Conformation, Self-Organization and Thermoresponsibility of Polymethacrylate Molecular Brushes with Oligo(ethylene glycol)-block-oligo(propylene glycol) Side Chains
Abstract
:1. Introduction
2. Materials and Methods
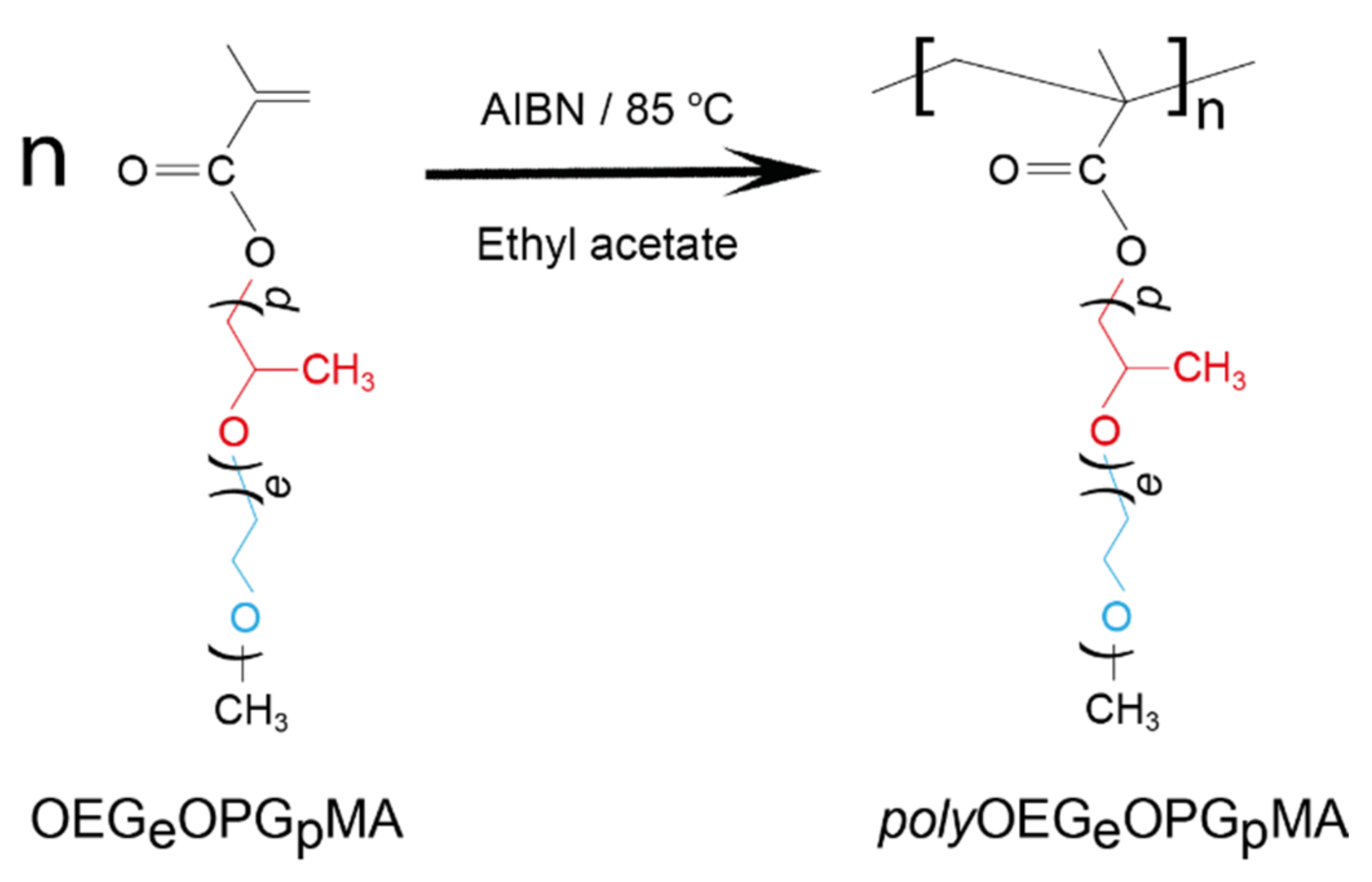
2.1. Copolymer Synthesis
2.2. Methods Molecular Hydrodynamics and Optics
2.3. Investigation of the Self-Assembly of PolyOEGeOPGpMA in Water Solutions
3. Result and Discussion
3.1. Molar-Masses and Hydrodynamic Characteristics
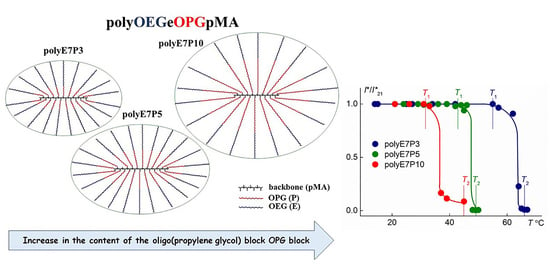
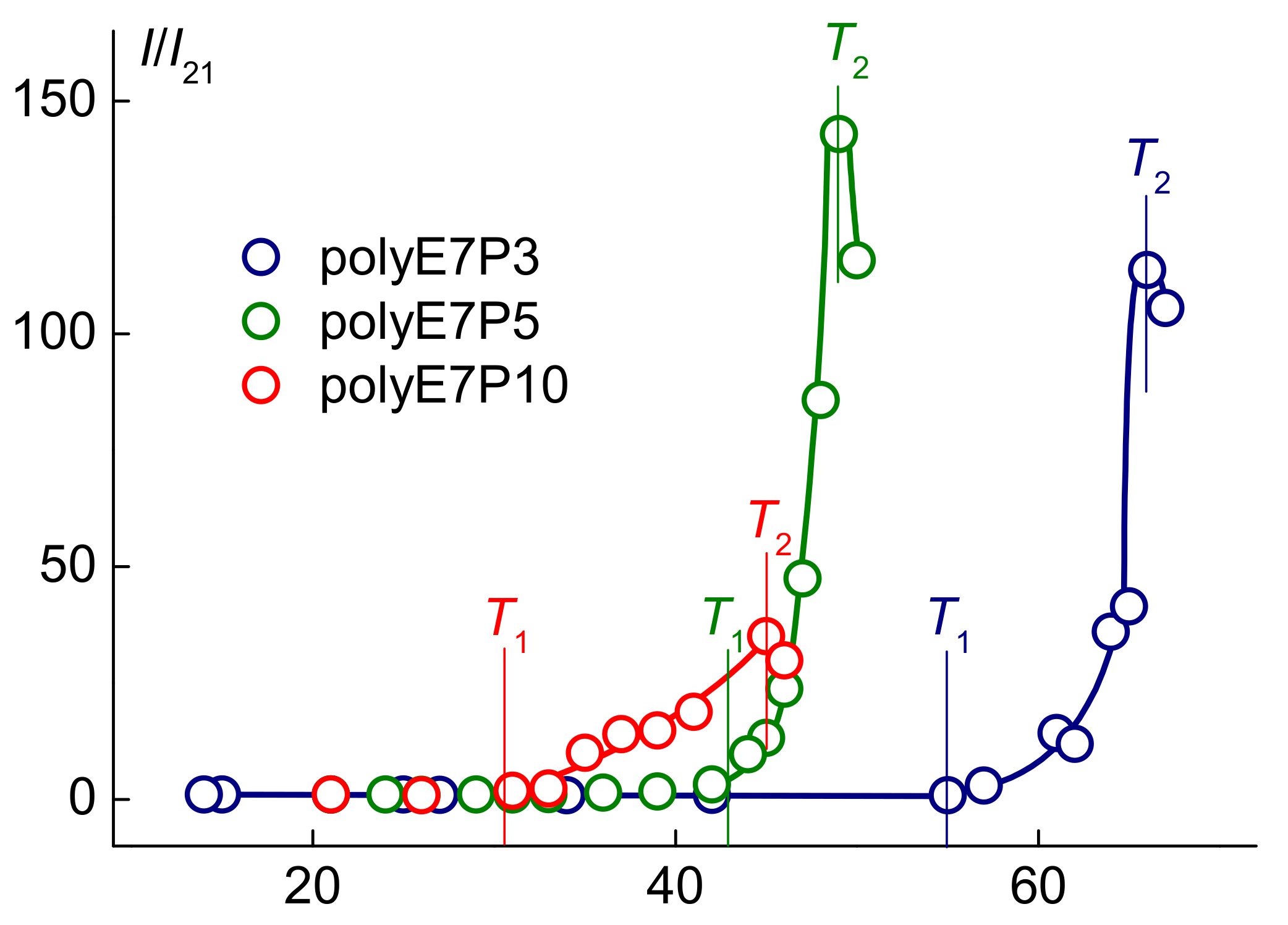
3.2. Properties of the Aqueous Solutions of PolyOEGeOPGpMA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ρ0 | density | g∙cm−3 |
| η0 | dynamic viscosity | cP |
| n0 | refractive index | - |
| Rh-D | hydrodynamic radii of macromolecule | nm |
| Rh-m | hydrodynamic radius of micelle | nm |
| D0 | translation diffusion coefficient | cm2 × g−1 |
| T | absolute temperature | °K |
| T | temperature | °C |
| T1 | the onset of phase separation | °C |
| T2 | the finishing of phase separation | °C |
| Mw | molar mass | g·mol−1 |
| M0 | molar mass repeating units | g·mol−1 |
| A2 | second virial coefficient | cm3·mol·g−2 |
| I90 | the excessive intensity of light scattered at an angle of 90 | Hz |
| dn/dc | refractive index increment | cm3·g−1 |
| [η] | intrisic viscosity | cm3·g−1 |
| A0 | hydrodynamic invariant | erg·K−1mol−1/3 |
| λ0 | wavelength | nm |
| Lb | length of backbone | nm |
| Lsc | lengths of the side chains | nm |
| Nb | polymerization degree | - |
| I*/I*21 | relative optical transmission | - |
| I/I21 | relative light scattering intensity | - |
| I* | intensity of optical transmission | Hz |
| I | light scattering intensity | Hz |
| I21 | light scattering intensity at 21 °C | Hz |
