Surface Analyses of PVDF/NMP/[EMIM][TFSI] Solid Polymer Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
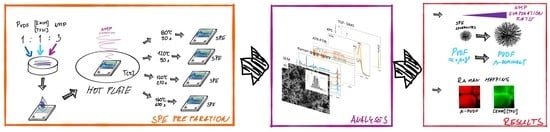
2.1. Sample Preparation
2.2. Scanning Electron Microscopy (SEM)
2.3. Raman Spectroscopy
2.4. Fourier Transform Infrared Spectroscopy
2.5. Differential Scanning Calorimetry
2.6. X-ray Photoelectron Spectroscopy
2.7. Secondary Ion Mass Spectroscopy (SIMS)
3. Results and Discussion
3.1. Raman Spectroscopy
3.2. Fourier Transform Infrared Spectroscopy
3.3. Differential Scanning Calorimetry
3.4. X-ray Photoelectron Spectroscopy
3.5. Secondary Ion Mass Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Y.S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid–Polymer Composites: A New Platform for Multifunctional Applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Josef, E.; Yan, Y.; Stan, M.C.; Wellmann, J.; Vizintin, A.; Winter, M.; Johansson, P.; Dominko, R.; Guterman, R. Ionic Liquids and their Polymers in Lithium-Sulfur Batteries. Isr. J. Chem. 2019, 59, 832–842. [Google Scholar] [CrossRef] [Green Version]
- Austin Suthanthiraraj, S.; Johnsi, M. Nanocomposite polymer electrolytes. Ionics 2017, 23, 2531–2542. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, Z. PVDF-based dielectric polymers and their applications in electronic materials. IET Nanodielectr. 2018, 1, 17–31. [Google Scholar] [CrossRef]
- Kammoun, M.; Berg, S.; Ardebili, H. Flexible thin-film battery based on graphene-oxide embedded in solid polymer electrolyte. Nanoscale 2015, 7, 17516–17522. [Google Scholar] [CrossRef]
- Park, J.; Ahn, D.B.; Kim, J.; Cha, E.; Bae, B.S.; Lee, S.Y.; Park, J.U. Printing of wirelessly rechargeable solid-state supercapacitors for soft, smart contact lenses with continuous operations. Sci. Adv. 2019, 5, eaay0764. [Google Scholar] [CrossRef] [Green Version]
- Kuberský, P.; Syrový, T.; Hamáček, A.; Nešpůrek, S.; Syrová, L. Towards a fully printed electrochemical NO2 sensor on a flexible substrate using ionic liquid based polymer electrolyte. Sens. Actuators B Chem. 2015, 209, 1084–1090. [Google Scholar] [CrossRef]
- Luo, B.; Xiao, M.; Huang, X.; Hu, H.; Knibbe, R.; Wang, S.; Lyu, M.; Wang, L.; Sun, D. An Integrated Strategy towards Enhanced Performance of the Lithium–Sulfur Battery and its Fading Mechanism. Chem. A Eur. J. 2018, 24, 18544–18550. [Google Scholar]
- Luo, R.; Li, Q.; Du, B.; Zhou, S.; Chen, Y. Preparation and Characterization of Solid Electrolyte Doped With Carbon Nanotubes and its Preliminary Application in NO2 Gas Sensors. Front. Mater. 2019, 6, 113. [Google Scholar] [CrossRef]
- Vonau, C.; Zosel, J.; Paramasivam, M.; Ahlborn, K.; Gerlach, F.; Vashook, V.; Guth, U. Polymer based materials for solid electrolyte sensors. Solid State Ion. 2012, 225, 337–341. [Google Scholar] [CrossRef]
- Navratil, J.; Kubersky, P.; Sedlak, P.; Hamacek, A. Preparation of Nitrogen Dioxide Sensor Utilizing Aerosol Jet Printing Technology. In Proceedings of the Proceedings of the International Spring Seminar on Electronics Technology, Demanovska Valley, Slovakia, 14–15 May 2020. [Google Scholar]
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B Chem. 2011, 156, 527–538. [Google Scholar] [CrossRef]
