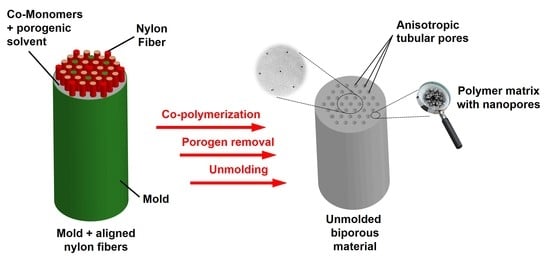
Wood-Mimicking Bio-Based Biporous Polymeric Materials with Anisotropic Tubular Macropores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Vanillin Methacrylate
2.3. Preparation of Monoporous VMA-Based Materials
2.4. Preparation of Monoporous HEMA-Based Materials
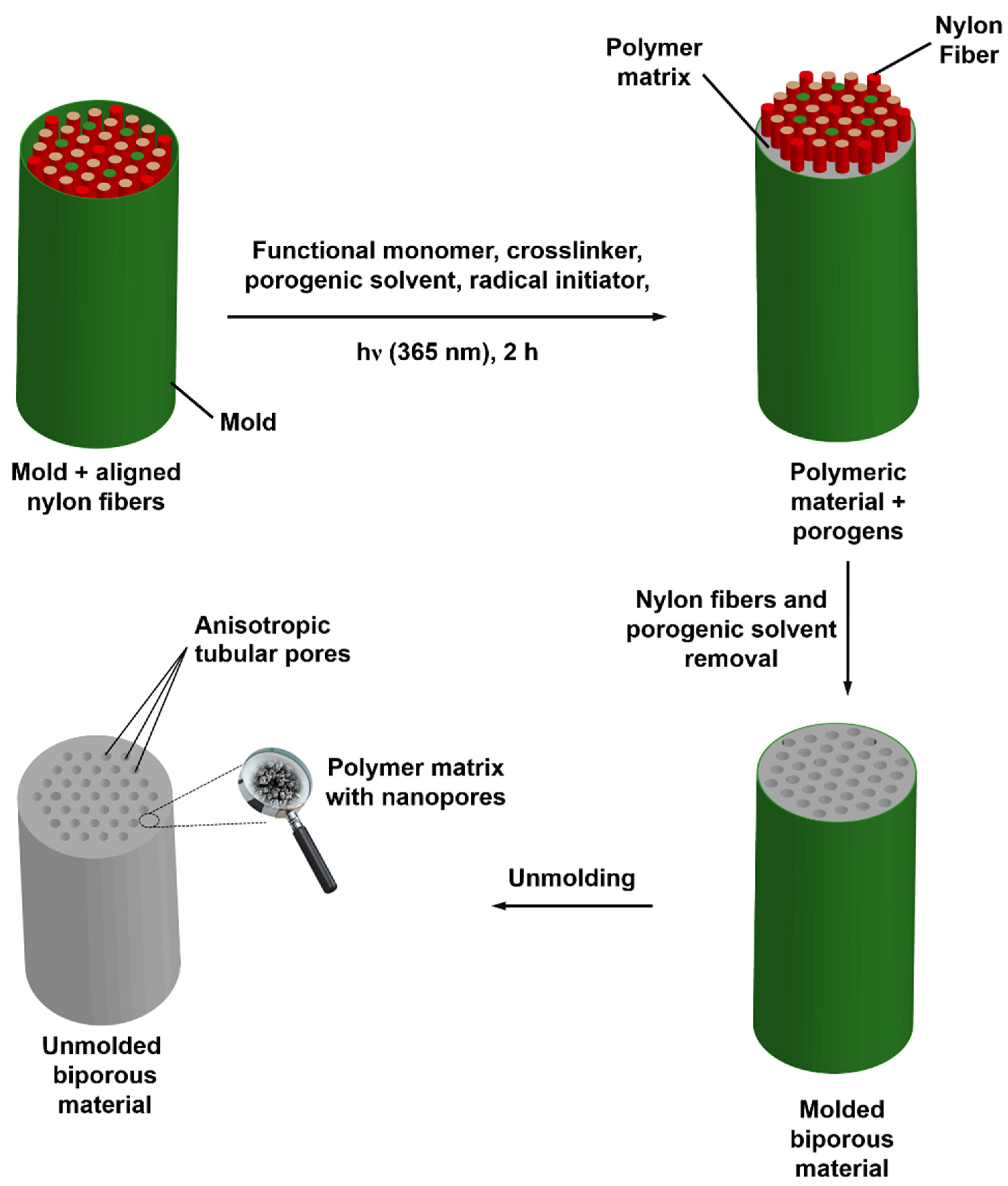
2.5. Preparation of Biporous HEMA- Or VMA-Based Materials
2.6. Instrumentation
3. Results and Discussion
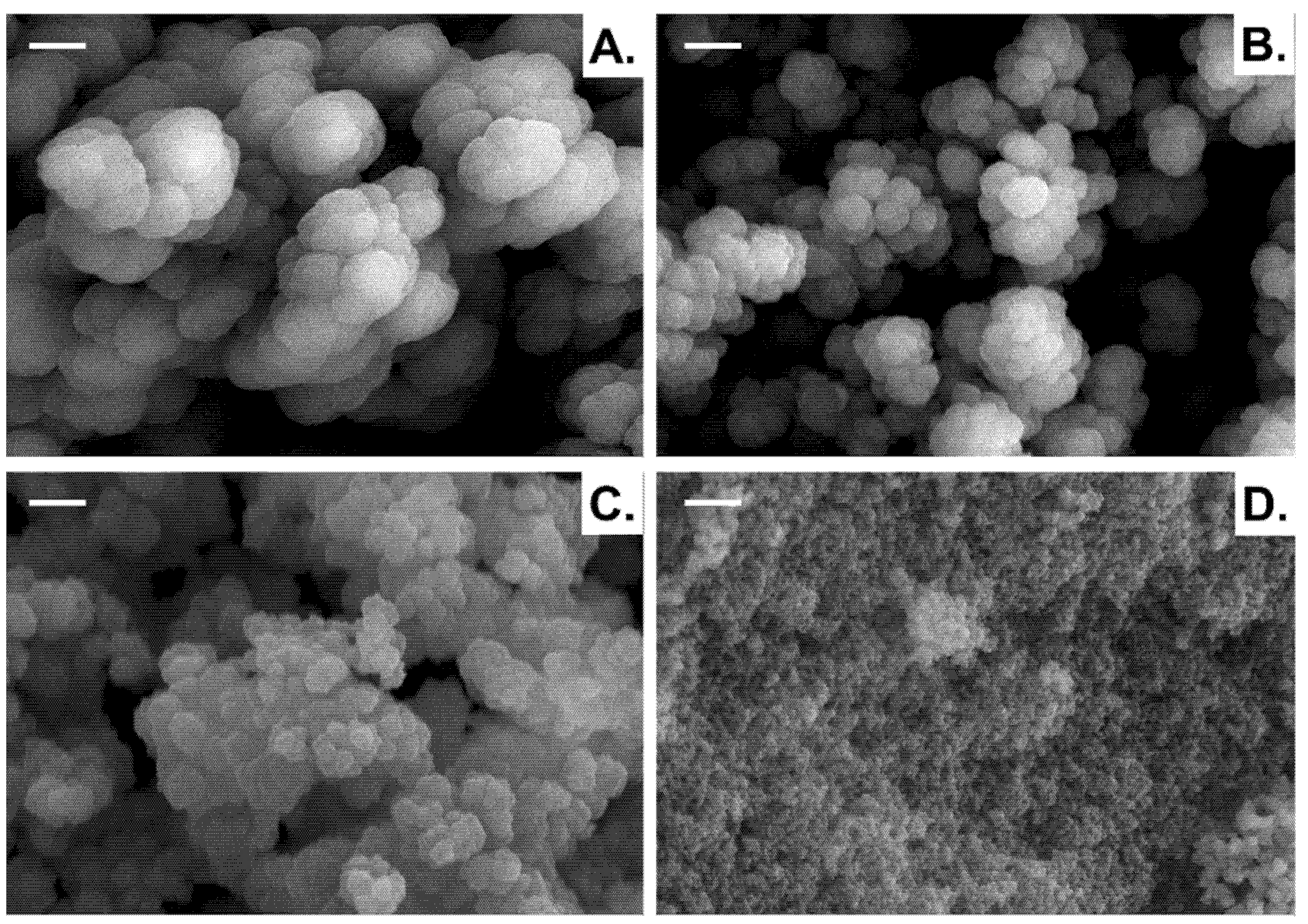
3.1. Effect of Porogenic Solvent Nature on Porous Features of Vanillin-Based Polymeric Materials
3.2. Effect of Co-Monomers/Solvent Volume Ratio on Nanoporous Vanillin-Based Materials
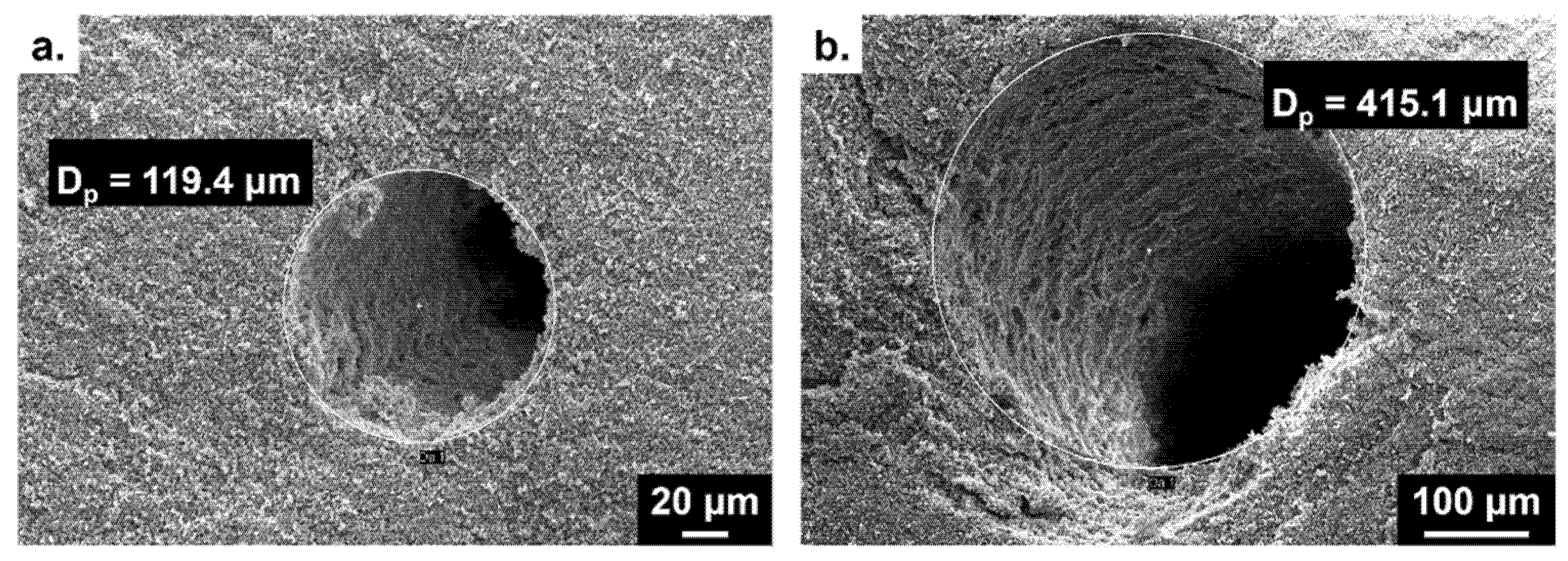
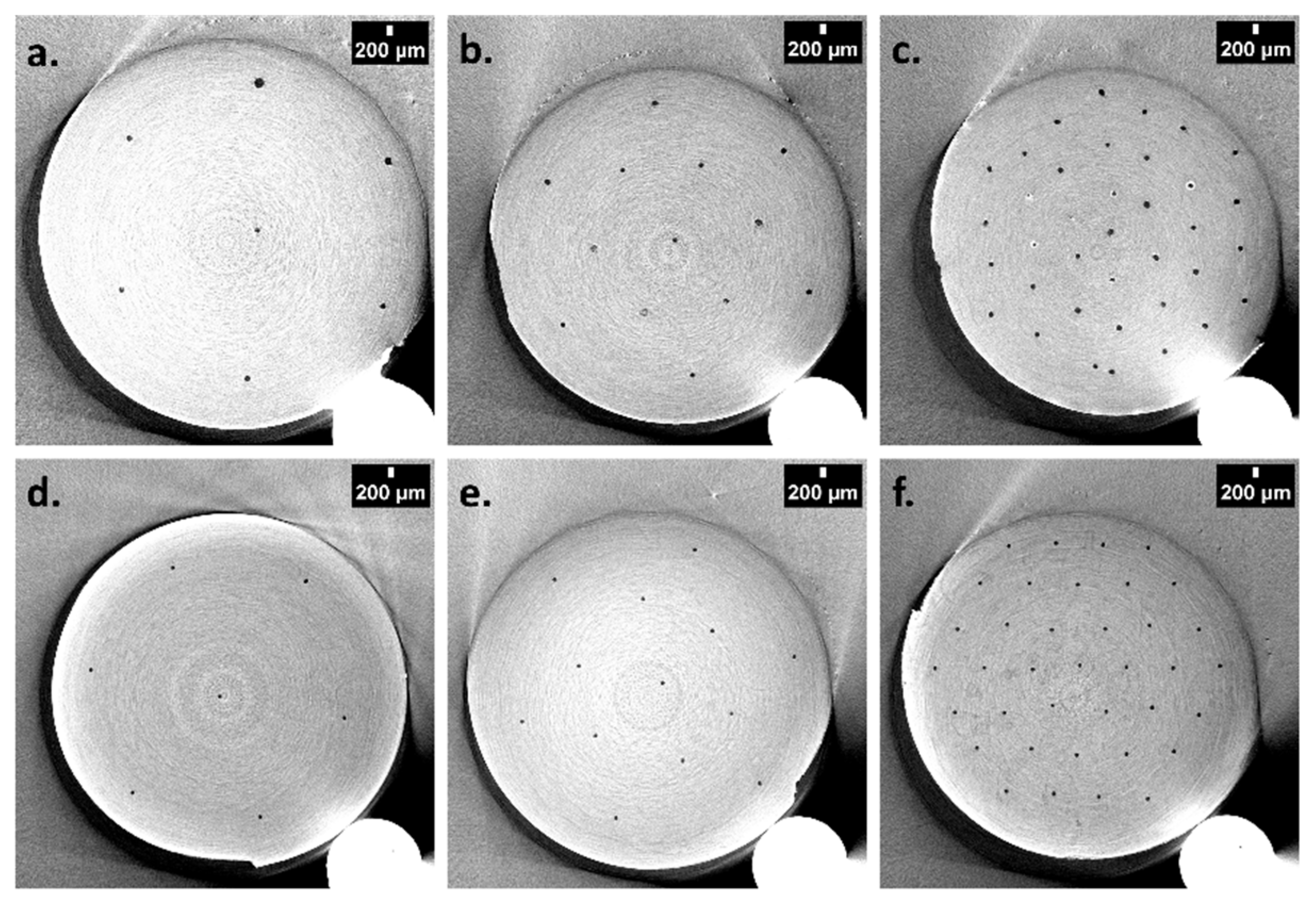
3.3. Design of Doubly Porous Materials with Varying Macroporogen Sizes and Patterns
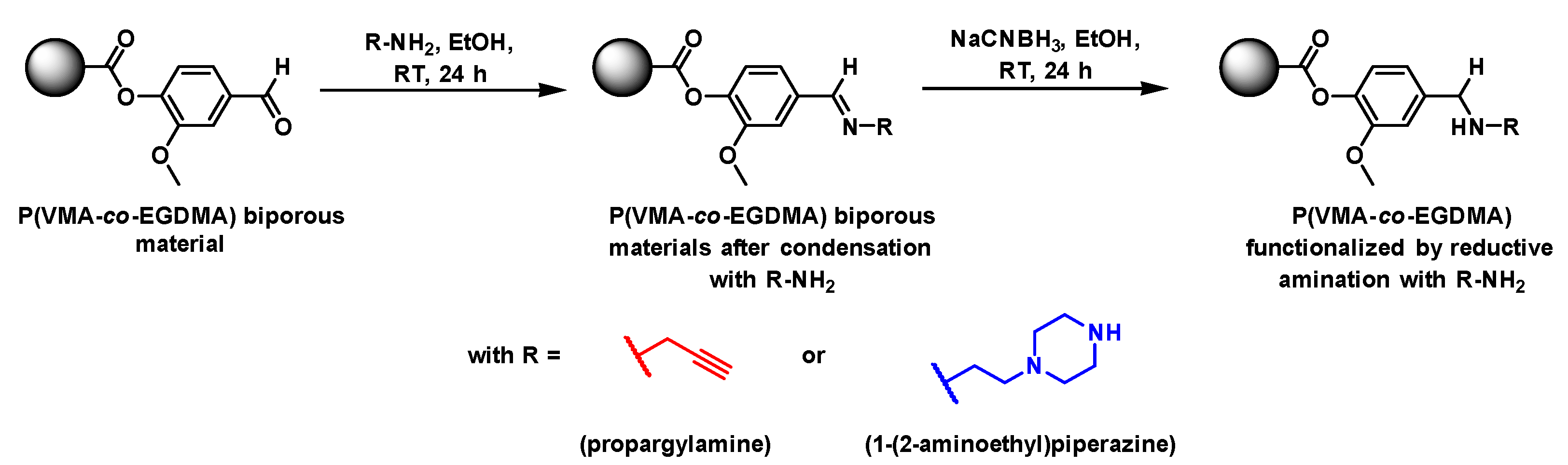
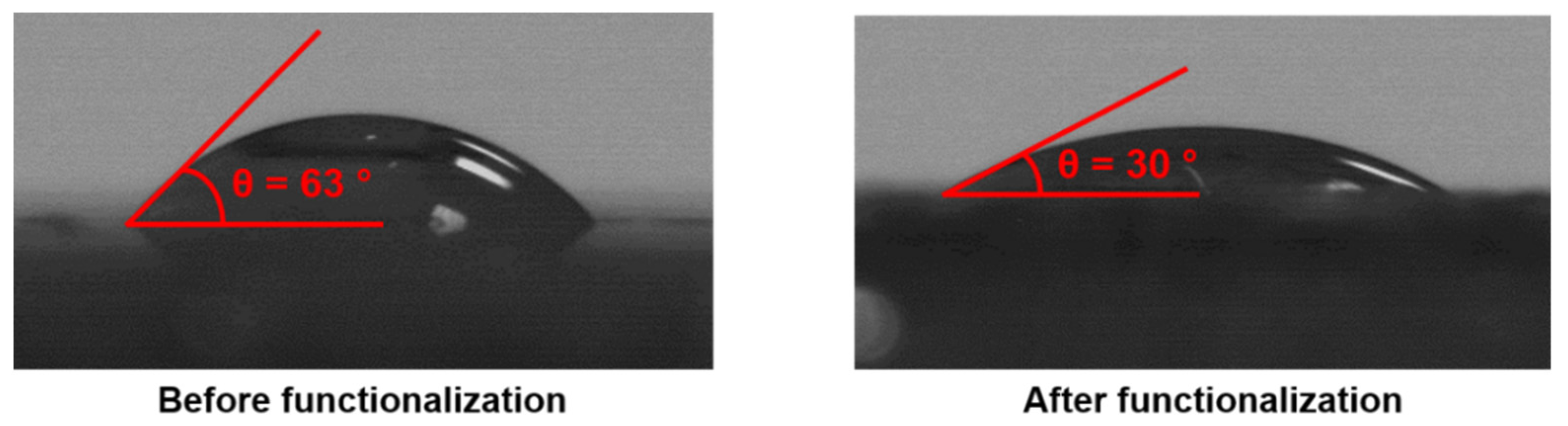
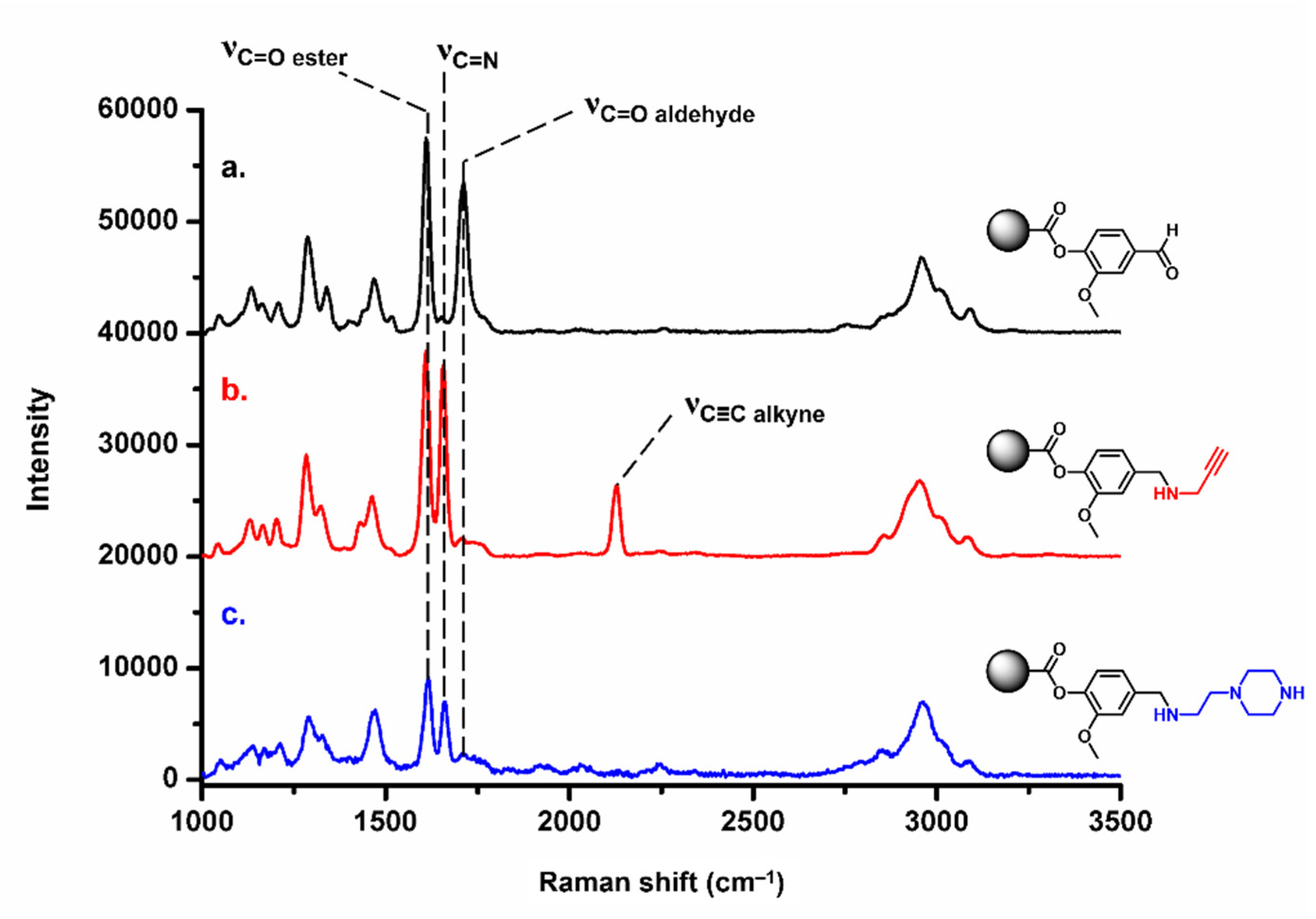
3.4. Functionalization of Vanillin-Based Doubly Porous Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cochard, H.; Delzon, S. Hydraulic failure and repair are not routine in trees. Ann. For. Sci. 2013, 70, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Stroock, A.D.; Pagay, V.V.; Zwieniecki, M.A.; Holbrook, M.N. The Physicochemical Hydrodynamics of Vascular Plants. Annu. Rev. Fluid Mech. 2014, 46, 615–642. