Investigation on the Properties and on the Photo-Oxidation Behaviour of Polypropylene/Fumed Silica Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Processing
2.2. Photo-Oxidation
2.3. Characterisation
3. Results and Discussion
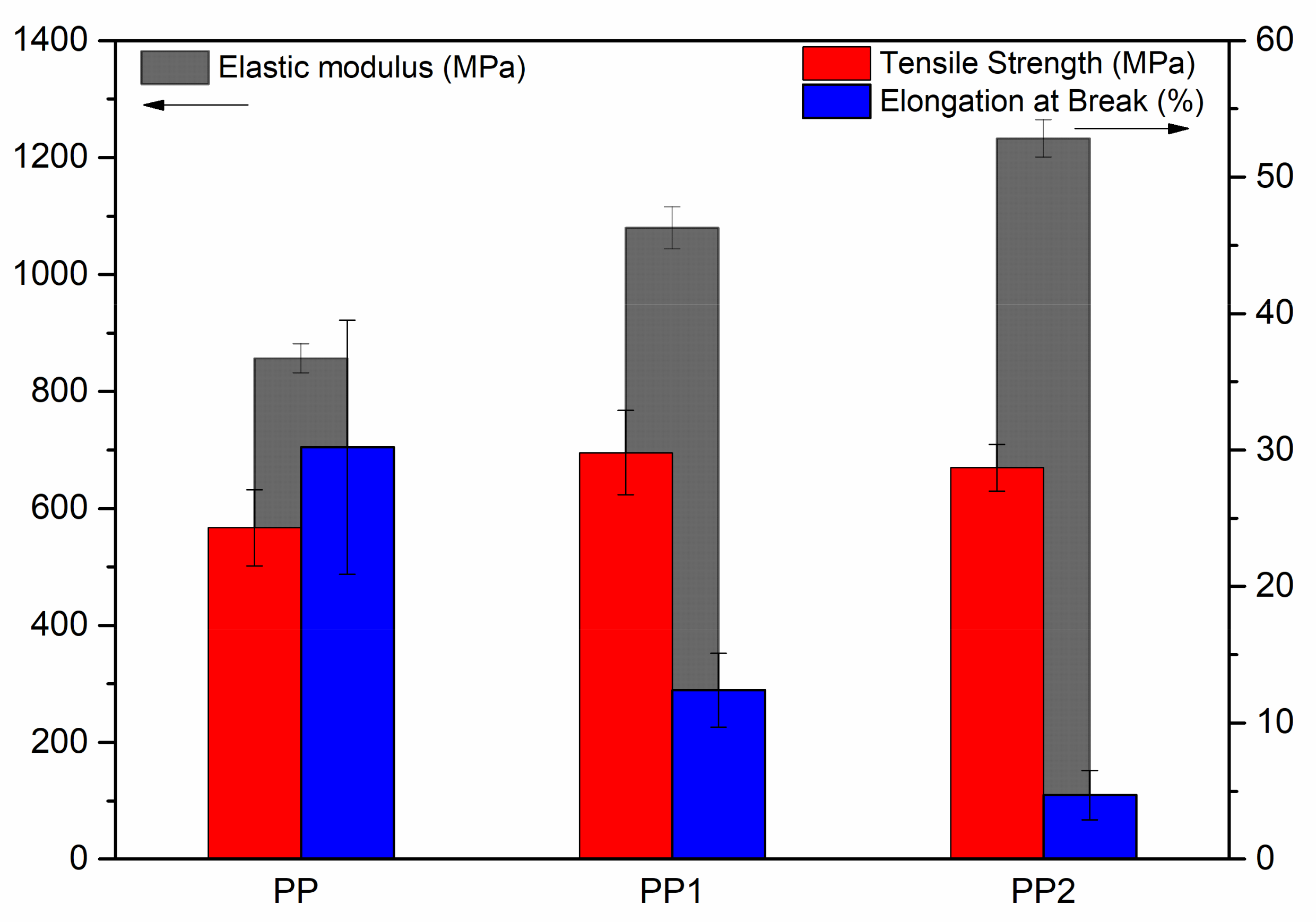
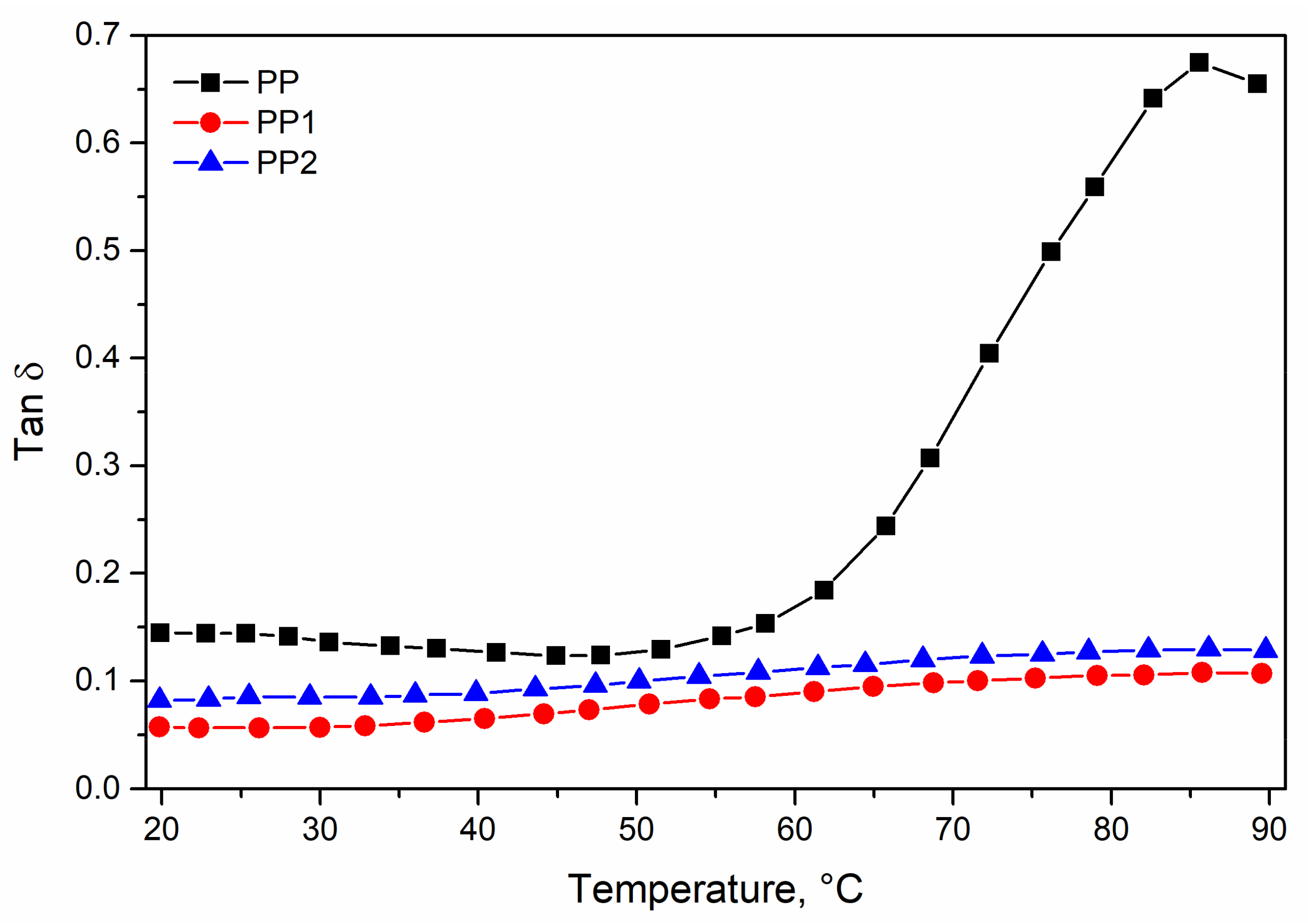
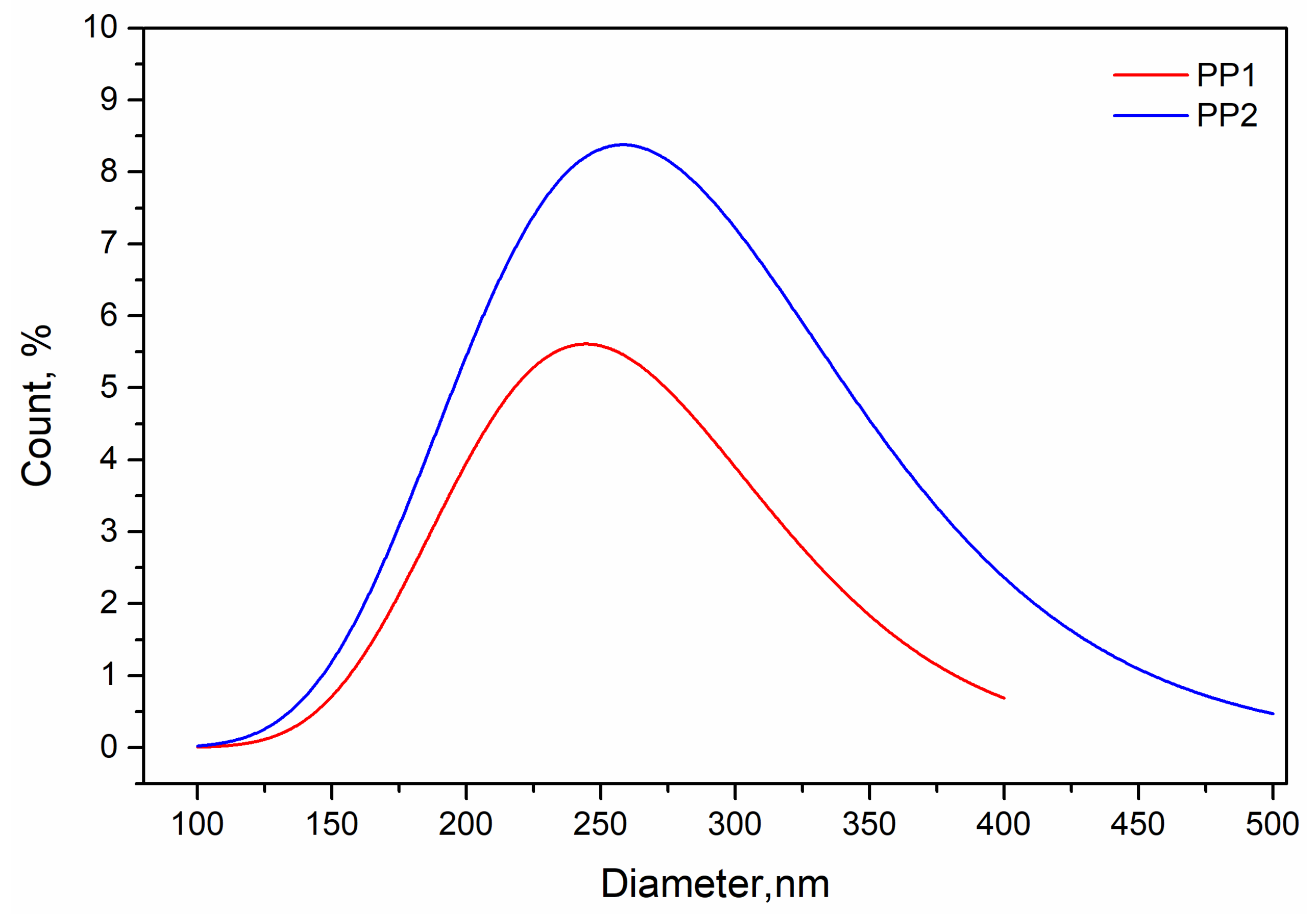
3.1. Characterisation of Polypropylene Nanocomposites
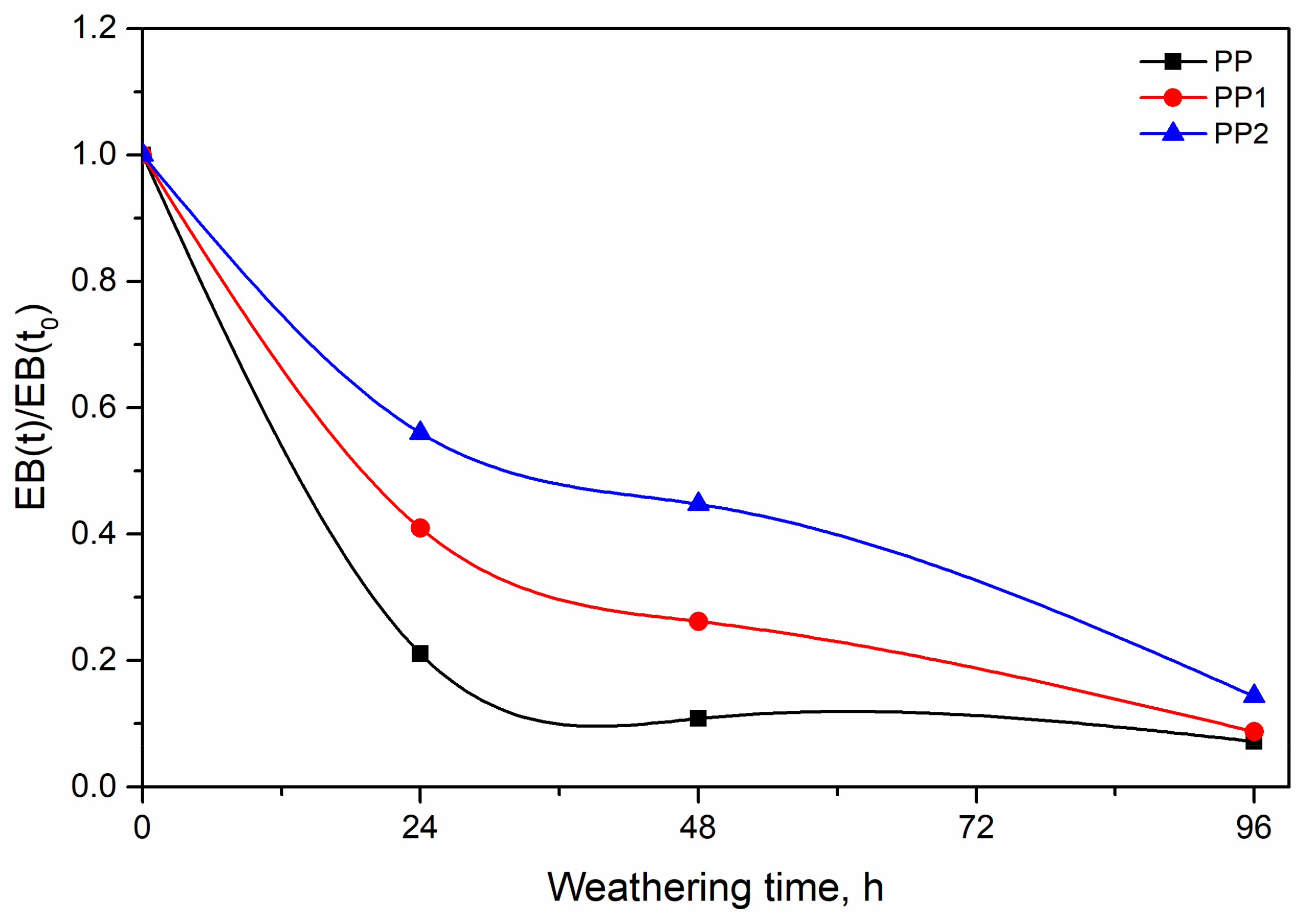
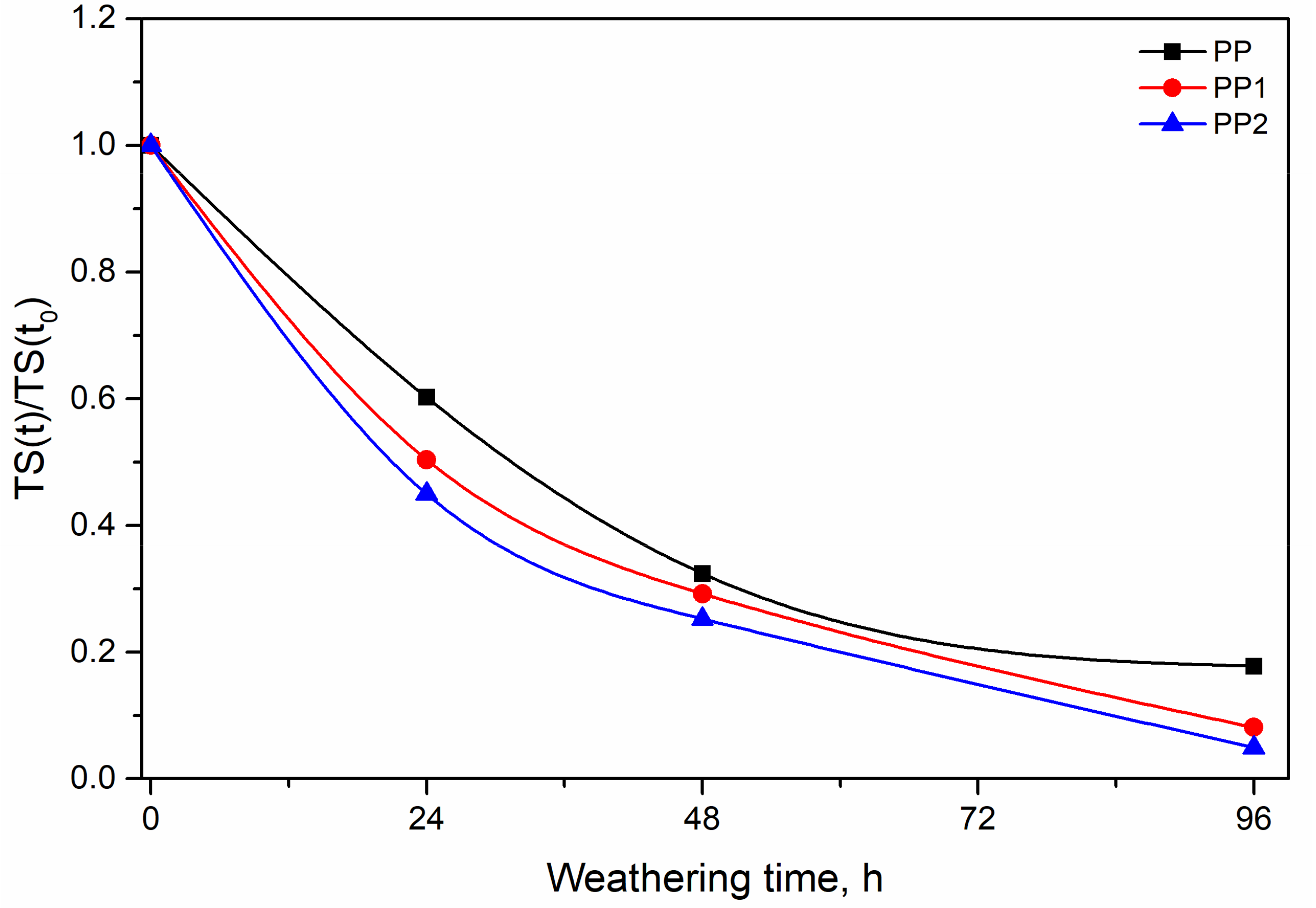
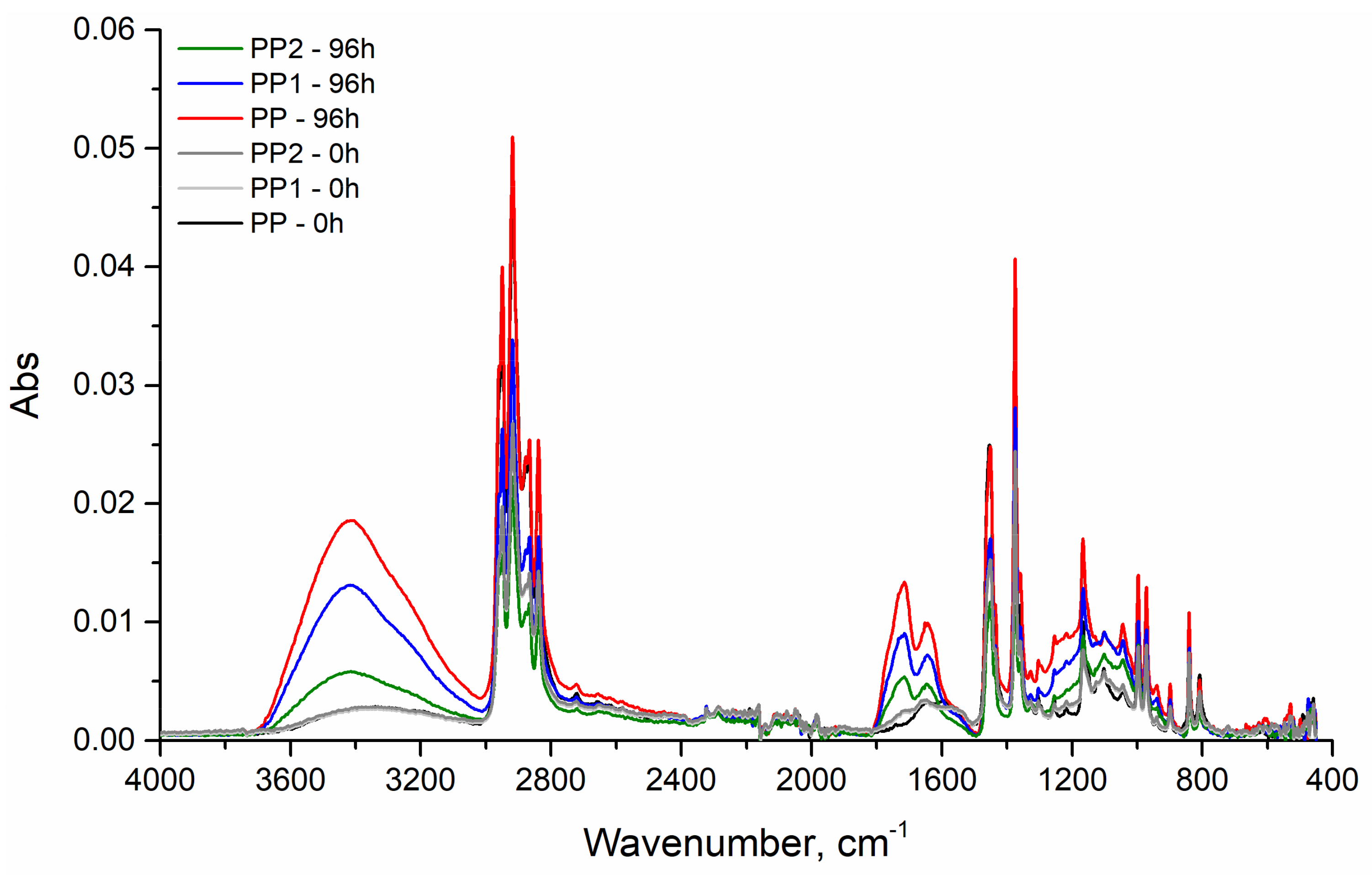
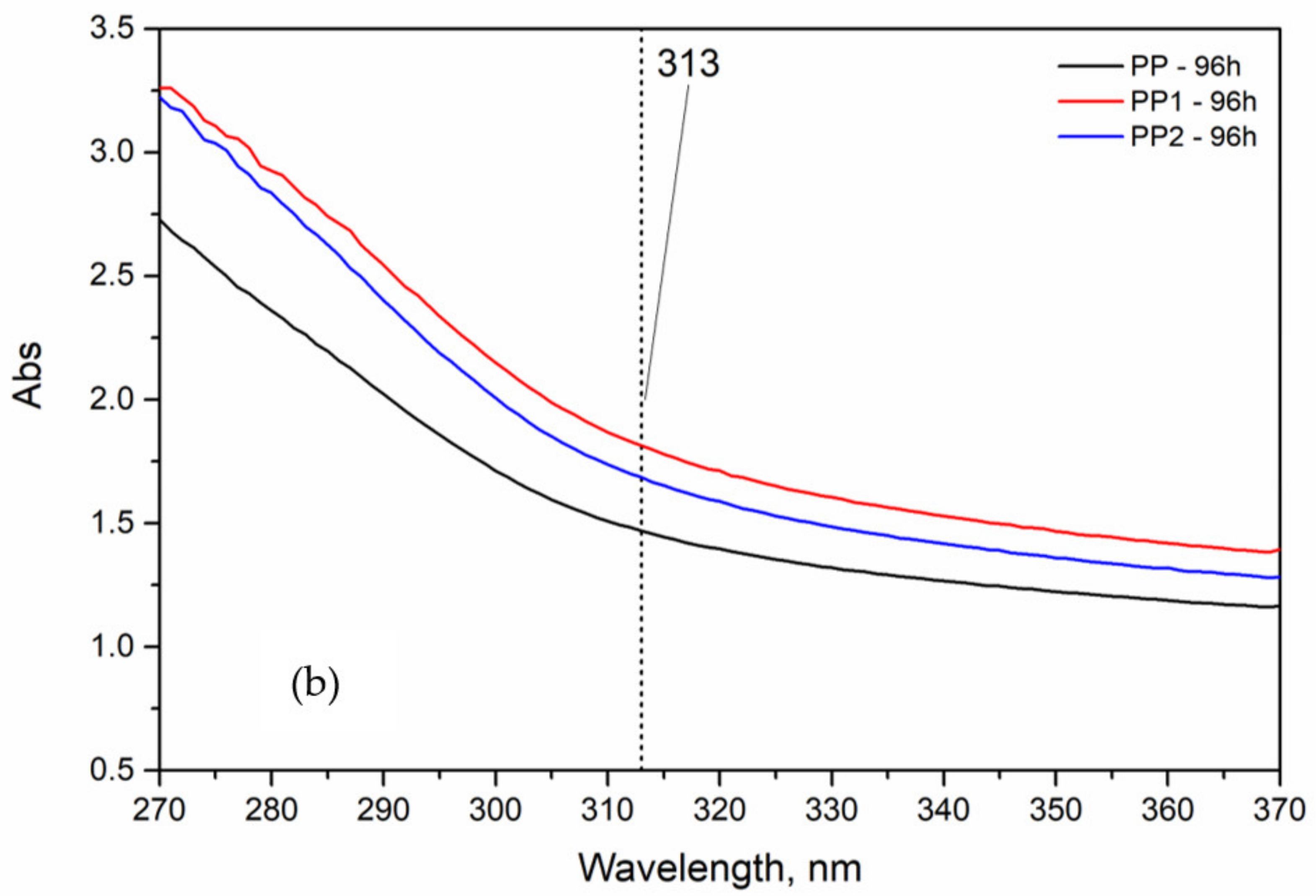
3.2. Photo-Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crosby, A.J.; Lee, J.Y. Polymer Nanocomposite: The effect on mechanical properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Hassanabadi, H.M.; Rodrigue, D. Effect of Particle Size and Shape on the Reinforcing Efficiency of Nanoparticles in Polymer Nanocomposites, Macromol. Mater. Eng. 2014, 299, 1220–1231. [Google Scholar]
- Suhailatmh, K.; Ramesan, M.T. Investigations on the structural, mechanical, thermal, and electrical properties of Ce-doped TiO2/poly(n-butyl methacrylate) nanocomposites. J. Therm. Anal. Calorim. 2019, 135, 2159–2169. [Google Scholar] [CrossRef]