- Luo, R.; Li, H.; Du, B.; Zhou, S.; Chen, Y. A printed and flexible NO2 sensor based on a solid polymer electrolyte. Front. Chem. 2019, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.K.; Gupta, S. Natural polymer-based electrolytes for electrochemical devices: A review. Ionics 2011, 17, 479–483. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, H.J.; Kim, E.; Oh, B.; Cho, J.H. Photocured PEO-based solid polymer electrolyte and its application to lithium-polymer batteries. J. Power Sources 2001, 92, 255–259. [Google Scholar] [CrossRef]
- Manjunatha, H.; Damle, R.; Pravin, K.; Kumaraswamy, G.N. Modification in the transport and morphological properties of solid polymer electrolyte system by low-energy ion irradiation. Ionics 2018, 24, 3027–3037. [Google Scholar] [CrossRef]
- Sedlak, P.; Gajdos, A.; Macku, R.; Majzner, J.; Sedlakova, V.; Holcman, V.; Kuberský, P. The effect of thermal treatment on ac/dc conductivity and current fluctuations of PVDF/NMP/ [EMIM][TFSI] solid polymer electrolyte. Sci. Rep. 2020, 10, 21140. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Fu, W.; Hu, Y.; Ding, Y. Effect of annealing treatment on crystalline and dielectric properties of PVDF/PEG-containing ionic liquid composites. Compos. Sci. Technol. 2018, 158, 1–8. [Google Scholar] [CrossRef]
- Lewandowski, A.; Świderska, A. New composite solid electrolytes based on a polymer and ionic liquids. Solid State Ion. 2004, 169, 21–24. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Singh, R.K.; Chandra, S. Effect of ionic liquid on the crystallization kinetics behaviour of polymer poly(ethylene oxide). CrystEngComm 2013, 15, 6022–6034. [Google Scholar] [CrossRef]
- Correia, D.M.; Costa, C.M.; Lizundia, E.; Sabater i Serra, R.; Gómez-Tejedor, J.A.; Biosca, L.T.; Meseguer-Dueñas, J.M.; Gomez Ribelles, J.L.; Lanceros-Méndez, S. Influence of Cation and Anion Type on the Formation of the Electroactive β-Phase and Thermal and Dynamic Mechanical Properties of Poly(vinylidene fluoride)/Ionic Liquids Blends. J. Phys. Chem. C 2019, 123, 45. [Google Scholar] [CrossRef]
- Correia, D.M.; Barbosa, J.C.; Costa, C.M.; Reis, P.M.; Esperança, J.M.S.S.; De Zea Bermudez, V.; Lanceros-Méndez, S. Ionic Liquid Cation Size-Dependent Electromechanical Response of Ionic Liquid/Poly(vinylidene fluoride)-Based Soft Actuators. J. Phys. Chem. C 2019, 123, 12744–12752. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, M.; Zhao, L.; You, J.; Cao, X.; Li, Y. Ionic liquid modified poly(vinylidene fluoride): Crystalline structures, miscibility, and physical properties. Polym. Chem. 2013, 4, 5726–5734. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Singh, R.K.; Chandra, S. Ionic liquid assisted modification in ionic conductivity, phase transition temperature and crystallization kinetics behaviour of polymer poly(ethylene oxide). Solid State Ion. 2014, 262, 790–794. [Google Scholar] [CrossRef]
- Pickford, T.; Gu, X.; Heeley, E.L.; Wan, C. Effects of an ionic liquid and processing conditions on the β-polymorph crystal formation in poly(vinylidene fluoride). CrystEngComm 2019, 21, 5418–5428. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Hassankiadeh, N.T.; Zhuang, Y.; Drioli, E.; Lee, Y.M. Crystalline polymorphism in poly(vinylidenefluoride) membranes. Prog. Polym. Sci. 2015, 51, 94–126. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, Q.; Yu, C.; Peng, J.; Ma, J.; Ju, X.; Zhai, M. Effect of ionic liquid on the properties of poly(vinylidene fluoride)-based gel polymer electrolytes. Ionics 2013, 19, 1587–1593. [Google Scholar] [CrossRef]
