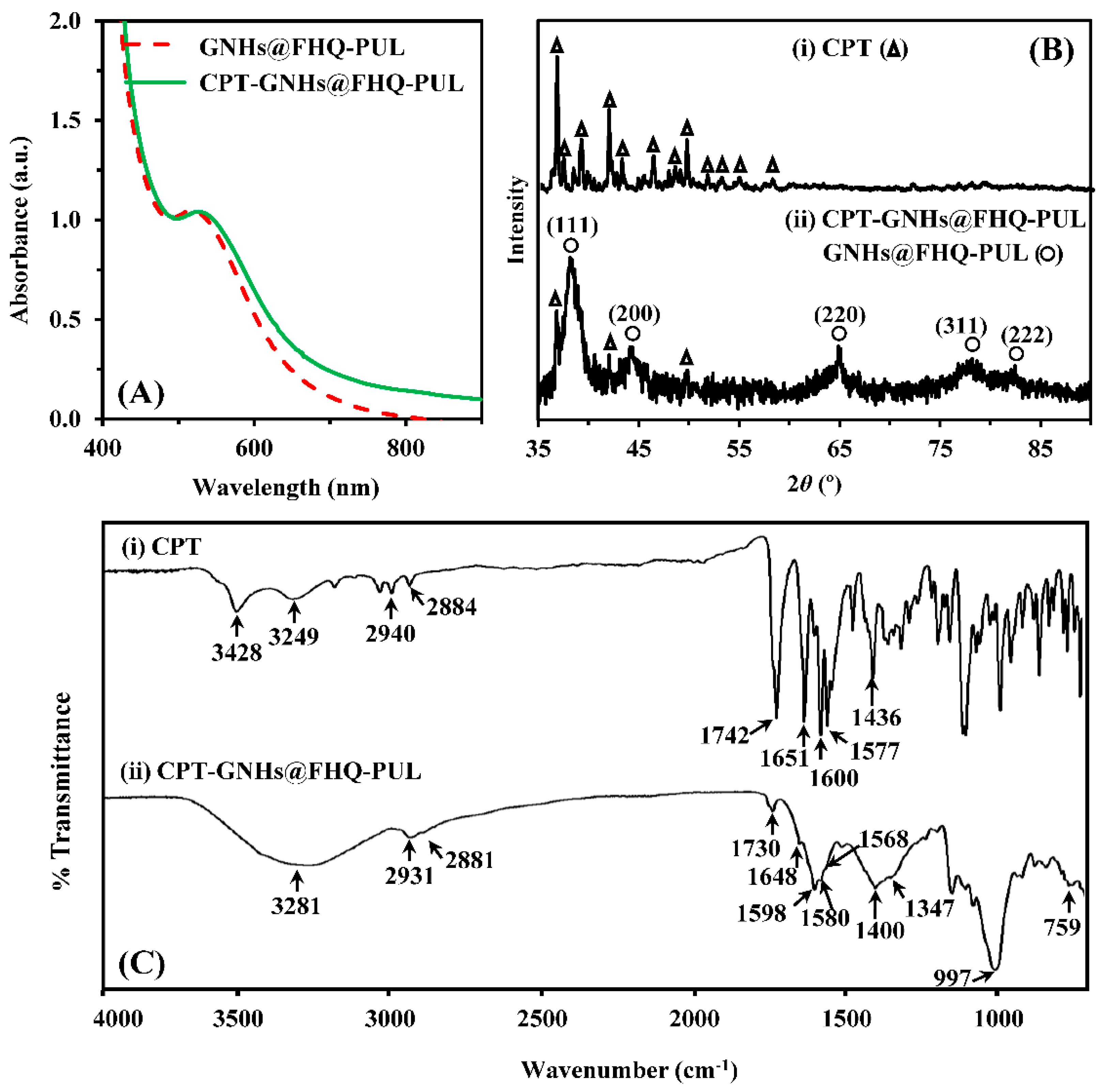
2.3. Characterization of CPT-GNHs@FHQ-PUL
2.4. Cell Culture
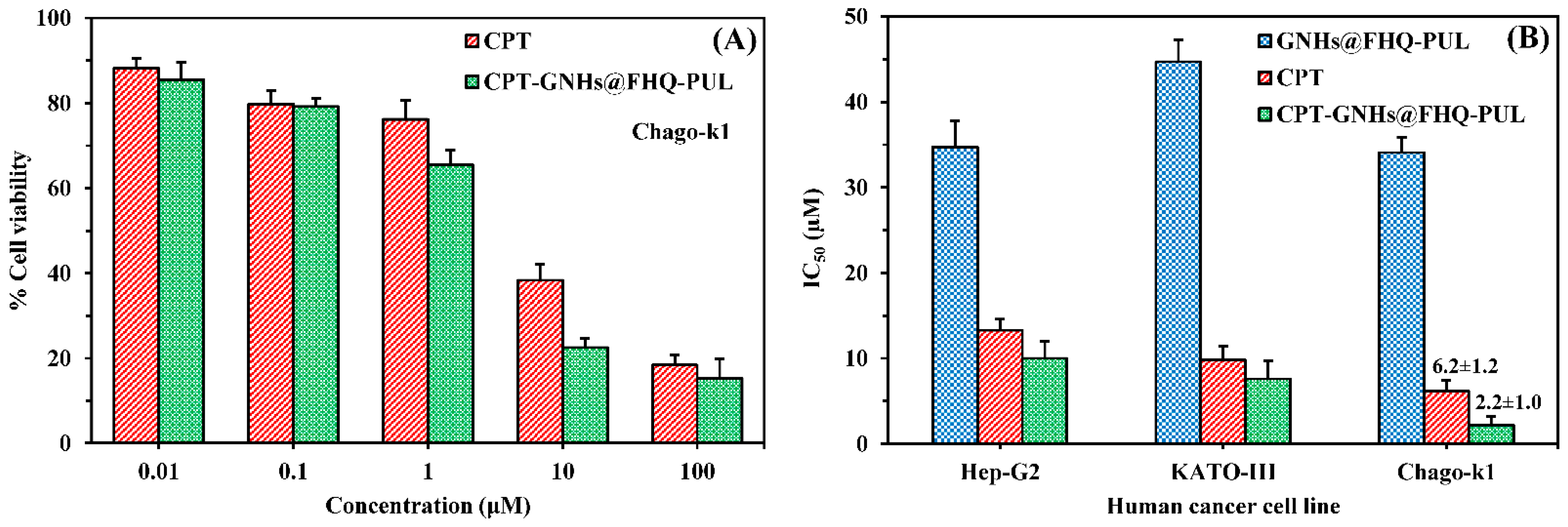
2.5. Cytotoxicity Study
2.6. Colony Formation Assay
2.7. Intracellular Uptake Study
2.8. Confocal Microscopy
2.9. In Vitro Release Behaviors of CPT-GNHs@FHQ-PUL
2.10. Cell Cycle Analysis
2.11. Apoptosis Assay
3. Results and Discussion
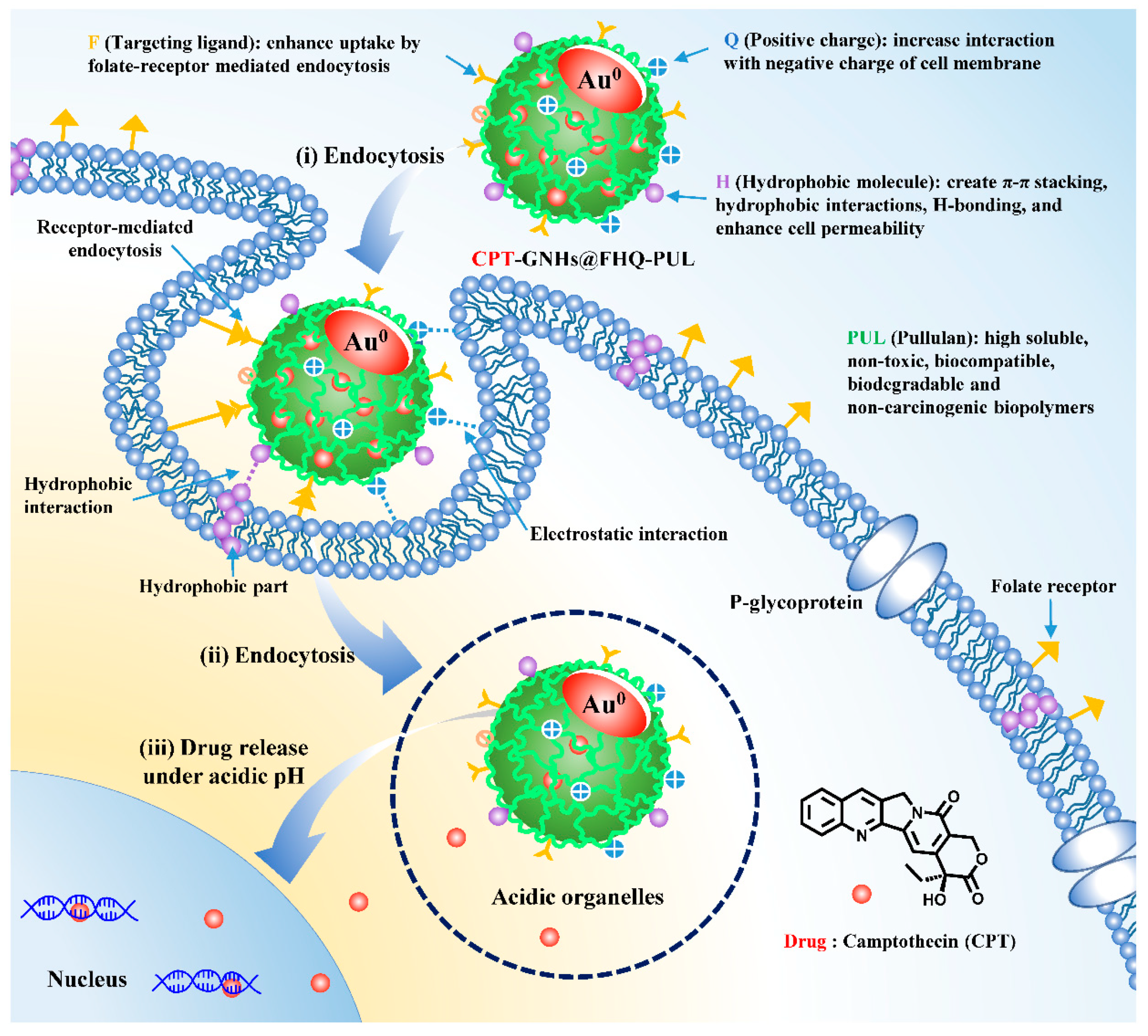
3.1. Preparation and Characterization of CPT-GNHs@FHQ-PUL
3.2. Cytotoxicity Study
3.3. Cell Morphology and Colony Formation Assay
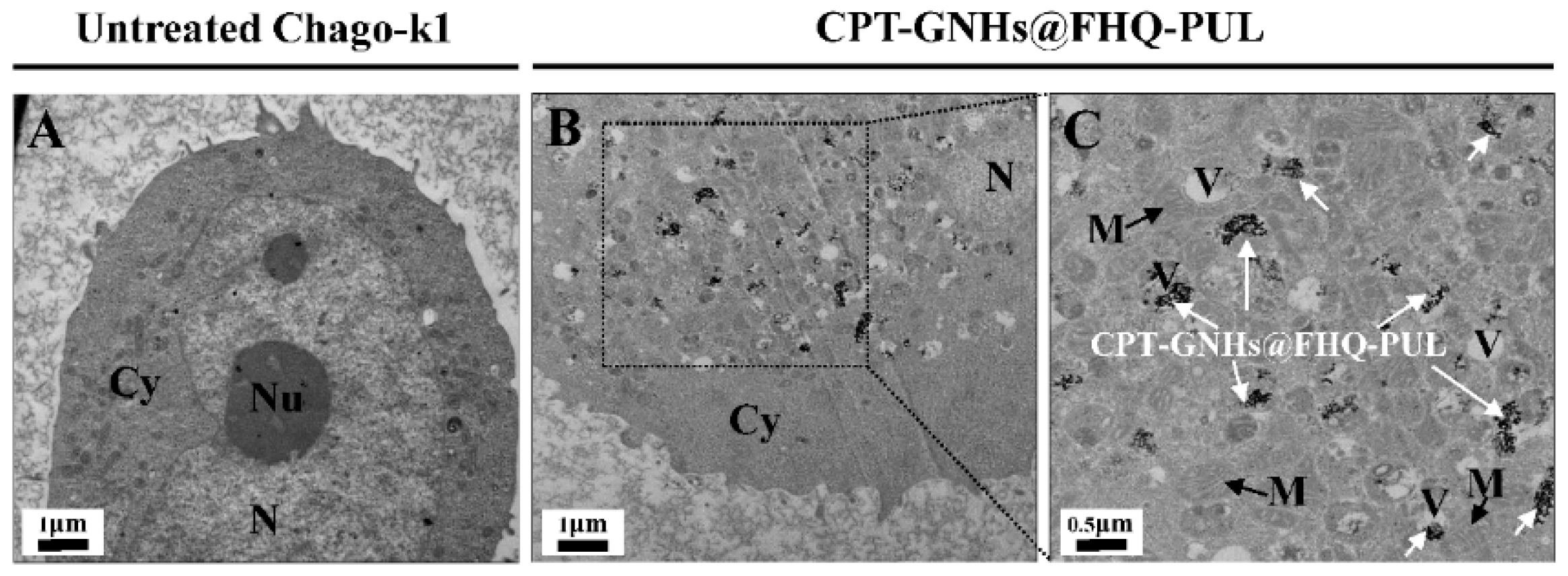
3.4. Intracellular Uptake Study
3.5. Confocal Microscopy
3.6. The Release Behaviors of CPT-GNHs@FHQ-PUL
3.7. Cell Cycle Analysis
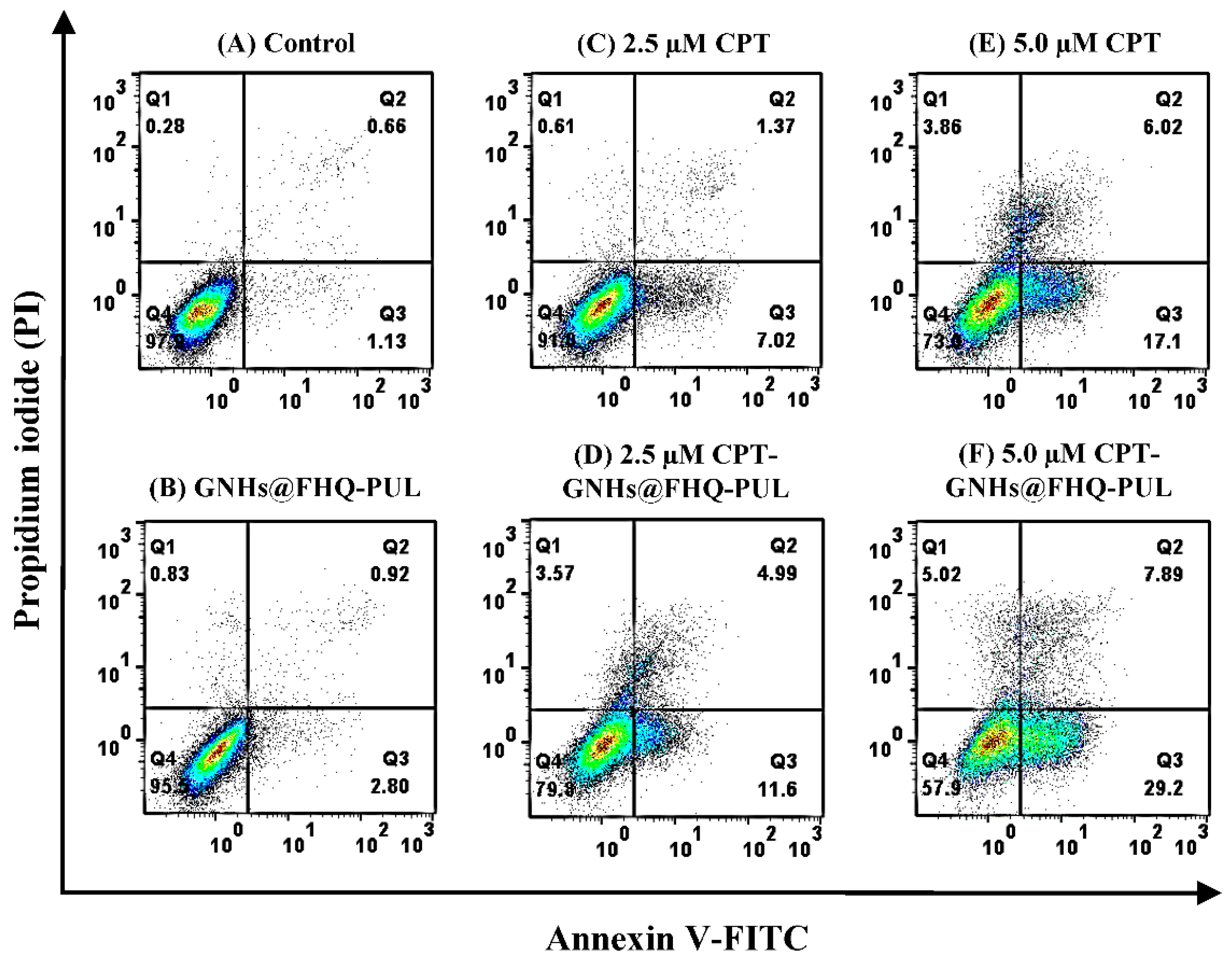
3.8. Apoptosis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 2017, 17, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.C.; de Sousa, M.; Maia, D.L.S.; Visani de Luna, L.; Alves, O.L. Understanding the driving forces of camptothecin interactions on the surface of nanocomposites based on graphene oxide decorated with silica nanoparticles. Nanoscale Adv. 2020, 2, 1290–1300. [Google Scholar] [CrossRef]
- Madhusudhan, A.; Reddy, G.; Venkatesham, M.; Veerabhadram, G.; Kumar, D.; Natarajan, S.; Yang, M.-Y.; Hu, A.; Singh, S. Efficient pH Dependent Drug Delivery to Target Cancer Cells by Gold Nanoparticles Capped with Carboxymethyl Chitosan. Int. J. Mol. Sci. 2014, 15, 8216–8234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delshadi, R.; Bahrami, A.; McClements, D.J.; Moore, M.D.; Williams, L. Development of nanoparticle-delivery systems for antiviral agents: A review. J. Control. Release 2021, 331, 30–44. [Google Scholar] [CrossRef]