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Caré, S.; Courtier-Murias, D.; Faure, P.; Rodts, S.; Coussot, P. Magnetic resonance imaging evidences of the impact of water sorption on hardwood capillary imbibition dynamics. Wood Sci. Technol. 2018, 52, 929–955. [Google Scholar] [CrossRef]
- Zhou, M.; Caré, S.; King, A.; Courtier-Murias, D.; Rodts, S.; Gerber, G.; Aimedieu, P.; Bonnet, M.; Bornert, M.; Coussot, P. Wetting enhanced by water adsorption in hygroscopic plantlike materials. Phys. Rev. Res. 2019, 1, 033190. [Google Scholar] [CrossRef] [Green Version]
- Penvern, H.; Zhou, M.; Maillet, B.; Courtier-Murias, D.; Scheel, M.; Perrin, J.; Weitkamp, T.; Bardet, S.; Caré, S.; Coussot, P. How Bound Water Regulates Wood Drying. Phys. Rev. Appl. 2020, 14, 054051. [Google Scholar] [CrossRef]
- Holmberg, A.L.; Nguyen, N.A.; Karavolias, M.G.; Reno, K.H.; Wool, R.P.; Epps, T.H. Softwood Lignin-Based Methacrylate Polymers with Tunable Thermal and Viscoelastic Properties. Macromolecules 2016, 49, 1286–1295. [Google Scholar] [CrossRef]