| Si | contribution to the integral scattering intensity | % |
References
- Zhang, M.; Muller, A.H.E. Cylindrical Polymer Brushes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3461–3469. [Google Scholar] [CrossRef]
- Xie, G.; Martinez, M.R.; Olszewski, M.; Sheiko, S.S.; Matyjaszewski, K. Molecular bottlebrushes as novel materials. Biomacromolecules 2019, 20, 27–54. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Meleshko, T.K.; Kashina, A.V.; Yakimansky, A.V. Amphiphilic multicomponent molecular brushes. Russ. Chem. Rev. 2019, 88, 1248–1290. [Google Scholar] [CrossRef]
- Verduzco, R.; Li, X.; Pesek, S.L.; Stein, G.E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, H.; Nie, T.; Chen, Y.; Wang, Z.; Huang, H.; Liu, L.; Chen, Y. Molecular bottlebrush as a unimolecular vehicle with tunable shape for photothermal cancer therapy. Biomaterials 2018, 178, 620–629. [Google Scholar] [CrossRef]
- Müllner, M.; Dodds, S.J.; Nguyen, T.-H.; Senyschyn, D.; Porter, C.J.H.; Boyd, B.J.; Caruso, F. Size and rigidity of cylindrical polymer brushes dictate long circulating properties In Vivo. ACS Nano 2015, 9, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Müllner, M. Molecular polymer brushes in nanomedicine. Macromol. Chem. Phys. 2016, 217, 2209–2222. [Google Scholar] [CrossRef]
- Huang, K.; Canterbury, D.P.; Rzayev, J. Organosoluble polypyrrole nanotubes from core–shell bottlebrush copolymers. Chem. Commun. 2010, 46, 6326–6328. [Google Scholar] [CrossRef] [PubMed]
- Müllner, M.; Yuan, J.; Weiss, S.; Walther, A.; Förtsch, M.; Drechsler, M.; Müller, A.H.E. Water-soluble organo–silica hybrid nanotubes templated by cylindrical polymer brushes. J. Am. Chem. Soc. 2010, 132, 16587–16592. [Google Scholar] [CrossRef]