- Landgraf, M.; Lahr, C.A.; Kaur, I.; Shafiee, A.; Sanchez-Herrero, A.; Janowicz, P.W.; Ravichandran, A.; Howard, C.B.; Cifuentes-Rius, A.; McGovern, J.A.; et al. Targeted camptothecin delivery via silicon nanoparticles reduces breast cancer metastasis. Biomaterials 2020, 240, 119791. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-C.; Cabral, H.; Mi, P.; Toh, K.; Matsumoto, Y.; Liu, X.; Koori, H.; Kim, A.; Miyazaki, K.; Miura, Y.; et al. Light-Induced Cytosolic Activation of Reduction-Sensitive Camptothecin-Loaded Polymeric Micelles for Spatiotemporally Controlled in Vivo Chemotherapy. ACS Nano 2014, 8, 11591–11602. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Taki, H.; Okada, H. Nose-to-brain drug delivery system with ligand/cell-penetrating peptide-modified polymeric nano-micelles for intracerebral gliomas. Eur. J. Pharm. Biopharm. 2020, 152, 85–94. [Google Scholar] [CrossRef]
- Bai, S.; Jia, D.; Ma, X.; Liang, M.; Xue, P.; Kang, Y.; Xu, Z. Cylindrical polymer brushes-anisotropic unimolecular micelle drug delivery system for enhancing the effectiveness of chemotherapy. Bioact. Mater. 2021, 6, 2894–2904. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Huang, R.; Cang, H.; Cai, Z.; Sun, B. pH-sensitive prodrug conjugated polydopamine for NIR-triggered synergistic chemo-photothermal therapy. Eur. J. Pharm. Biopharm. 2018, 128, 260–271. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Pan, G.; Wang, P.; Li, Y.; Zhu, X.; Zhang, C. Injectable Drug-Conjugated DNA Hydrogel for Local Chemotherapy to Prevent Tumor Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 21441–21449. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, W.; Xu, Y.; Zhu, S.; Wu, Y.; Chen, T.; Xiao, Y.; Lu, W.; Zhang, X.; Yu, J. Dynamic core crosslinked camptothecin prodrug micelles with reduction sensitivity and boronic acid-mediated enhanced endocytosis: An intelligent tumor-targeted delivery nanoplatform. Int. J. Pharm. 2020, 580, 119250. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ying, X.; Su, Y.; Jin, X.; Xu, Q.; Li, Y. Kinetically-stable small-molecule prodrug nanoassemblies for cancer chemotherapy. Int. J. Pharm. 2021, 597, 120369. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Z.; Zhao, Z.; Li, Q.; Wu, Y.; Yan, S.; Shen, Y.; Huang, P. Enzyme-Triggered Transcytosis of Dendrimer–Drug Conjugate for Deep Penetration into Pancreatic Tumors. ACS Nano 2020, 14, 4890–4904. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Kaceli, T.; Mondal, A.; Farzaei, M.H.; Bishayee, A. Recent Advances in Improved Anticancer Efficacies of Camptothecin Nano-Formulations: A Systematic Review. Biomedicines 2021, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Liu, Z.; Zu, Y.; Fu, Y.; Zhao, C.; Zhao, X.; Meng, R.; Tan, S. Synthesis of camptothecin-loaded gold nanomaterials. Appl. Surf. Sci. 2010, 256, 3917–3920. [Google Scholar] [CrossRef]
- Han, H.; Davis, M.E. Single-Antibody, Targeted Nanoparticle Delivery of Camptothecin. Mol. Pharm. 2013, 10, 2558–2567. [Google Scholar] [CrossRef] [Green Version]
- Marinescu, L.; Ficai, D.; Oprea, O.; Marin, A.; Ficai, A.; Andronescu, E.; Holban, A.-M. Optimized Synthesis Approaches of Metal Nanoparticles with Antimicrobial Applications. J. Nanomater. 2020, 2020, 6651207. [Google Scholar] [CrossRef]
- Nurakhmetova, Z.A.; Azhkeyeva, A.N.; Klassen, I.A.; Tatykhanova, G.S. Synthesis and Stabilization of Gold Nanoparticles Using Water-Soluble Synthetic and Natural Polymers. Polymers 2020, 12, 2625. [Google Scholar] [CrossRef]
- Cyganowski, P.; Dzimitrowicz, A.; Jamroz, P.; Jermakowicz-Bartkowiak, D.; Pohl, P. Polymerization-Driven Immobilization of dc-APGD Synthesized Gold Nanoparticles into a Quaternary Ammonium-Based Hydrogel Resulting in a Polymeric Nanocomposite with Heat-Transfer Applications. Polymers 2018, 10, 377. [Google Scholar] [CrossRef] [Green Version]
- Fuller, M.; Kӧper, I. Polyelectrolyte-Coated Gold Nanoparticles: The Effect of Salt and Polyelectrolyte Concentration on Colloidal Stability. Polymers 2018, 10, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Yang, X. Synthesis of Chitosan-Stabilized Gold Nanoparticles in the Absence/Presence of Tripolyphosphate. Biomacromolecules 2004, 5, 2340–2346. [Google Scholar] [CrossRef]