- Holmberg, A.L.; Reno, K.H.; Nguyen, N.A.; Wool, R.P.; Epps, T.H. Syringyl Methacrylate, a Hardwood Lignin-Based Monomer for High-Tg Polymeric Materials. ACS Macro Lett. 2016, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.-Z.; Nishihara, H.; Iwamura, S.; Sekiguchi, T.; Sato, A.; Isogai, A.; Kang, F.; Kyotani, T.; Yang, Q.-H. Cellulose Nanofiber as a Distinct Structure-Directing Agent for Xylem-like Microhoneycomb Monoliths by Unidirectional Freeze-Drying. ACS Nano 2016, 10, 10689–10697. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-L.; Yang, N.; Zhou, L.-C.; Ma, Z.-Y.; Zhu, Y.-B.; Lu, Y.-Y.; Qin, B.; Xing, W.-Y.; Ma, T.; Li, S.-C.; et al. Bioinspired polymeric woods. Sci. Adv. 2018, 4, eaat7223. [Google Scholar] [CrossRef] [Green Version]
- Compton, B.G.; Lewis, J.A. 3D-Printing of Lightweight Cellular Composites. Adv. Mater. 2014, 26, 5930–5935. [Google Scholar] [CrossRef]
- Zander, N.E. Hierarchically Structured Electrospun Fibers. Polymers 2013, 5, 19–44. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Zhang, H.; Zhao, Z.; Han, C.C. Construction of hierarchical structures by electrospinning or electrospraying. Polymer 2012, 53, 546–554. [Google Scholar] [CrossRef]
- Auriault, J.L.; Boutin, C. Deformable porous media with double porosity. Quasi-statics. I: Coupling effects. Transp. Porous Media 1992, 7, 63–82. [Google Scholar] [CrossRef] [Green Version]
