Functionalization of Silicone Surface with Drugs and Polymers for Regulation of Capsular Contracture
Abstract
:1. Introduction
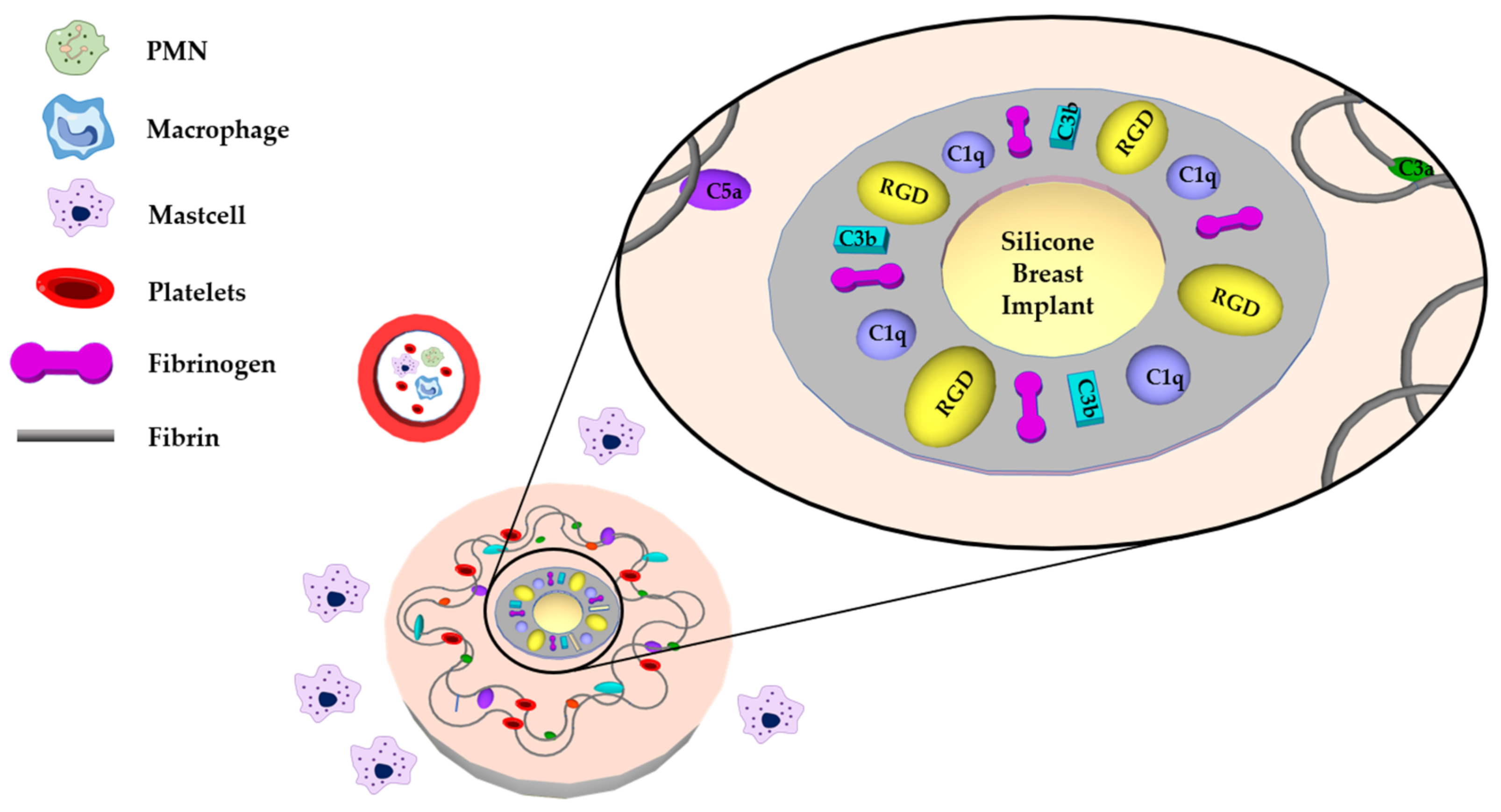
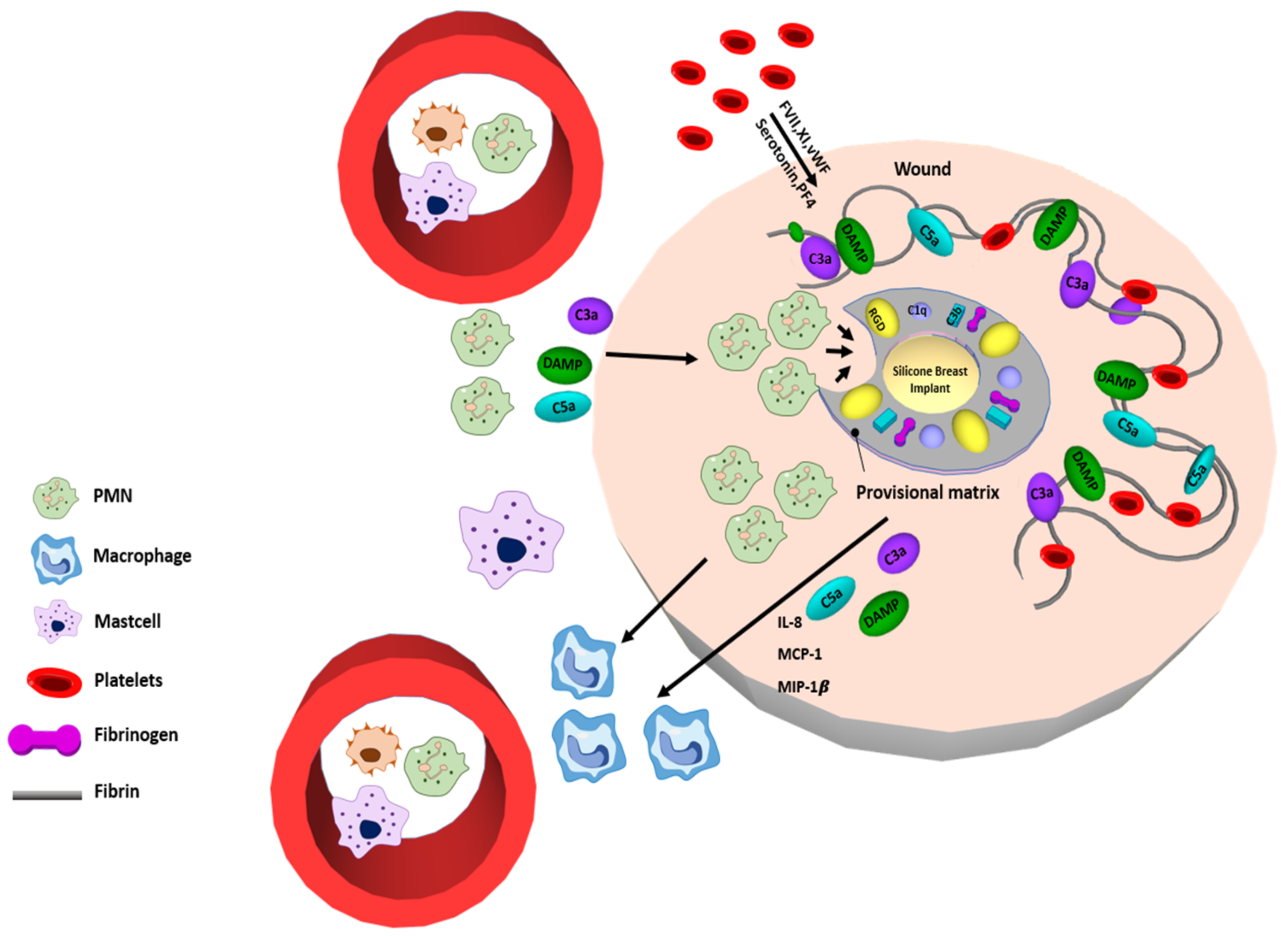
2. The Procedure of Fibrosis Formation
3. Surface Modification Using Drugs
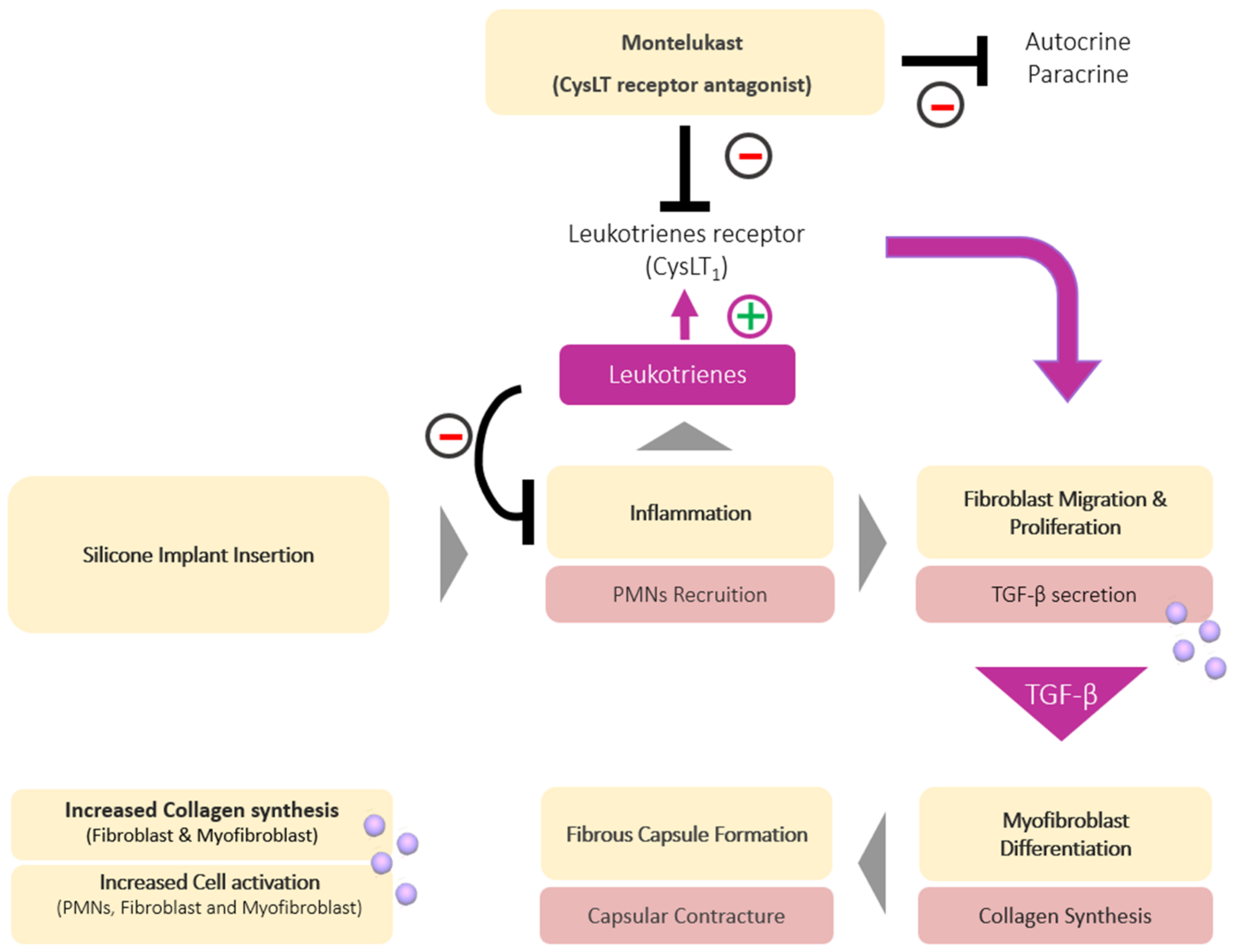
3.1. Montelukast
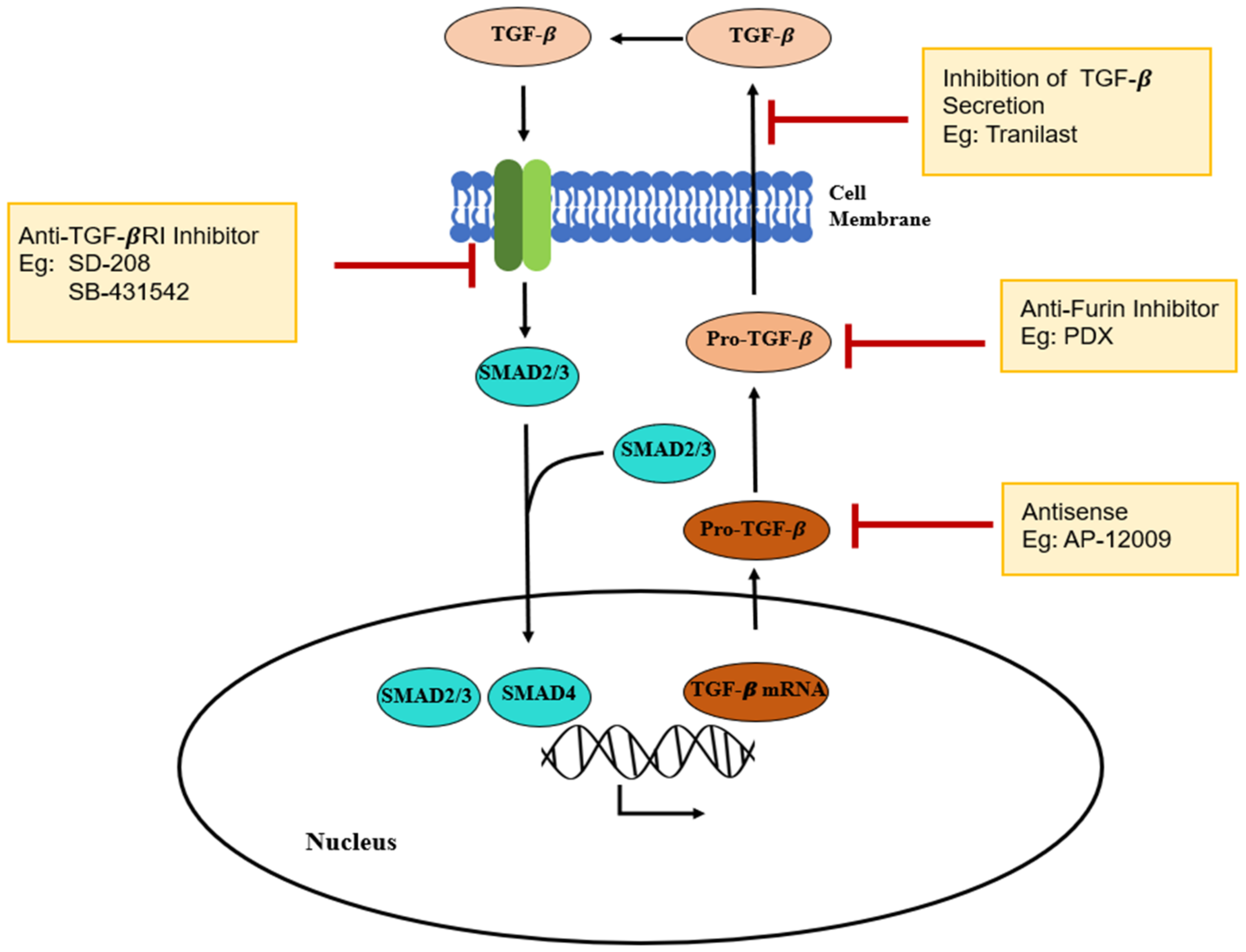
3.2. Tranilast
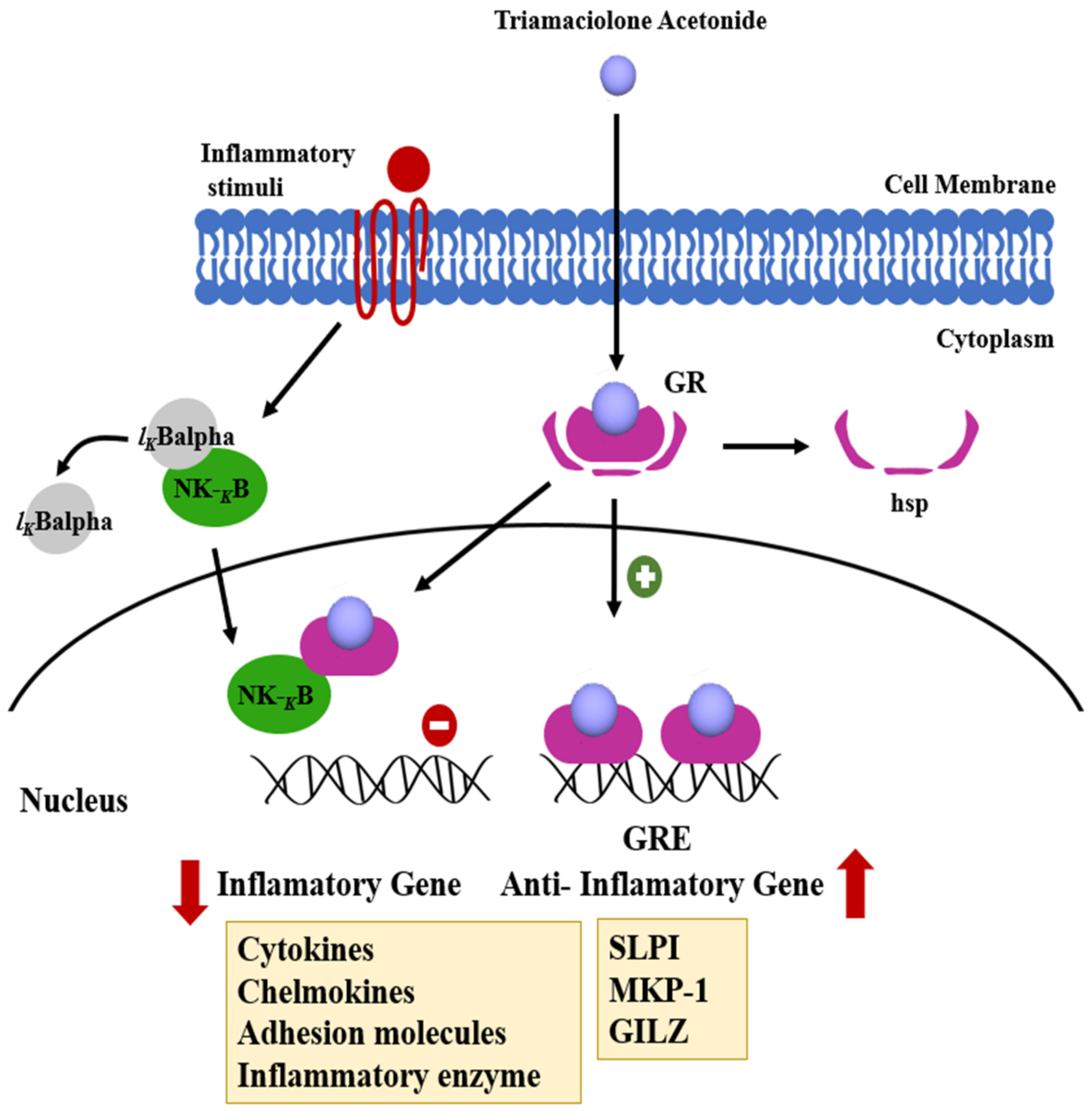
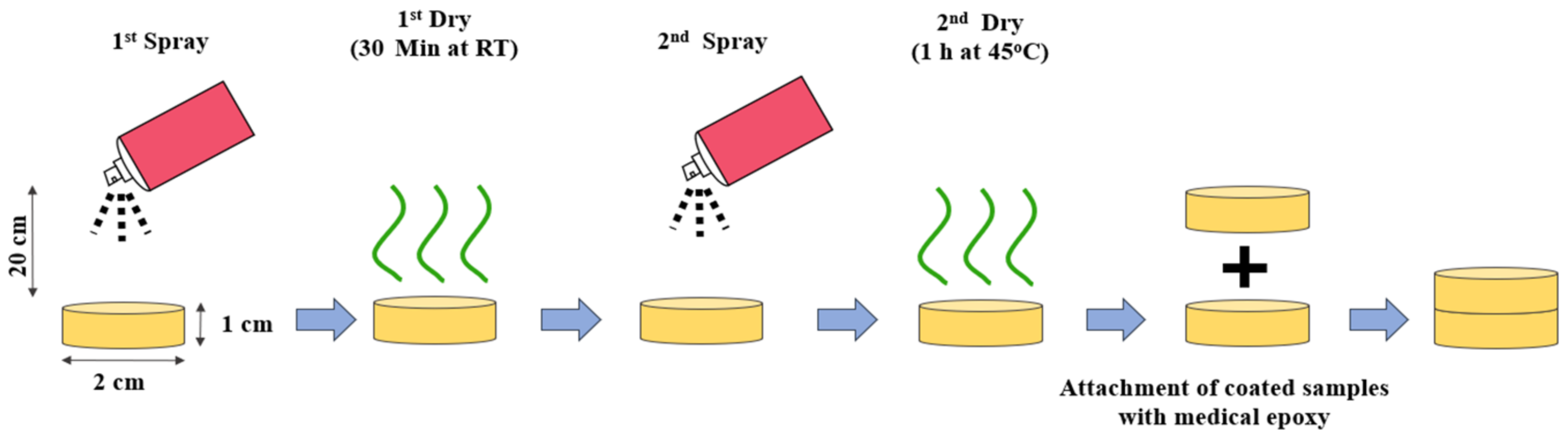