- Laksee, S.; Sansanaphongpricha, K.; Puthong, S.; Sangphech, N.; Palaga, T.; Muangsin, N. New organic/inorganic nanohybrids of targeted pullulan derivative/gold nanoparticles for effective drug delivery systems. Int. J. Biol. Macromol. 2020, 162, 561–577. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef]
- Tavakoli Naeini, A.; Adeli, M.; Vossoughi, M. Synthesis of gold nanoparticle necklaces using linear—Dendritic copolymers. Eur. Polym. J. 2010, 46, 165–170. [Google Scholar] [CrossRef]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamalakannan, R.; Mani, G.; Muthusamy, P.; Susaimanickam, A.A.; Kim, K. Caffeine-loaded gold nanoparticles conjugated with PLA-PEG-PLA copolymer for in vitro cytotoxicity and anti-inflammatory activity. J. Ind. Eng. Chem. 2017, 51, 113–121. [Google Scholar] [CrossRef]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Singh, M.; Sureshkumar, S.; Anand, M.; Ramakritinan, C.M.; Quero, F.; Kumaraguru, A.K. Chitosan mediated gold nanoparticles against pathogenic bacteria, fungal strains and MCF-7 cancer cells. Int. J. Biol. Macromol. 2020, 146, 560–568. [Google Scholar] [CrossRef]
- Sun, L.; Pu, S.; Li, J.; Cai, J.; Zhou, B.; Ren, G.; Ma, Q.; Zhong, L. Size controllable one step synthesis of gold nanoparticles using carboxymethyl chitosan. Int. J. Biol. Macromol. 2019, 122, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.Z.; Shamshad, H. Preparation and characterization of doxorubicin functionalized gold nanoparticles. Eur. J. Med. Chem. 2011, 46, 1857–1860. [Google Scholar] [CrossRef]
- Kumar, C.S.; Raja, M.D.; Sundar, D.S.; Gover Antoniraj, M.; Ruckmani, K. Hyaluronic acid co-functionalized gold nanoparticle complex for the targeted delivery of metformin in the treatment of liver cancer (HepG2 cells). Carbohydr. Polym. 2015, 128, 63–74. [Google Scholar] [CrossRef]