- Auriault, J.L.; Boutin, C. Deformable porous media with double porosity. Quasi-statics. II: Memory effects. Transp. Porous Media 1993, 10, 153–169. [Google Scholar] [CrossRef]
- Auriault, J.L.; Boutin, C. Deformable porous media with double porosity III: Acoustics. Transp. Porous Media 1994, 14, 143–162. [Google Scholar] [CrossRef] [Green Version]
- Stanzione, J.F., III; Sadler, J.M.; La Scala, J.J.; Wool, R.P. Lignin Model Compounds as Bio-Based Reactive Diluents for Liquid Molding Resins. ChemSusChem 2012, 5, 291–1297. [Google Scholar] [CrossRef]
- Ly, H.B.; le Droumaguet, B.; Monchiet, V.; Grande, D. Facile fabrication of doubly porous polymeric materials with controlled nano- and macro-porosity. Polymer 2015, 78, 13–21. [Google Scholar] [CrossRef]
- Guerrouache, M.; Khalil, A.M.; Kebe, S.; le Droumaguet, B.; Mahouche-Chergui, S.; Carbonnier, B. Monoliths bearing hydrophilic surfaces for in vitro biomedical samples analysis. Surf. Innov. 2015, 3, 84–102. [Google Scholar] [CrossRef]
- Zou, H.F.; Huang, X.D.; Ye, M.L.; Luo, Q.Z. Monolithic stationary phases for liquid chromatography and capillary electrochromatography. J. Chromatogr. A 2002, 954, 5–32. [Google Scholar] [CrossRef]