- Gregorio, R.; Borges, D.S. Effect of crystallization rate on the formation of the polymorphs of solution cast poly(vinylidene fluoride). Polymer 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Kuberský, P.; Hamáček, A.; Nešpůrek, S.; Soukup, R.; Vik, R. Effect of the geometry of a working electrode on the behavior of a planar amperometric NO2 sensor based on solid polymer electrolyte. Sens. Actuators B Chem. 2013, 187, 546–552. [Google Scholar] [CrossRef]
- Kuberský, P.; Sedlák, P.; Hamáček, A.; Nešpůrek, S.; Kuparowitz, T.; Šikula, J.; Majzner, J.; Sedlaková, V.; Grmela, L.; Syrový, T. Quantitative fluctuation-enhanced sensing in amperometric NO2 sensors. Chem. Phys. 2015, 456, 111–117. [Google Scholar] [CrossRef]
- Sedlák, P.; Kuberský, P.; Mívalt, F. Effect of various flow rate on current fluctuations of amperometric gas sensors. Sens. Actuators B Chem. 2019, 283, 321–328. [Google Scholar] [CrossRef]
- Sedlák, P.; Kuberský, P. The Effect of the Orientation Towards Analyte Flow on Electrochemical Sensor Performance and Current Fluctuations. Sensors 2020, 20, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nespurek, S.; Mracek, L.; Kubersky, P.; Syrovy, T.; Hamacek, A. Ionic liquids in electrochemical gas sensors and transistors. Mol. Cryst. Liq. Cryst. 2019, 694, 1–20. [Google Scholar] [CrossRef]
- Nair, J.R.; Shaji, I.; Ehteshami, N.; Thum, A.; Diddens, D.; Heuer, A.; Winter, M. Solid Polymer Electrolytes for Lithium Metal Battery via Thermally Induced Cationic Ring-Opening Polymerization (CROP) with an Insight into the Reaction Mechanism. Chem. Mater. 2019, 31, 3118–3133. [Google Scholar] [CrossRef]
- Jurado-Meneses, N.M.; Delgado-Rosero, M.I.; Meléndez-Lira, M.A. Structural and vibrational studies on composites polymer electrolytes (PEO)10CF3COONa + x wt.% Al2O3. Rev. Fac. Ing. 2017, 2017, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Schaepe, K.; Jungnickel, H.; Heinrich, T.; Tentschert, J.; Luch, A.; Unger, W.E.S. Secondary ion mass spectrometry. In Characterization of Nanoparticles: Measurement Processes for Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 481–509. ISBN 9780128141830. [Google Scholar]
- Constantino, C.J.L.; Job, A.E.; Simões, R.D.; Simões, S.; Giacometti, J.A.; Zucolotto, V.; Oliveira, O.N.; Gozzi, G.; Chinaglia, D.L. Phase Transition in Poly(vinylidene fluoride) Investigated with Micro-Raman Spectroscopy. Appl. Spectrosc. 2005, 59, 275–279. [Google Scholar] [CrossRef]
- Nallasamy, P. Vibrational spectroscopic characterization of form II poly(vinylidene fluoride). IJPAP 2005, 43, 821–827. [Google Scholar]
- Peleš, A.; Aleksić, O.; Pavlović, V.P.; Djoković, V.; Dojčilović, R.; Nikolić, Z.; Marinković, F.; Mitrić, M.; Blagojević, V.; Vlahović, B.; et al. Structural and electrical properties of ferroelectric poly(vinylidene fluoride) and mechanically activated ZnO nanoparticle composite films. Phys. Scr. 2018, 93, 105801. [Google Scholar] [CrossRef]