- Ryu, H.J.; Hang, N.T.; Lee, J.H.; Choi, J.Y.; Choi, G.; Choy, J.H. Effect of organo-smectite clays on the mechanical properties and thermal stability of EVA nanocomposites. Appl. Clay Sci. 2020, 196, 105750. [Google Scholar] [CrossRef]
- Pandey, S.; Jana, K.K.; Answal, V.K.; Rana, D.; Maiti, P. Effect of nanoparticle on the mechanical and gas barrier properties of thermoplastic polyurethane. Appl. Clay Sci. 2017, 146, 468–474. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; Lopez, D.; Lopez, J.; Kenny, J.M. An overview of nanoparticles role in the improvement of barrier properties of bioplastics for food packaging applications. Food Packag. 2017, 391, 424. [Google Scholar]
- Ezzeddine, I.; Ghorbel, N.; Ilsouk, M.; Arous, M.; Lahcini, M.; Bouharras, F.Z.; Raihane, M.; Kallel, A. Dielectric and thermal characteristics of Beidellite nanoclay-reinforced poly(butylene succinate). Mater. Chem. Phys. 2021, 258, 123855. [Google Scholar] [CrossRef]
- Chan, C.M.; Wu, J.; Li, J.X.; Cheung, Y.K. Polypropylene/calcium carbonate nanocomposites. Polymer 2002, 43, 2981–2992. [Google Scholar] [CrossRef]
- Chafidz, A.; Kaavessina, M.; Al-Zahrani, S.; Al-Otaibi, M.N. Rheological and mechanical properties of polypropylene/calcium carbonate nanocomposites prepared from masterbatch. J. Thermoplast. Compos. Mater. 2016, 29, 593–622. [Google Scholar] [CrossRef]
- Qi, K.Y.; Li, Y.G.; Sun, Y.; Feng, D. Morphology and mechanical properties of polypropylene/calcium carbonate nanocomposites. Mater. Lett. 2006, 60, 805–809. [Google Scholar]
- Tarapow, J.A.; Bernal, C.R.; Alvarez, V.A. Mechanical properties of polypropylene/clay nanocomposites: Effect of clay content, polymer/clay compatibility, and processing conditions. J. Appl. Polym. Sci. 2008, 111, 768–778. [Google Scholar] [CrossRef]
- Hong, C.H.; Lee, Y.B.; Bae, J.W.; Jho, J.Y.; Nam, B.U.; Hwang, T.W. Preparation and mechanical properties of polypropylene/clay nanocomposites for automotive parts application. J. Appl. Polym. Sci. 2005, 98, 427–433. [Google Scholar] [CrossRef]