- Poupart, R.; Grande, D.; Carbonnier, B.; le Droumaguet, B. Porous polymers and metallic nanoparticles: A hybrid wedding as a robust method toward efficient supported catalytic systems. Prog. Polym. Sci. 2019, 96, 21–42. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.A.B.d.; Zabkova, M.; Araújo, J.D.; Cateto, C.A.; Barreiro, M.F.; Belgacem, M.N.; Rodrigues, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Brazinha, C.; Barbosa, D.S.; Crespo, J.G. Sustainable recovery of pure natural vanillin from fermentation media in a single pervaporation step. Green Chem. 2011, 13, 2197–2203. [Google Scholar] [CrossRef]
- Peng, H.; Xiong, H.; Li, J.; Xie, M.; Liu, Y.; Bai, C.; Chen, L. Vanillin cross-linked chitosan microspheres for controlled release of resveratrol. Food Chem. 2010, 121, 23–28. [Google Scholar] [CrossRef]
- le Droumaguet, B.; Lacombe, R.; Ly, H.-B.; Carbonnier, B.; Grande, D. Novel Polymeric Materials with Double Porosity: Synthesis and Characterization. Macromol. Symp. 2014, 340, 18–27. [Google Scholar] [CrossRef]
- le Droumaguet, B.; Lacombe, R.; Ly, H.-B.; Guerrouache, M.; Carbonnier, B.; Grande, D. Engineering functional doubly porous PHEMA-based materials. Polymer 2014, 55, 373–379. [Google Scholar] [CrossRef]