- Barnakov, Y.A.; Paul, O.; Joaquim, A.; Falconer, A.; Barnakov, V.Y.; Dikin, D.; Petranovskii, V.P.; Zavalin, A.; Ueda, A.; Williams, F.; et al. Nanoplasmonics: Past, present, and glimpse into future. Int. J. Smart Nano Mater. 2011, 19, 1–17. [Google Scholar]
- Boccaccio, T.; Bottino, A.; Capannelli, G.; Piaggio, P. Characterization of PVDF membranes by vibrational spectroscopy. J. Memb. Sci. 2002, 210, 315–329. [Google Scholar] [CrossRef]
- Elashmawi, I.S.; Gaabour, L.H. Raman, morphology and electrical behavior of nanocomposites based on PEO/PVDF with multi-walled carbon nanotubes. Results Phys. 2015, 5, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, P.; Sobola, D.; Částková, K.; Knápek, A.; Burda, D.; Orudzhev, F.; Dallaev, R.; Tofel, P.; Trčka, T.; Grmela, L.; et al. Characterization of Polyvinylidene Fluoride (PVDF) Electrospun Fibers Doped by Carbon Flakes. Polymers 2020, 12, 2766. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Fries, J.; Leipertz, A. Experimental vibrational study of imidazolium-based ionic Liquids: Raman and infrared spectra of 1-ethyl-3methylimidazolium bis(trifluoromethylsulfonyl) imide and 1-ethyl-3-methylimidazolium ethylsulfate. Appl. Spectrosc. 2007, 61, 1306–1311. [Google Scholar] [CrossRef]
- Rey, I.; Johansson, P.; Lindgren, J.; Lassègues, J.C.; Grondin, J.; Servant, L. Spectroscopic and theoretical study of (CF3SO2)2N- (TFSI-) and (CF3SO2)2NH (HTFSI). J. Phys. Chem. A 1998, 102, 3249–3258. [Google Scholar] [CrossRef]
- Lassègues, J.C.; Grondin, J.; Holomb, R.; Johansson, P. Raman and ab initio study of the conformational isomerism in the 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide ionic liquid. J. Raman Spectrosc. 2007, 38, 551–558. [Google Scholar] [CrossRef]
- Huang, H.C.; Yen, Y.C.; Chang, J.C.; Su, C.W.; Chang, P.Y.; Sun, I.W.; Hsieh, C.T.; Lee, Y.L.; Teng, H. An ether bridge between cations to extend the applicability of ionic liquids in electric double layer capacitors. J. Mater. Chem. A 2016, 4, 19160–19169. [Google Scholar] [CrossRef]
- Xu, P.; Fu, W.; Cui, Z.; Ding, Y. Synergistic promotion of polar phase crystallization of PVDF by ionic liquid with PEG segment. Appl. Surf. Sci. 2018, 444, 480–484. [Google Scholar] [CrossRef]
- Revathi, V.; Dinesh Kumar, S.; Chithra Lekha, P.; Subramanian, V.; Natarajan, T.S.; Muthamizhchelvan, C. Structural, dielectric, and magnetic studies on electrospun magnesium ferrite-polyvinylidene fluoride core-shell composite fibers. Acta Metall. Sin. 2014, 27, 557–562. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Castkova, K.; Kastyl, J.; Sobola, D.; Petrus, J.; Stastna, E.; Riha, D.; Tofel, P. Structure–properties relationship of electrospun pvdf fibers. Nanomaterials 2020, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Benz, M.; Euler, W.B. Determination of the crystalline phases of poly(vinylidene fluoride) under different preparation conditions using differential scanning calorimetry and infrared spectroscopy. J. Appl. Polym. Sci. 2003, 89, 1093–1100. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, K.; Zhou, Y.; Qu, Z.; Wang, H.; Zhang, Y.; Zhou, H.; Yan, C. Facile preparation of highly oriented poly(vinylidene fluoride) uniform films and their ferro- and piezoelectric properties. RSC Adv. 2017, 7, 17038–17043. [Google Scholar] [CrossRef] [Green Version]