- Al-Saleh, M.A.; Yussuf, A.A.; Al-Enezi, S.; Kazemi, R.; Wahit, M.U.; Al-Shammari, T.; Al-banna, A. Polypropylene/Graphene Nanocomposites: Effects of GNP Loading and Compatibilizers on the Mechanical and Thermal Properties. Materials 2019, 12, 3924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Achaby, M.; Arrakhiz, F.; Vaudreuil, S.; Qaiss, A.; Bousmina, M.; Fassi-Fehri, O. Mechanical, thermal, and rheological properties of graphene-based polypropylene nanocomposites prepared by melt mixing. Polym. Compos. 2012, 33, 733–744. [Google Scholar] [CrossRef]
- Zaccone, M.; Armentano, I.; Cesano, F.; Scarano, D.; Frache, A.; Torre, L.; Montig, M. Effect of injection molding conditions on crystalline structure and electrical resistivity of PP/MWCNT nanocomposites. Polymers 2020, 12, 1685. [Google Scholar] [CrossRef]
- Gkourmpis, T.; Gaska, K.; Tranchida, D.; Gitsas, A.; Müller, C.; Matic, A.; Kádár, R. Melt-mixed 3D hierarchical graphene/polypropylene nanocomposites with low electrical percolation threshold. Nanomaterials 2019, 9, 1766. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Renbo, Z.; Yafei, L. Mechanical Properties and Crystallization Behavior of Polypropylene/Nano-SiO2 Composites. J. Reinf. Plast. Compos. 2006, 25, 1001–1012. [Google Scholar] [CrossRef]