- Lertsarawut, P.; Rattanawongwiboon, T.; Tangthong, T.; Laksee, S.; Kwamman, T.; Phuttharak, B.; Romruensukharom, P.; Suwanmala, P.; Hemvichian, K. Starch-Based Super Water Absorbent: A Promising and Sustainable Way to Increase Survival Rate of Trees Planted in Arid Areas. Polymers 2021, 13, 1314. [Google Scholar] [CrossRef]
- Muttaqin, H.; Gopakumar, D.A.; Arumughan, V.; Pottathara, Y.B.; Sisanth, K.S.; Pasquini, D.; Bračič, M.; Seantier, B.; Nzihou, A.; Thomas, S.; et al. Robust Superhydrophobic Cellulose Nanofiber Aerogel for Multifunctional Environmental Applications. Polymers 2019, 11, 495. [Google Scholar]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [Green Version]
- Laksee, S.; Puthong, S.; Teerawatananond, T.; Palaga, T.; Muangsin, N. Highly efficient and facile fabrication of monodispersed Au nanoparticles using pullulan and their application as anticancer drug carriers. Carbohydr. Polym. 2017, 173, 178–191. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, G.; Shi, X.; Guo, R. Hyaluronic Acid-Decorated Laponite ® Nanocomposites for Targeted Anticancer Drug Delivery. Polymers 2019, 11, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.-Y.; Jia, X.-W.; Liu, Q.; Kong, B.-H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Di Martino, A.; Guselnikova, O.A.; Trusova, M.E.; Postnikov, P.S.; Sedlarik, V. Organic-inorganic hybrid nanoparticles controlled delivery system for anticancer drugs. Int. J. Pharm. 2017, 526, 380–390. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Ghosh, B.; Biswas, S. Current trends in using polymer coated gold nanoparticles for cancer therapy. Int. J. Pharm. 2015, 484, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Alea-Reyes, M.E.; González, A.; Calpena, A.C.; Ramos-López, D.; de Lapuente, J.; Pérez-García, L. Gemini pyridinium amphiphiles for the synthesis and stabilization of gold nanoparticles for drug delivery. J. Colloid. Interface Sci. 2017, 502, 172–183. [Google Scholar] [CrossRef]