- Mayerhöfer, T.G. Employing Theories Far beyond Their Limits–Linear Dichroism Theory. ChemPhysChem 2018, 19, 2123–2130. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Structural, microstructural and electrochemical properties of dispersed-type polymer nanocomposite films. J. Phys. D. Appl. Phys. 2018, 51, 044504. [Google Scholar] [CrossRef] [Green Version]
- Mejri, R.; Dias, J.C.; Hentati, S.B.; Martins, M.S.; Costa, C.M.; Lanceros-Mendez, S. Effect of anion type in the performance of ionic liquid/poly(vinylidene fluoride) electromechanical actuators. J. Non. Cryst. Solids 2016, 453, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Sa’Adun, N.N.; Subramaniam, R.; Kasi, R. Development and characterization of poly(1-vinylpyrrolidone-co-vinyl acetate) copolymer based polymer electrolytes. Sci. World J. 2014, 2014, 254215. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Ao, M.; Sung, W.; Moon, B.; Ahlström, B.; Johansson, P.; Ouchi, Y.; Kim, D. Structures of ionic liquid-water mixtures investigated by IR and NMR spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 9591–9601. [Google Scholar] [CrossRef]
- Ponzio, E.A.; Echevarria, R.; Morales, G.M.; Barbero, C. Removal of N-methylpyrrolidone hydrogen-bonded to polyaniline free-standing films by protonation-deprotonation cycles or thermal heating. Polym. Int. 2001, 50, 1180–1185. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Bhattarai, B.; Suri, R.P.S. Environmentally Friendly β-Cyclodextrin-Ionic Liquid Polyurethane-Modified Magnetic Sorbent for the Removal of PFOA, PFOS, and Cr(VI) from Water. ACS Sustain. Chem. Eng. 2017, 5, 9223–9232. [Google Scholar] [CrossRef]
- Hong, Y.; Fang, Y.; Sun, D.; Zhou, X. Ionic liquids modified cobalt/ZSM-5 as a highly efficient catalyst for enhancing the selectivity towards KA oil in the aerobic oxidation of cyclohexane. Open Chem. 2019, 17, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Hao, D.; Wang, X.; Liu, X.; Zhu, X.; Sun, S.; Li, J.; Yue, O. A novel eco-friendly imidazole ionic liquids based amphoteric polymers for high performance fatliquoring in chromium-free tanned leather production. J. Hazard. Mater. 2020, 399, 123048. [Google Scholar] [CrossRef]
- Sobola, D.; Kaspar, P.; Částková, K.; Dallaev, R.; Papež, N.; Sedlák, P.; Trčka, T.; Orudzhev, F.; Kaštyl, J.; Weiser, A.; et al. PVDF Fibers Modification by Nitrate Salts Doping. Polymers 2021, 13, 2439. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Compromising Science by Ignorant Instrument Calibration—Need to Revisit Half a Century of Published XPS Data. Angew. Chem. Int. Ed. 2020, 59, 5002–5006. [Google Scholar] [CrossRef] [PubMed]
- Weber, I.; Kim, J.; Buchner, F.; Schnaidt, J.; Behm, R.J. Surface Science and Electrochemical Model Studies on the Interaction of Graphite and Li-Containing Ionic Liquids. ChemSusChem 2020, 13, 2589–2601. [Google Scholar] [CrossRef] [PubMed]
- Göktürk, P.A. X-ray Photoelectron Spectroscopy for Chemical and Electrical Characterization of Devices Extended to Liquid/Solid Interfaces. Ph.D. Thesis, Bilkent University, Ankara, Turkey, 2018. [Google Scholar]
- Seo, S.; Park, J.; Kang, Y.C. Chemical Analysis of Ionic Liquids Using Photoelectron Spectroscopy. Bull. Korean Chem. Soc. 2016, 37, 355–360. [Google Scholar] [CrossRef]