- Müllner, M.; Lunkenbein, T.; Schieder, M.; Gröschel, A.H.; Miyajima, N.; Förtsch, M.; Breu, J.; Caruso, F.; Müller, A.H.E. Template-directed mild synthesis of anatase hybrid nanotubes within cylindrical core–shell–corona polymer brushes. Macromolecules 2012, 45, 6981–6988. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers Hydrodynamic and Optical Properties in Solution, 1st ed.; Plenum Press: New York, NY, USA, 1989; pp. 1–512. [Google Scholar]
- Simonova, M.; Ivanov, I.; Meleshko, T.; Kopyshev, A.; Santer, S.; Yakimansky, A.; Filippov, A. Self-Assembly of Molecular Brushes with Polyimide Backbone and Amphiphilic Block-Copolymer Side Chains in Selective Solvents. Polymers 2020, 12, 2922. [Google Scholar] [CrossRef]
- Rodchenko, S.; Amirova, A.; Kurlykin, M.; Tenkovtsev, A.; Milenin, S.; Filippov, A. Amphiphilic Molecular Brushes with Regular Polydimethylsiloxane Backbone and Poly-2-isopropyl2-oxazoline Side Chains. 2. Self-Organization in Aqueous Solutions on Heating. Polymers 2021, 13, 31. [Google Scholar]
- Filippov, A.P.; Belyaeva, E.V.; Krasova, A.S.; Simonova, M.A.; Meleshko, T.K.; Ilgach, D.M.; Bogorad, N.N.; Yakimansky, A.V.; Larin, S.V.; Darinskii, A.A. Conformations of Molecular Brushes Based on Polyimide and Poly(methyl methacrylate) in Selective Solvents: Experiment and Computer Simulation. Polym. Sci. Ser. A 2014, 56, 393–404. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Krasova, A.S.; Simonova, M.A.; Tarabukina, E.B.; Meleshko, T.K.; Ilgach, D.M.; Bogorad, N.N.; Yakimansky, A.V. Synthesis and investigation of the solution behavior of graft block-copolymers of polyimide and poly(methyl methacrylate. Polym. Sci. Ser. A. 2014, 56, 1–9. [Google Scholar] [CrossRef]
- Meleshko, T.K.; Ivanov, I.V.; Kashina, A.V.; Bogorad, N.N.; Simonova, M.A.; Zakharova, N.V.; Filippov, A.P.; Yakimansky, A.V. Diphilic macromolecular brushes with a polyimide backbone and poly(methacrylic acid) blocks in side chains. Polym. Sci. Ser. B 2018, 60, 35–50. [Google Scholar] [CrossRef]