- Lerouge, T.; Maillet, B.; Coutier-Murias, D.; Grande, D.; le Droumaguet, B.; Pitois, O.; Coussot, P. Drying of a Compressible Biporous Material. Phys. Rev. Appl. 2020, 13, 044061. [Google Scholar] [CrossRef]
- Lerouge, T.; Pitois, O.; Grande, D.; le Droumaguet, B.; Coussot, P. Synergistic actions of mixed small and large pores for capillary absorption through biporous polymeric materials. Soft Matter 2018, 14, 8137–8146. [Google Scholar] [CrossRef] [Green Version]
- Ly, H.B.; le Droumaguet, B.; Monchiet, V.; Grande, D. Designing and modeling doubly porous polymeric materials. Eur. Phys. J. Spec. Top. 2015, 224, 1689–1706. [Google Scholar] [CrossRef]
- Ly, H.B.; le Droumaguet, B.; Monchiet, V.; Grande, D. Tailoring doubly porous poly(2-hydroxyethyl methacrylate)-based materials via thermally induced phase separation. Polymer 2016, 86, 138–146. [Google Scholar] [CrossRef]
- Mezhoud, S.; Le Droumaguet, B.; Aimedieu, P.; Monchiet, V.; Bornert, M.; Grande, D. Investigation of morphology associated with biporous polymeric materials obtained by the double porogen templating approach. Colloid Polym. Sci. 2021, 299, 537–550. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Prog. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Grulke, E.A. Polymer Handbook, 4th ed.; Brandrup, E.H., Immergut, E.H., Grulke, E.A., Eds.; Wiley: New York, NY, USA, 1999; p. VII/688. [Google Scholar]