- Höfft, O.; Bahr, S.; Himmerlich, M.; Krischok, S.; Schaefer, J.A.; Kempter, V. Electronic structure of the surface of the ionic liquid [EMIM][Tf 2N] studied by metastable Impact Electron Spectroscopy (MIES), UPS, and XPS. Langmuir 2006, 22, 7120–7123. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.M.; Han, H.J.; Yim, S.; Choi, M.-J.; Jeon, J.; Jung, Y.S. Long-Term Stable 2H-MoS2 Dispersion: Critical Role of Solvent for Simultaneous Phase Restoration and Surface Functionalization of Liquid-Exfoliated MoS2. ACS Omega 2017, 2, 4678–4687. [Google Scholar] [CrossRef]
- Yakimchuk, E.; Volodin, V.; Antonova, I. New graphene derivative with N-methylpyrrolidone: Suspension, structural, optical and electrical properties. Phys. Chem. Chem. Phys. 2019, 21, 12494–12504. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy C. D. Wanger, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979. 190 pp. $195. Surf. Interface Anal. 1981, 3. [Google Scholar] [CrossRef]
- Souda, R. Phase transition of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide thin films on highly oriented pyrolytic graphite. J. Phys. Chem. B 2009, 113, 12973–12977. [Google Scholar] [CrossRef] [PubMed]
- Bundaleski, N.; Caporali, S.; Chenakin, S.P.; Moutinho, A.M.C.; Teodoro, O.M.N.D.; Tolstogouzov, A. Ion-induced fragmentation of imidazolium ionic liquids: TOF-SIMS study. Int. J. Mass Spectrom. 2013, 353, 19–25. [Google Scholar] [CrossRef]
- Günster, J.; Höfft, O.; Krischok, S.; Souda, R. A time-of-flight secondary ion mass spectroscopy study of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide RT-ionic liquid. Surf. Sci. 2008, 602, 3403–3407. [Google Scholar] [CrossRef]
- Feng, J.; Chan, C.M.; Weng, L.T. Influence of chain sequence structure of polymers on ToF-SIMS spectra. Polymer 2000, 41, 2695–2699. [Google Scholar] [CrossRef]








| Sample | SPE 80 °C 90 s | SPE 120 °C 90 s | SPE 120 °C 210 s | SPE 160 °C 600 s |
|---|---|---|---|---|
| β-phase, % | 84.11 | 56.23 | 73.03 | 98.80 |
| γ-phase, % | 15.89 | 43.77 | 26.97 | 1.20 |
| Sample | Element Content [%] | ||||
|---|---|---|---|---|---|
| S2p | C1s | N1s | O1s | F1s | |
| SPE 80 °C 90 s | 3.28 | 54.15 | 5.75 | 16.02 | 20.79 |
| SPE 120 °C 90 s | 3.44 | 53.24 | 5.68 | 14.91 | 22.72 |
| SPE 120 °C 210 s | 5.97 | 45.02 | 9.25 | 14.96 | 24.80 |
| SPE 160 °C 600 s | 6.94 | 37.53 | 11.12 | 17.91 | 26.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlak, P.; Sobola, D.; Gajdos, A.; Dallaev, R.; Nebojsa, A.; Kubersky, P. Surface Analyses of PVDF/NMP/[EMIM][TFSI] Solid Polymer Electrolyte. Polymers 2021, 13, 2678. https://doi.org/10.3390/polym13162678
Sedlak P, Sobola D, Gajdos A, Dallaev R, Nebojsa A, Kubersky P. Surface Analyses of PVDF/NMP/[EMIM][TFSI] Solid Polymer Electrolyte. Polymers. 2021; 13(16):2678. https://doi.org/10.3390/polym13162678
Chicago/Turabian StyleSedlak, Petr, Dinara Sobola, Adam Gajdos, Rashid Dallaev, Alois Nebojsa, and Petr Kubersky. 2021. "Surface Analyses of PVDF/NMP/[EMIM][TFSI] Solid Polymer Electrolyte" Polymers 13, no. 16: 2678. https://doi.org/10.3390/polym13162678
APA StyleSedlak, P., Sobola, D., Gajdos, A., Dallaev, R., Nebojsa, A., & Kubersky, P. (2021). Surface Analyses of PVDF/NMP/[EMIM][TFSI] Solid Polymer Electrolyte. Polymers, 13(16), 2678. https://doi.org/10.3390/polym13162678