- Benke, B.P.; Madhavan, N. Aminobenzoic acid incorporated octapeptides for cation transport. Bioorg. Med. Chem. 2015, 23, 1413–1420. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Fatahi, Y.; Atyabi, F.; Dinarvand, R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. J. Control. Release 2019, 295, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Laksee, S.; Puthong, S.; Kongkavitoon, P.; Palaga, T.; Muangsin, N. Facile and green synthesis of pullulan derivative-stabilized Au nanoparticles as drug carriers for enhancing anticancer activity. Carbohydr. Polym. 2018, 198, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Banihashem, S.; Nezhati, M.N.; Panahia, H.A. Synthesis of chitosan-grafted-poly(N-vinylcaprolactam) coated on the thiolated gold nanoparticles surface for controlled release of cisplatin. Carbohydr. Polym. 2020, 227, 115333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Muhammad, N.; Li, T.; Wang, H.; Liu, Y.; Liu, B.; Zhan, H. Hyaluronic Acid-Coated Camptothecin Nanocrystals for Targeted Drug Delivery to Enhance Anticancer Efficacy. Mol. Pharm. 2020, 17, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Meng, Q.; Hu, H.; Xu, T.; Shen, Y.; Cong, H. Construction of Dimeric Drug-Loaded Polymeric Micelles with High Loading Efficiency for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, M.; Zhao, H.; Peng, L.; Zou, X.; Zhao, Y.; Sun, L. Loading of Au/Ag Bimetallic Nanoparticles within and Outside of the Flexible SiO2 Electrospun Nanofibers as Highly Sensitive, Stable, Repeatable Substrates for Versatile and Trace SERS Detection. Polymers 2020, 12, 3008. [Google Scholar] [CrossRef]
- Ma, M.; Shang, W.; Xing, P.; Li, S.; Chu, X.; Hao, A.; Liu, G.; Zhang, Y. A supramolecular vesicle of camptothecin for its water dispersion and controllable releasing. Carbohydr. Res. 2015, 402, 208–214. [Google Scholar] [CrossRef]