- Garcia, M.; van Vliet, G.; Jain, S.; Schrauwen, B.A.G.; Sarkissov, A.; van Zyl, W.E.; Boukamp, B. Polypropylene/SiO2 nanocomposites with improved mechanical properties. Rev. Adv. Mater. Sci. 2004, 6, 169–175. [Google Scholar]
- Wang, N.; Shao, Y.; Zhang, J.; Li, H. Preparation and property of polypropylene/modified nano-SiO2 composite. Petrochem. Technol. 2008, 37, 624–627. [Google Scholar]
- Bracho, D.; Dougnac, V.N.; Palza, H.; Quijada, R. Functionalization of Silica Nanoparticles for Polypropylene Nanocomposite Applications. J. Nanomater. 2012, 2012, 19. [Google Scholar] [CrossRef]
- D’Amato, M.; Dorigato, A.; Fambri, L.; Pegoretti, A. High performance polyethylene nanocomposite fibers. Express Polym. Lett. 2012, 6, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Bikiaris, D.N.; Vassiliou, A.; Pavlidou, E.; Karayannidis, G.P. Compatibilisation effect of PP-g-MA copolymer on iPP/SiO2 nanocomposites prepared by melt mixing. Eur. Polym. J. 2005, 41, 1965–1978. [Google Scholar] [CrossRef]