3.3. Triamcinolone
3.4. Itaconic Acid
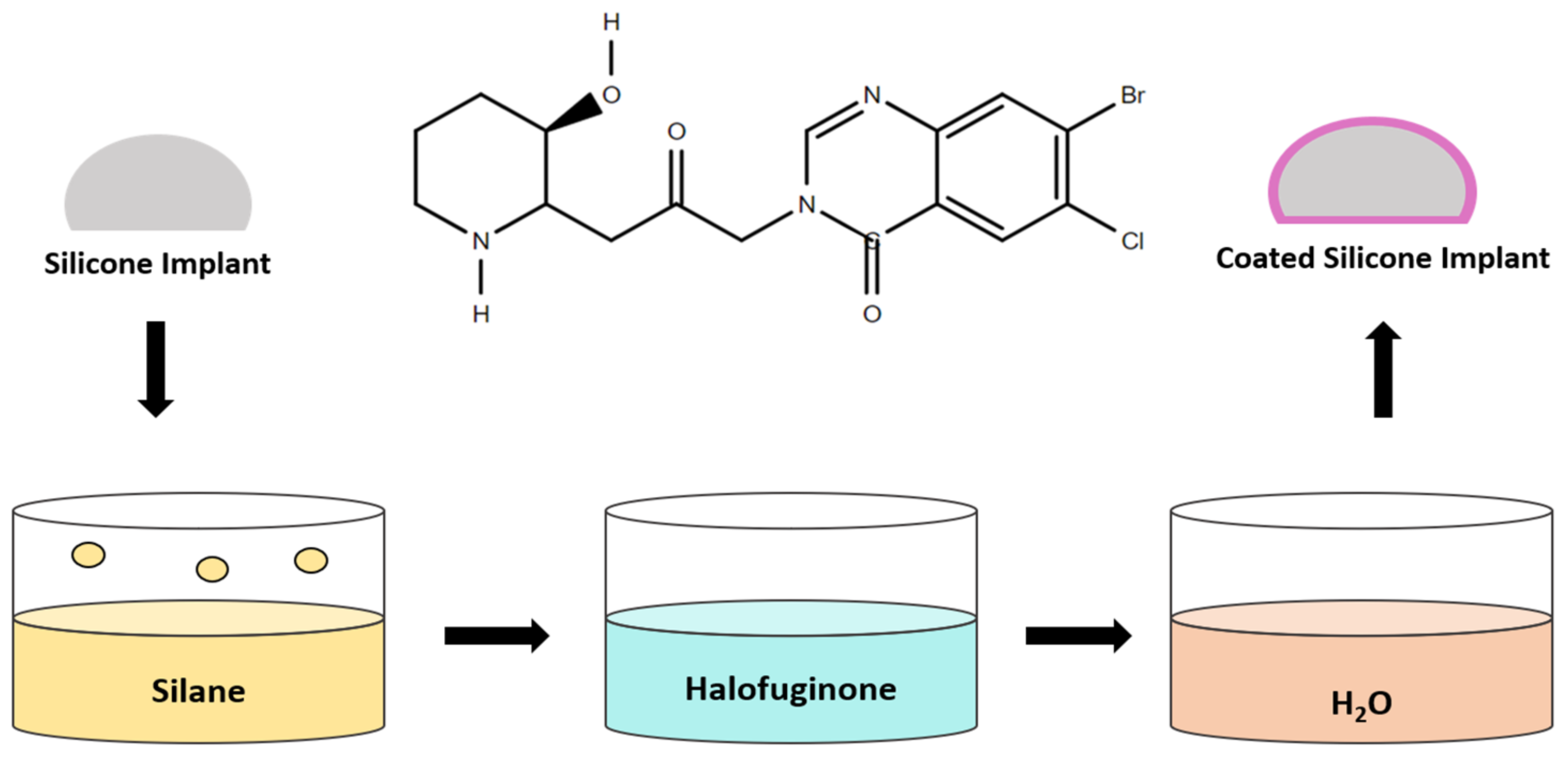
3.5. Halofuginone
4. Surface Modification Using Polymers
4.1. Natural Polymers
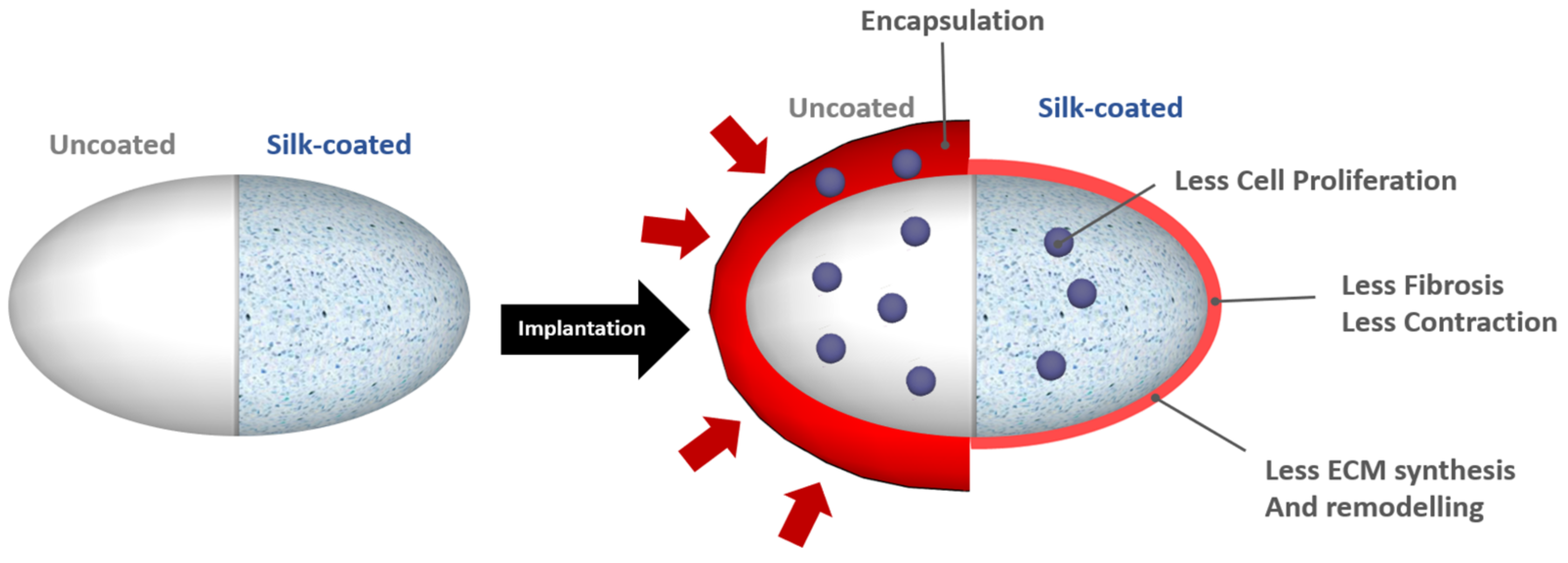
4.1.1. Spider Silk
4.1.2. Interleukin-4(IL-4)
4.2. Neutral Hydrophilic Polymers
4.2.1. Poly(glycerol monomethacrylate)
4.2.2. Polyethylene Glycol
4.3. Zwitterionic Polymer Coating
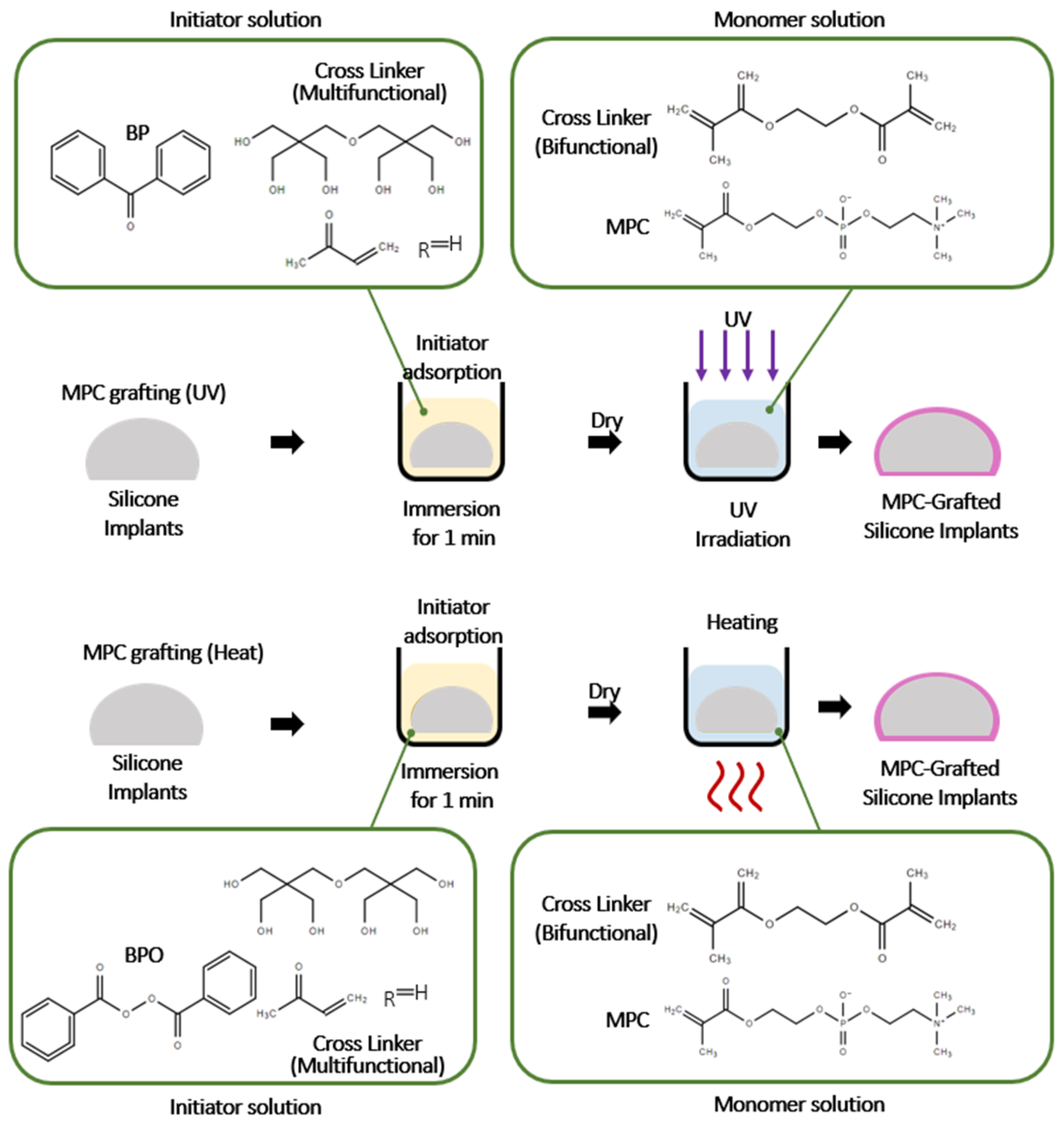
4.3.1. Methacryloxyethyl Phosphorylcholine (MPC)