- Zakharova, N.V.; Simonova, M.A.; Zelinskii, S.N.; Annenkov, V.V.; Filippov, A.P. Synthesis, molecular characteristics, and stimulus-sensitivity of graft copolymer of chitosan and poly (N, N-diethylacrylamide). J. Mol. Liquids 2019, 292, 111355. [Google Scholar] [CrossRef]
- Kudryavtseva, A.A.; Kurlykin, M.P.; Tarabukina, E.B.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of thermosensitive graft copolymer with aromatic polyester backbone and poly-2-ethyl-2-oxazoline side chains in aqueous solutions. Int. J. Polym. Anal. Charact. 2017, 22, 526–533. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethyleneglycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Zhang, X.J.; Dai, Y. Recent development of brush polymers via polymerization of poly(ethylene glycol)-based macromonomers. Polym. Chem. 2019, 10, 2212–2222. [Google Scholar] [CrossRef]
- Badi, N. Non-linear PEG-based thermoresponsive polymer systems. Prog. Polym. Sci. 2017, 66, 54–79. [Google Scholar] [CrossRef]
- Liu, M.; Leroux, J.C.; Gauthier, M.A. Conformation-function relationships for the combshaped polymer pOEGMA. Prog. Polym. Sci. 2015, 48, 111–121. [Google Scholar] [CrossRef]
- Lutz, J.F.; Hoth, A.; Schade, K. Design of Oligo(ethylene glycol)-Based Thermoresponsive Polymers: An Optimization Study. Des. Monomers Polym. 2009, 12, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Neugebauer, D. Graft copolymers with poly(ethylene oxide) Segments. Polym. Int. 2007, 56, 1469–1498. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, W.; Zhou, S. Engineering oligo(ethylene glycol)-based thermosensitive microgels for drug delivery applications. Polymer 2010, 51, 3926–3933. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Z.C.; Chen, D.J. Thermo- and pH-sensitive behavior of hydrogels based on oligo (ethylene glycol) methacrylates and acrylic acid. J. Mat. Sci. 2012, 47, 1280–1288. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Orekhov, D.V.; Kazantsev, O.A.; Sivokhin, A.P.; Savinova, M.V. Features of the acidcatalyzed hydrolysis of mono- and poly(ethylene glycol) methacrylates. Europ. Polym. J. 2018, 100, 18–24. [Google Scholar] [CrossRef]
- Shibata, M.; Matsumoto, M.; Hirai, Y.; Takenaka, M.; Sawamoto, M.; Terashima, T. Intramolecular Folding or Intermolecular Self-Assembly of Amphiphilic Random Copolymers: On-Demand Control by Pendant Design. Macromolecules 2018, 51, 3738–3745. [Google Scholar] [CrossRef]
- Matsumoto, M.; Takenaka, M.; Sawamoto, M.; Terashima, T. Self-assembly of amphiphilic block pendant polymers as microphase separation materials and folded flower micelles. Polym Chem. 2019, 10, 4954–4961. [Google Scholar] [CrossRef]
- Sivokhin, A.P.; Orekhov, D.V.; Kazantsev, O.A.; Gubanova, O.V.; Kamorin, D.M.; Zarubina, I.S.; Bolshakova, E.A.; Zaitsev, S.D. Amphiphilic thermoresponsive copolymer bottlebrushes: Synthesis, characterization and study of their self-assembly into flower-like micelles. Polym. J. 2021, 53, 655–665. [Google Scholar] [CrossRef]
- Paris, R.; Quijada-Garrido, I. Synthesis and aggregation properties in water solution of comblike methacrylic polymers with oligo(propylene glycol)-block-oligo(ethylene glycol) as side chains. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1928–1932. [Google Scholar] [CrossRef]
- Kazantsev, O.A.; Bolshakova, E.A.; Orekhov, D.V.; Simagin, A.S.; Kamorin, D.M.; Sivokhin, A.P. Synthesis and thermoresponsive properties of polymethacrylate molecular brushes with oligo(ethylene glycol)-block-oligo(propylene glycol) side chains. Polym. Bulletin. 2021, in press. [Google Scholar]