- Zoukrami, F.; Haddaoui, N.; Vanzeveren, C.; Sclavons, M.; Devaux, J. Effect of compatibilizer on the dispersion of untreated silica in a polypropylene matrix. Polym. Int. 2007, 57, 756–763. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Kim, Y.C.; Park, O.O. Polymer/nanoporous silica nanocomposite blue-light-emitting diodes. Nanotechnology 2005, 16, 1793. [Google Scholar] [CrossRef]
- ASTM International. ASTM D638-14, Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Titone, V.; la Mantia, F.P.; Mistretta, M.C. The effect of calcium carbonate on the photo-oxidative behavior of poly(butylene adipate-co-terephthalate). Macromol. Mater. Eng. 2020, 35, 2000358. [Google Scholar] [CrossRef]
- Wunderlich, B. Thermal Analysis; Academic Press: New York, NY, USA, 1990; p. 418. [Google Scholar]
- Winter, H.H. Three views of viscoelasticity for Cox–Merz materials. Rheol. Acta 2009, 48, 241–243. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Arrigo, R.; Morreale, M. Effect of the orientation and rheological behaviour of biodegradable polymer nanocomposites. Eur. Polym. J. 2014, 54, 11–17. [Google Scholar] [CrossRef]
- Ren, J.; Krishnamoorti, R. Nonlinear viscoelastic properties of layered silicate-based intercalated nanocomposites. Macromolecules 2003, 36, 4443–4451. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Botta, L.; Mistretta, M.C.; Di Fiore, A.; Titone, V. Recycling of a biodegradable polymer blend. Polymers 2020, 12, 2297. [Google Scholar] [CrossRef]
- Mistretta, M.C.; Botta, L.; Vinci, A.D.; Ceraulo, M.; la Mantia, F.P. Photo-oxidation of polypropylene/graphene nanoplatelets composites. Polym. Degrad. Stab. 2019, 160, 35–43. [Google Scholar] [CrossRef]
- Mistretta, M.C.; la Mantia, F.P.; Titone, V.; Botta, L.; Pedeferri, M.; Morreale, M. Effect of ultraviolet and moisture action on biodegradable polymers and their blend. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020926653. [Google Scholar] [CrossRef]
- Mistretta, M.C.; la Mantia, F.P.; Titone, V.; Megna, B.; Botta, L.; Morreale, M. Durability of Biodegradable Polymers for the Conservation of Cultural Heritage. Front. Mater. 2019, 6, 151. [Google Scholar] [CrossRef]








| Tm, °C | ΔHm, J/g | XC, % | |
|---|---|---|---|
| PP (100/0 wt/wt%) | 176.4 | 89.4 ± 0.9 | 43.1 |
| PP1 (99/1 wt/wt%) | 177.2 | 91.8 ± 1.2 | 44.7 |