4.3.2. Poly(carboxybetaine methacrylate) and Poly(sulfobetaine methacrylate) (pSBMA)
5. Surface Modification Techniques
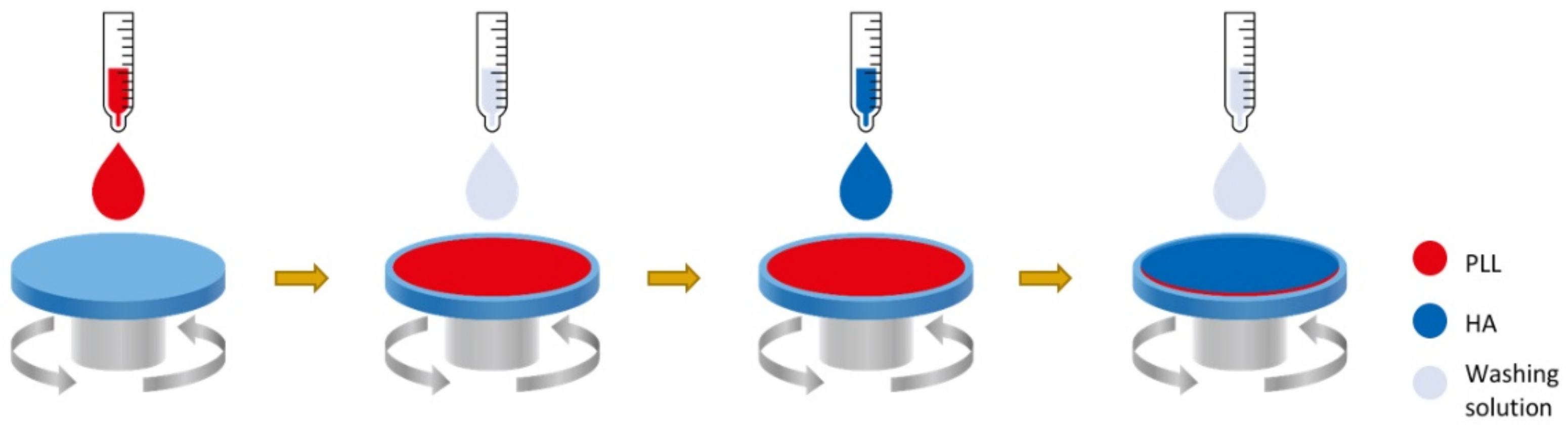
5.1. Layer-by-Layer Deposition Techniques (LBL)
5.2. Microgrooved Pattern
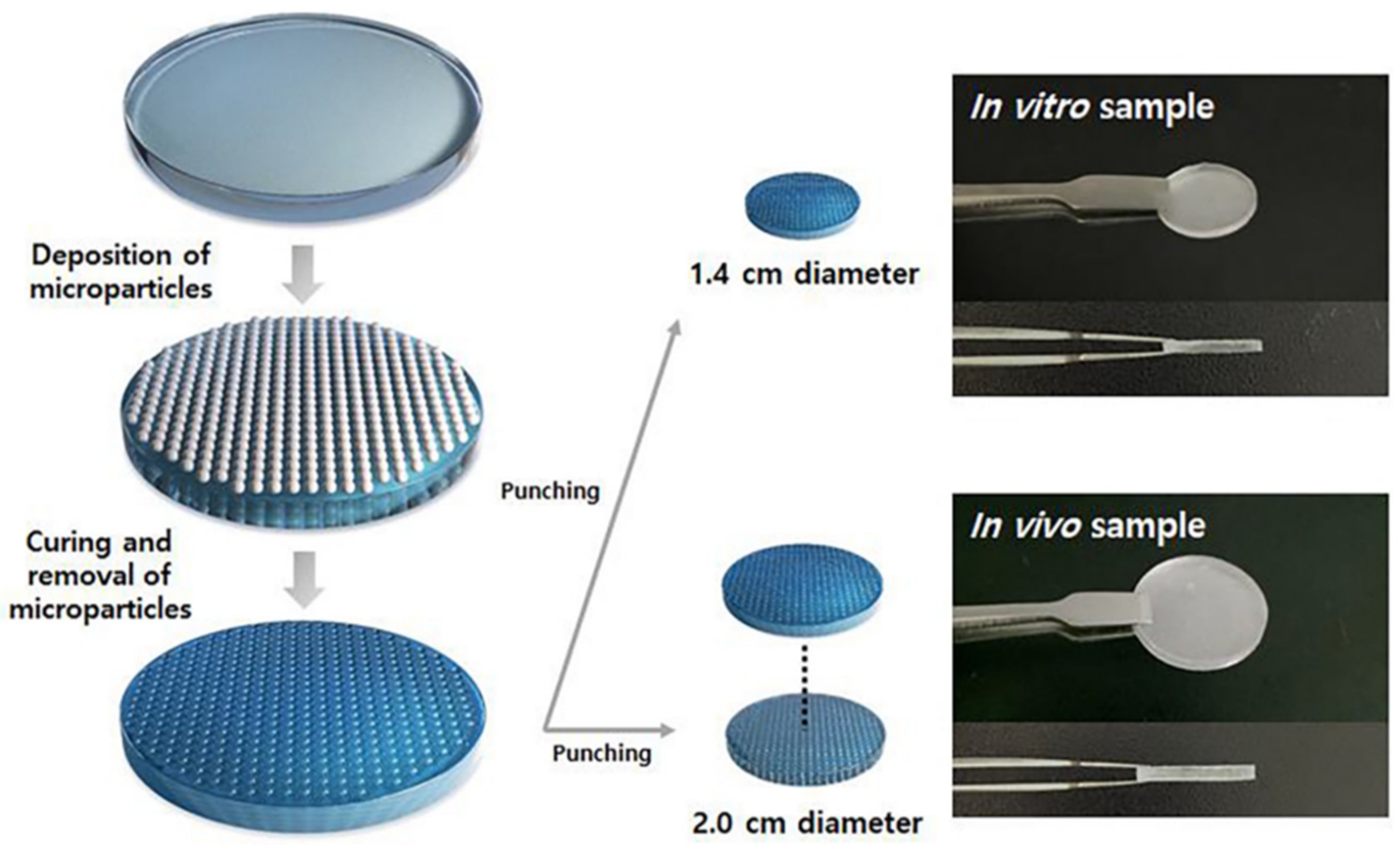
5.3. Dot Pattern
5.4. Drug Delivery Net (DDN) Method
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baino, F. The Use of Polymers in the Treatment of Retinal Detachment: Current Trends and Future Perspectives. Polymers 2010, 2, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Baino, F. Scleral buckling biomaterials and implants for retinal detachment surgery. Med. Eng. Phys. 2010, 32, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Manish, C.C.; Wendy, W.W.; Michael, E.H.; Subhas, C.G. The evolution of breast reconstruction: A historical perspective. World J. Surg. 2012, 36, 730–742. [Google Scholar]
- Malahias, M.; Jordan, D.J.; Hughes, L.C.; Hindocha, S.; Juma, A. A literature review and summary of capsular contracture: An ongoing challenge to breast surgeons and their patients. Int. J. Surg. Open 2016, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pajkos, A.; Deva, A.K.; Vickery, K.; Cope, C.; Chang, L.; Cossart, Y.E. Detection of subclinical infection in significant breast implant capsules. Plast. Reconstr. Surg. 2003, 111, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA News Release: FDA Approves New Silicone Gel-Filled Breast Implant; Food and Drug Administration: Washington, DC, USA, 2012.
- United States Centers for Disease Control and Prevention. Occupational Health Guideline for Soluble Platinum Salts (as Platinum). Available online: http://www.cdc.gov/niosh/docs/81-123/pdfs/0520.pdf (accessed on 6 June 2012).
- Afssaps Launches the Alert for Defective Breast Prostheses. AFP. 31 March 2010. Available online: https://www.lemonde.fr/societe/article/2010/03/31/l-afssaps-lance-l-alerte-aux-protheses-mammaires-defectueuses_1326644_3224.html (accessed on 6 June 2012).
- Macrae, F.; Randall, C. Thousands of British women in dangerous breast implants alert. Daily Mail, 18 June 2010. [Google Scholar]
- Global Market Insight. 2019. Available online: file:///H:/Review%20paper/Breast%20Implaant/Manuscript/Submission/Global%20Breast%20Implant%20Market%20to%20Surpass%20$4.9%20Billion%20and.html (accessed on 6 June 2012).
- Research Dive. 2020. Available online: https://www.researchdive.com/download-sample/41 (accessed on 6 June 2012).