- Weast, R.C. (Ed.) Handbook of Chemistry and Physics, 45th ed.; Chemical Rubber Co.: Cleveland, OH, USA, 1964; p. E-31. [Google Scholar]
- Scipioni, M.; Kay, G.; Megson, I.; Lin, P.K.T. Novel vanillin derivatives: Synthesis, anti-oxidant, DNA and cellular protection properties. Eur. J. Med. Chem. 2018, 143, 745–754. [Google Scholar] [CrossRef]
- Poupart, R.; Benlahoues, A.; le Droumaguet, B.; Grande, D. Porous Gold Nanoparticle-Decorated Nanoreactors Prepared from Smartly Designed Functional Polystyrene-block-Poly(d,l-Lactide) Diblock Copolymers: Toward Efficient Systems for Catalytic Cascade Reaction Processes. ACS Appl. Mater. Interfaces 2017, 9, 31279–31290. [Google Scholar] [CrossRef] [PubMed]


| Solvent | Porosity Ratio 1 (%) | Total Pore Volume 1 (mL.g−1) | Peak Pore Size 1 (µm) | Δ 2 (MPa0.5) | E 3 |
|---|---|---|---|---|---|
| MeOH | 69 | 2.35 | 2.05 | 14.5 | 32.7 |
| EtOH | 64 | 2.50 | 1.61 | 12.7 | 24.5 |
| i-PrOH | 67 | 2.59 | 1.29 | 11.5 | 17.9 |
| t-BuOH | 71 | 2.78 | 1.45 | 10.6 | 15.8 |
| Octanol | 71 | 1.70 | 0.37 | 10.3 | 10.3 |
| Dodecanol | 67 | 1.46 | 0.52 | 9.8 | 6.5 |
| EtOAc | 63 | 1.30 | 0.10 | 9.1 | 6.0 |
| Dioxane | 49 | 0.69 | 0.03 | 7.9 | 2.2 |