| PP2 (98/2 wt/wt%) | 177.3 | 91.1 ± 1.0 | 44.9 |
| Tm, °C | ΔHm, J/g | XC, % | XC (t)/XC (t0) | |
|---|---|---|---|---|
| PP (100/0 wt/wt%) | 157.3 | 129.5 ± 1.2 | 62.5 | 1.45 |
| PP1 (99/1 wt/wt%) | 154.2 | 130.0 ± 1.0 | 63.4 | 1.42 |
| PP2 (98/2 wt/wt%) | 154.3 | 127.6 ± 0.6 | 62.9 | 1.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titone, V.; Mistretta, M.C.; Botta, L.; Mantia, F.P.L. Investigation on the Properties and on the Photo-Oxidation Behaviour of Polypropylene/Fumed Silica Nanocomposites. Polymers 2021, 13, 2673. https://doi.org/10.3390/polym13162673
Titone V, Mistretta MC, Botta L, Mantia FPL. Investigation on the Properties and on the Photo-Oxidation Behaviour of Polypropylene/Fumed Silica Nanocomposites. Polymers. 2021; 13(16):2673. https://doi.org/10.3390/polym13162673
Chicago/Turabian StyleTitone, Vincenzo, Maria Chiara Mistretta, Luigi Botta, and Francesco Paolo La Mantia. 2021. "Investigation on the Properties and on the Photo-Oxidation Behaviour of Polypropylene/Fumed Silica Nanocomposites" Polymers 13, no. 16: 2673. https://doi.org/10.3390/polym13162673