- Zhao, C.L.; Winnik, M.A.; Riess, G.; Croucher, M.D. Fluorescence probe techniques used to study micelle formation in water-soluble block copolymers. Langmuir 1990, 6, 514–516. [Google Scholar] [CrossRef]
- Naksuriya, O.; Shi, Y.; van Nostrum, C.F.; Anuchapreeda, S.; Hennink, W.E.; Okonogi, S. HPMA-based polymeric micelles for curcumin solubilization and inhibition of cancer cell growth. Europ. J. Pharm. Biopharm. 2015, 94, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Filippov, A.; Tarabukina, E.; Simonova, M.; Kirila, T.; Fundueanu, G.; Harabagiu, V.; Constantin, M.; Popescu, I. Synthesis and Investigation of double stimuli-responsive behavior of N-Isopropylacrylamide and maleic acid copolymer in solutions. J. Macromol. Sci. Part B 2015, 54, 1105–1121. [Google Scholar] [CrossRef]
- Simonova, M.A.; Tarasova, E.V.; Dudkina, M.M.; Tenkovtsev, A.V.; Filippov, A.P. Synthesis and hydrodynamic and conformation properties of star-shaped polystyrene with calix[8]arene core. Int. J. Polym. Anal. Charact. 2019, 24, 87–95. [Google Scholar] [CrossRef]
- Burchard, W. Solution properties of branched macromolecules. Adv. Polym. Sci. 1999, 143, 113–194. [Google Scholar]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. A hydrodynamic invariant of polymeric molecules. Russ. Chem. Rev. 1982, 51, 975–993. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic invariant of polymer molecules. J. Polym. Sci. 1984, 22, 3447–3486. [Google Scholar] [CrossRef]
- Kirila, T.; Smirnova, A.; Razina, A.; Tenkovtsev, A.; Filippov, A. Synthesis and conformational characteristics of thermosensitive star-shaped six-arm polypeptoids. Polymers 2020, 12, 800. [Google Scholar] [CrossRef] [Green Version]