- Wójcik, M.; Lewandowski, W.; Król, M.; Pawłowski, K.; Mieczkowski, J.; Lechowski, R.; Zabielska, K. Enhancing Anti-Tumor Efficacy of Doxorubicin by Non-Covalent Conjugation to Gold Nanoparticles—In Vitro Studies on Feline Fibrosarcoma Cell Lines. PLoS ONE 2015, 10, e0124955. [Google Scholar] [CrossRef]
- Yen, H.-J.; Young, Y.-A.; Tsai, T.-N.; Cheng, K.-M.; Chen, X.-A.; Chen, Y.-C.; Chen, C.-C.; Young, J.-J.; Hong, P.-D. Positively charged gold nanoparticles capped with folate quaternary chitosan: Synthesis, cytotoxicity, and uptake by cancer cells. Carbohydr. Polym. 2018, 183, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Liu, Y.; Wang, Y.; Wu, J.; Li, R.; Yang, J.; Zhang, N. pH-sensitive pullulan-based nanoparticles for intracellular drug delivery. Polym. Chem. 2014, 5, 423–432. [Google Scholar] [CrossRef]
- Checa-Chavarria, E.; Rivero-Buceta, E.; Sanchez Martos, M.A.; Martinez Navarrete, G.; Soto-Sánchez, C.; Botella, P.; Fernández, E. Development of a Prodrug of Camptothecin for Enhanced Treatment of Glioblastoma Multiforme. Mol. Pharm. 2021, 18, 1558–1572. [Google Scholar] [CrossRef]
- Jones, C.B.; Clements, M.K.; Wasi, S.; Daoud, S.S. Enhancement of camptothecin-induced cytotoxicity with UCN-01 in breast cancer cells: Abrogation of S/G2 arrest. Cancer Chemother. Pharmacol. 2000, 45, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-S.; Kang, S.-I.; Yoo, K.-D.; Lee, M.-Y.; Yoo, H.-S.; Hong, J.-T.; Shin, H.-S.; Kim, B.; Yun, Y.-P. Camptothecin inhibits platelet-derived growth factor-BB-induced proliferation of rat aortic vascular smooth muscle cells through inhibition of PI3K/Akt signaling pathway. Exp. Cell Res. 2013, 319, 982–991. [Google Scholar] [CrossRef]