| Vol.% Comonomers (VMA/EGDMA) | Vol.% EtOH | Porosity Ratio 1 (%) | Total Pore Volume 1 (mL.g−1) | Peak Pore Size 1 (µm) |
|---|---|---|---|---|
| 5 | 95 | 86 | 3.17 | 1.29 |
| 10 | 90 | 71 | 2.50 | 1.29 |
| 20 | 80 | 64 | 2.30 | 1.61 |
| 25 | 75 | 57 | 2.10 | 2.27 |
| 30 | 70 | 48 | 1.56 | 6.04 |
| 35 | 65 | 37 | 0.85 | 6.67 |
| Number of Threads | Macropore Diameter 1 (µm) | |
|---|---|---|
| VMA-Based Materials | HEMA-Based Materials | |
| 7 | 119.3 | 170.9 |
| 13 | 120.8 | 166.2 |
| 37 | 122.0 | 168.8 |
| Mean value | 120.7 | 168.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srikanthan, V.; Pitois, O.; Coussot, P.; Le Droumaguet, B.; Grande, D. Wood-Mimicking Bio-Based Biporous Polymeric Materials with Anisotropic Tubular Macropores. Polymers 2021, 13, 2692. https://doi.org/10.3390/polym13162692
Srikanthan V, Pitois O, Coussot P, Le Droumaguet B, Grande D. Wood-Mimicking Bio-Based Biporous Polymeric Materials with Anisotropic Tubular Macropores. Polymers. 2021; 13(16):2692. https://doi.org/10.3390/polym13162692
Chicago/Turabian StyleSrikanthan, Vierajitha, Olivier Pitois, Philippe Coussot, Benjamin Le Droumaguet, and Daniel Grande. 2021. "Wood-Mimicking Bio-Based Biporous Polymeric Materials with Anisotropic Tubular Macropores" Polymers 13, no. 16: 2692. https://doi.org/10.3390/polym13162692