- Simonova, M.A.; Khayrullin, A.R.; Tyurina, V.O.; Filippov, A.P.; Sadikov, A.Y.; Kamorin, D.M.; Kamorina, S.I. Self-Organization Processes in Poly (N-[2-(diethylamino) ethyl] acrylamide) Buffer Solutions with Change in Concentration and pH of a Medium. Polym. Sci. 2020, 62, 24–31. [Google Scholar] [CrossRef]
- Simonova, M.A.; Khayrullin, A.R.; Turina, V.O.; Kamorina, S.I.; Kamorin, D.M.; Sadikov, A.Y.; Filippov, A.P. Self-organization in water solutions of thermosensitive linear copolymers based on N-(dimethylamino)ethyl methacrylate. Int. J. Polym. Anal. Charact. 2019, 24, 630–642. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, I.; Trzebicka, B.; Muller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Prog. Polym. Sci. 2007, 32, 1275–1343. [Google Scholar] [CrossRef]
- Winnik, M.; Yekta, A. Associative polymers in aqueous solution. Curr. Opin. Colloid Interface Sci. 1997, 2, 424–436. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly (2-oxazoline) s: A polymer class with numerous potential applications. Angew. Chem. Int. Ed. 2009, 48, 7978–7994. [Google Scholar] [CrossRef]
- Kratochvil, P. Classical Light Scattering from Polymer Solution, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 1–346. [Google Scholar]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions, 1st ed.; Springer: Berlin, Germany, 2007; pp. 1–187. [Google Scholar]
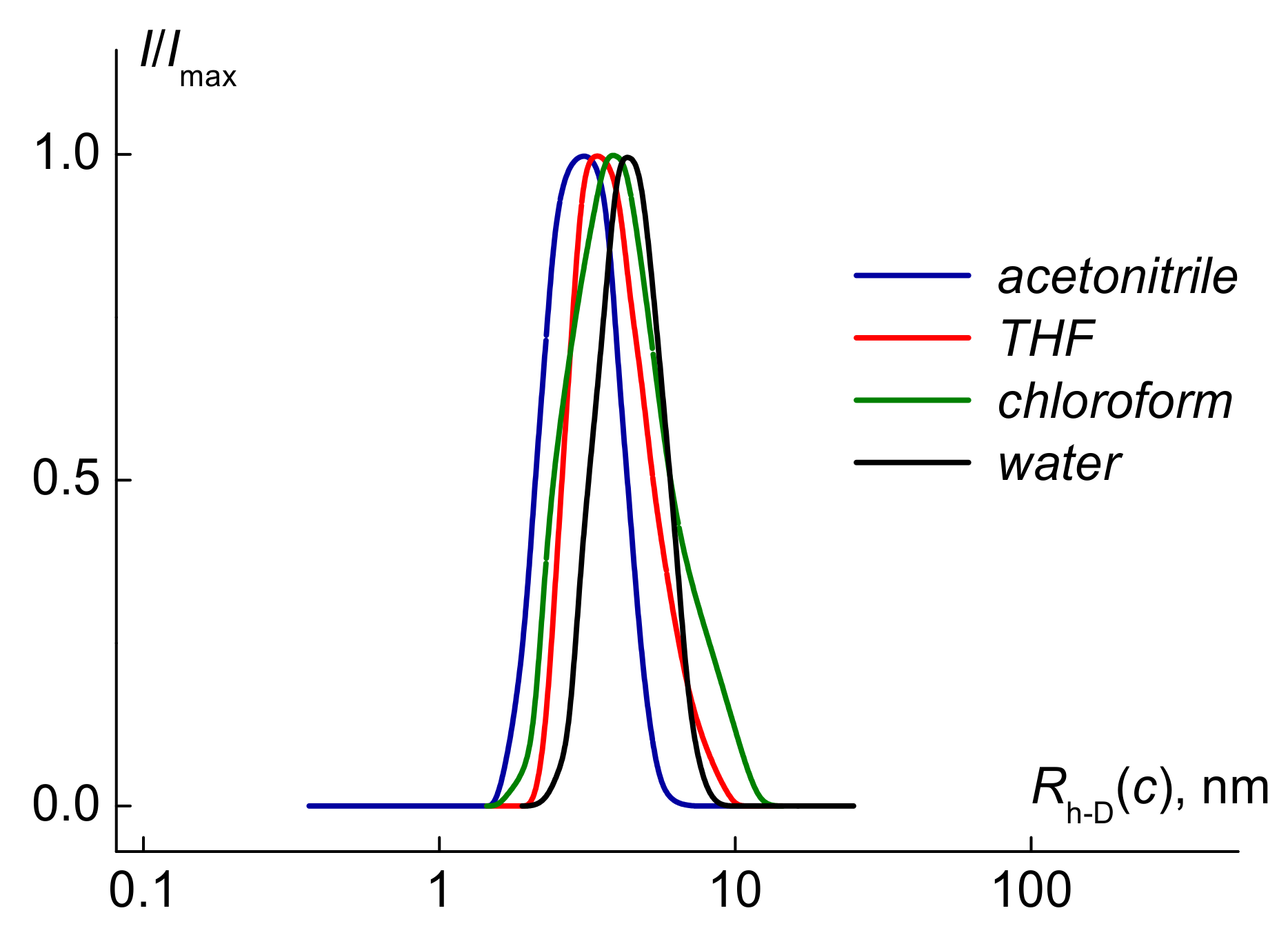



| Solvent | Mw, g·mol−1 | Rh-D, nm | [η], cm3·g−1 | A0 × 1010, erg·K−1mol−1/3 | CMC, wt% |
|---|---|---|---|---|---|
| polyE7P3 | |||||
| water | 33,000 ± 3000 | 3.2 ± 0.3 | 12 ± 0.6 | 3.9 | 3.4∙10−4 |
| chloroform | 20,000 ± 2000 | 2.1 ± 0.2 | 8.0 ± 0.4 | 4.1 | |
| THF | 10,000 ± 1000, 11,000 * | 2.1 ± 0.2 | 6.3 ± 0.3 | 3.0 | |
| acetonitrile | 12,000 ± 1200 | 2.1 ± 0.2 | 6.0 ± 0.3 | 2.9 | |