- Lu, L.; Li, B.; Lin, C.; Li, K.; Liu, G.; Xia, Z.; Luo, Z.; Cai, K. Redox-responsive amphiphilic camptothecin prodrug nanoparticles for targeted liver tumor therapy. J. Mater. Chem. B 2020, 8, 3918–3928. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Abnous, K.; Taghdisi, S.M.; Taghavi, S.; Saljooghi, A.S.; Ramezani, M.; Alibolandi, M. Targeted rod-shaped mesoporous silica nanoparticles for the co-delivery of camptothecin and survivin shRNA in to colon adenocarcinoma in vitro and in vivo. Eur. J. Pharm. Biopharm. 2020, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laksee, S.; Supachettapun, C.; Muangsin, N.; Lertsarawut, P.; Rattanawongwiboon, T.; Sricharoen, P.; Limchoowong, N.; Chutimasakul, T.; Kwamman, T.; Hemvichian, K. Targeted Gold Nanohybrids Functionalized with Folate-Hydrophobic-Quaternized Pullulan Delivering Camptothecin for Enhancing Hydrophobic Anticancer Drug Efficacy. Polymers 2021, 13, 2670. https://doi.org/10.3390/polym13162670
Laksee S, Supachettapun C, Muangsin N, Lertsarawut P, Rattanawongwiboon T, Sricharoen P, Limchoowong N, Chutimasakul T, Kwamman T, Hemvichian K. Targeted Gold Nanohybrids Functionalized with Folate-Hydrophobic-Quaternized Pullulan Delivering Camptothecin for Enhancing Hydrophobic Anticancer Drug Efficacy. Polymers. 2021; 13(16):2670. https://doi.org/10.3390/polym13162670
Chicago/Turabian StyleLaksee, Sakchai, Chamaiporn Supachettapun, Nongnuj Muangsin, Pattra Lertsarawut, Thitirat Rattanawongwiboon, Phitchan Sricharoen, Nunticha Limchoowong, Threeraphat Chutimasakul, Tanagorn Kwamman, and Kasinee Hemvichian. 2021. "Targeted Gold Nanohybrids Functionalized with Folate-Hydrophobic-Quaternized Pullulan Delivering Camptothecin for Enhancing Hydrophobic Anticancer Drug Efficacy" Polymers 13, no. 16: 2670. https://doi.org/10.3390/polym13162670
APA StyleLaksee, S., Supachettapun, C., Muangsin, N., Lertsarawut, P., Rattanawongwiboon, T., Sricharoen, P., Limchoowong, N., Chutimasakul, T., Kwamman, T., & Hemvichian, K. (2021). Targeted Gold Nanohybrids Functionalized with Folate-Hydrophobic-Quaternized Pullulan Delivering Camptothecin for Enhancing Hydrophobic Anticancer Drug Efficacy. Polymers, 13(16), 2670. https://doi.org/10.3390/polym13162670