| polyE7P5 | |||||
| water | 180,000 ± 1800 | 4.2 ± 0.3 | 15 ± 0.6 | 5.2 | 3.2∙10−4 |
| chloroform | 50,000 ± 4000 | 3.9 ± 0.3 | 9.9 ± 0.5 | 3.9 | |
| THF | 15,000 ± 1500, 14,000 * | 2.9 ± 0.3 | 8.0 ± 0.4 | 2.7 | |
| acetonitrile | 15,000 ± 1500 | 2.9 ± 0.3 | 8.0 ± 0.4 | 2.7 | |
| polyE7P10 | |||||
| water | 77,000 ± 7000 | 4.3 ± 0.4 | 19 ± 0.8 | 4.2 | 2.7∙10−4 |
| chloroform | 87,000 ± 8000 | 4.2 ± 0.4 | 12 ± 0.6 | 3.9 | |
| THF | 20,000 ± 2000 15,000 * | 3.2 ± 0.3 | 11 ± 0.5 | 3.0 | |
| acetonitrile | 17,000 ± 1700 | 3.0 ± 0.3 | 11 ± 0.5 | 2.8 | |
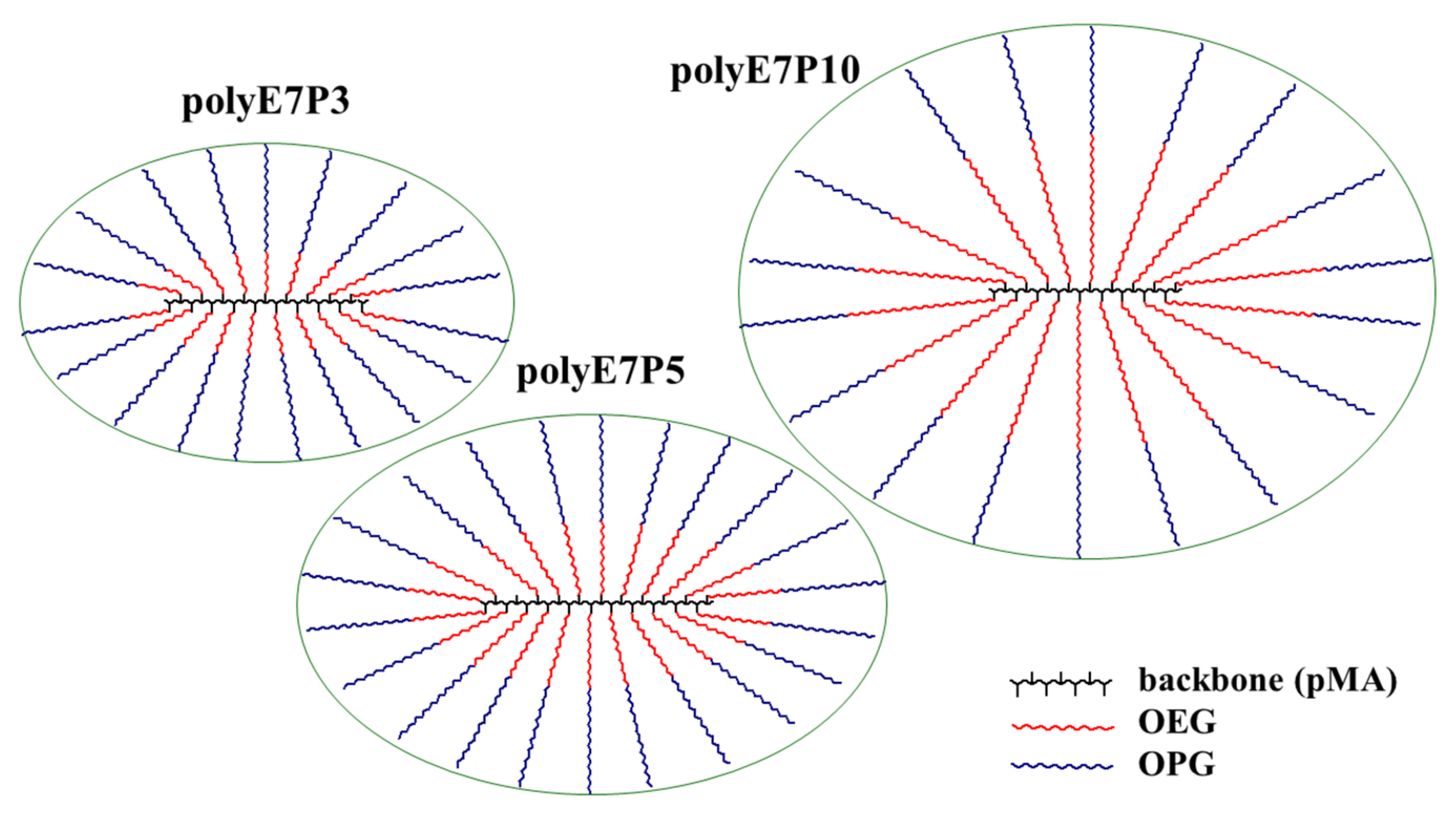
| Sample | Mw, g·mol−1 | Nb | Lb, nm | Lsc, nm |
|---|---|---|---|---|
| polyE7P3 | ~11,000 | 19 | 4.8 | 4.2 |
| polyE7P5 | 15,000 | 22 | 5.5 | 4.9 |
| polyE7P10 | 17,000 | 18 | 4.6 | 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonova, M.; Kamorin, D.; Kazantsev, O.; Nepomnyashaya, M.; Filippov, A. Conformation, Self-Organization and Thermoresponsibility of Polymethacrylate Molecular Brushes with Oligo(ethylene glycol)-block-oligo(propylene glycol) Side Chains. Polymers 2021, 13, 2715. https://doi.org/10.3390/polym13162715
Simonova M, Kamorin D, Kazantsev O, Nepomnyashaya M, Filippov A. Conformation, Self-Organization and Thermoresponsibility of Polymethacrylate Molecular Brushes with Oligo(ethylene glycol)-block-oligo(propylene glycol) Side Chains. Polymers. 2021; 13(16):2715. https://doi.org/10.3390/polym13162715
Chicago/Turabian StyleSimonova, Maria, Denis Kamorin, Oleg Kazantsev, Maria Nepomnyashaya, and Alexander Filippov. 2021. "Conformation, Self-Organization and Thermoresponsibility of Polymethacrylate Molecular Brushes with Oligo(ethylene glycol)-block-oligo(propylene glycol) Side Chains" Polymers 13, no. 16: 2715. https://doi.org/10.3390/polym13162715