- Bondurant, S.; Ernster, V.; Herdman, R. Safety of Silicone Breast Implants. Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants; National Academies Press: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- Lykissa, E.D.; Kala, S.V.; Hurley, J.B.; Lebovitz, R.M. Release of low molecular weight silicones and platinum from silicone breast implants. Anal. Chem. 1997, 1, 4912–4916. [Google Scholar] [CrossRef]
- International Organization for Standardization. Non-Active Surgical Implants—Mammary Implants Particular Requirements; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Coleman, D.J.; Foo, I.T.; Sharpe, D.T. Textured or smooth implants for breast augmentation? A prospective controlled trial. Br. J. Plast. Surg. 1991, 44, 444–448. [Google Scholar] [CrossRef]
- Carpaneda, C.A. Inflammatory reaction and capsular contracture around smooth silicone implants. Aesthetic. Plast. Surg. 1997, 21, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Hall-Findlay, E.J. Breast implant complication review: Double capsules and late seromas. Plast. Reconstr. Surg. 2011, 127, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-Y.; Zhang, X.; Faruq, O.; Chien, P.N.; Dönmez, N.; Heo, C.-Y. An Impact of Different Silicone Breast Implants on the Bacterial Attachment and Growth. J. Biomater. Nanobiotechnol. 2021, 12, 21–33. [Google Scholar] [CrossRef]
- Sforza, M.; Zaccheddu, R.; Alleruzzo, A.; Seno, A.; Mileto, D.; Paganelli, A.; Sulaiman, H.; Payne, M.; Maurovich-Horvat, L. Preliminary 3-Year Evaluation of Experience with SilkSurface and VelvetSurface Motiva Silicone Breast Implants: A Single-Center Experience With 5813 Consecutive Breast Augmentation Cases. Aesthet. Surg. J. 2018, 38, S62–S73. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, J.; Wang, Z.; Chen, S. Development of robust biocompatible silicone with high resistance to protein adsorption and bacterial adhesion. Acta Biomater. 2011, 7, 2053–2059. [Google Scholar] [CrossRef]
- Li, M.; Neoh, K.G.; Xu, L.Q.; Wang, R.; Kang, E.T.; Lau, T.; Olszyna, D.P.; Chiong, E. Surface Modification of Silicone for Biomedical Applications Requiring Long-Term Antibacterial, Antifouling, and Hemocompatible Properties. Langmuir 2012, 28, 16408–16422. [Google Scholar] [CrossRef]
- Gevaux, L.; Lejars, M.; Margaillan, A.; Briand, J.-F.; Bunet, R.; Bressy, C. Hydrolyzable Additive-Based Silicone Elastomers: A New Approach for Antifouling Coatings. Polymers 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Furkert, F.H.; Sörensen, J.H.; Arnoldi, J.; Robioneck, B.; Steckel, H. Antimicrobial efficacy of surface-coated external fixation pins. Curr. Microbiol. 2011, 62, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, G.P.; Sigurdson, L.J.; Barnsley, S.E. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: A meta-analysis of randomized controlled trials. Plast. Reconstr. Surg. 2006, 117, 2182–2190. [Google Scholar] [CrossRef]
- Wong, C.H.; Samuel, M.; Tan, B.K.; Song, C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: A systematic review. Plast. Reconstr. Surg. 2006, 118, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Ploner, C.; Lobenwein, S.; Sopper, S.; Hoertnagl, P.; Mayerl, C. Immunophenotypic characterization of human T cells after in vitro exposure to different silicone breast implant surfaces. PLoS ONE 2018, 13, e0192108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, D.J.; Oikonomou, A.; Hill, E.; Bayat, A. Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts. Biomaterials 2015, 52, 88–102. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Greisler, H.P. Interactions at the Blood/Material Interface. Ann. Vasc. Surg. 1990, 4, 98–103. [Google Scholar] [CrossRef]
- Hanson, S.R. Blood-material interactions. In Handbook of Biomaterial Properties; Black, J., Hastings, G., Eds.; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- Cristina, T.; Alessandra, M.; Marco, A.C. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Whittemore, M.K.; Dileepan, K.N.; Wood, J.G. Mast cell: A multi functional master cell. Front. Immunol. 2016, 6, 620. [Google Scholar]
- Raghavendrs, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials: Design, development and biomedical applications A2-Thomaz, Sabu. In Nanotechnology Applications for Tissue Engineering; Grohens, Y., Ninan, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 21–44. [Google Scholar]
- Anderson, M.J. Inflammation, Wound Healing, and the Foreign-Body Response. In Biomaterials Science; Academic Press: Cambridge, MA, USA, 2013; pp. 503–512. [Google Scholar]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Brandt, E.; Woerly, G.; Younes, A.B.; Loiseau, S.; Capron, M. IL-4 production by human polymorphonuclear neutrophils. J. Leukoc. Biol. 2000, 68, 125–130. [Google Scholar] [PubMed]
- Woerly, G.; Lacy, P.; Younes, A.B.; Roger, N.; Loiseau, S.; Moqbel, R.; Capron, M. Human eosinophils express and release IL-13 following CD28-dependent activation. J. Leukoc. Biol. 2002, 72, 769–779. [Google Scholar] [PubMed]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.L.; Sautter, N.B. Is montelukast indicated for treatment of chronic rhinosinusitis with polyposis. Laryngocope 2014, 124, 1735–1736. [Google Scholar] [CrossRef]
- Huang, C.K.; Handel, N. Effects of Singulair (montelukast) treatment for capsular contracture. Aesthet. Surg. J. 2010, 30, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Guo, Y.; Ha, B.; Zen, K.; Liu, Y. Regulation of the inflammatory response: Enhancing neutrophil infiltration under chronic inflammatory conditions. J. Immunol. 2012, 188, 844–853. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Gupta, S.; Dastidar, S.; Ray, A. Cysteinyl leukotrienes and their receptors: Molecular and functional characteristics. Pharmacology 2010, 85, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Peters-Goldern, M.; Gleason, M.M.; Togias, A. Cysteinyl leukotrienes: Multi-functional mediators in allergic rhinitis. Clin. Exp. Allergy 2006, 36, 689–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.H.; Park, M.; Park, H.J.; Lee, S.H.; Choi, S.Y.; Park, C.G.; Han, S.M.; Heo, C.Y.; Choy, Y.B. Prolonged, acute suppresssion of cysteinyl leukotrien to reduce capsular contracture around silicone implants. Acta Biomater. 2017, 21, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Kwon, O.H.; Lee, J.W.; Chung, H.Y.; Cho, B.C.; Park, H.Y.; Kim, T.G. The effect of montelukast and antiadhesion barrier solution on the capsule formation after insertion of silicone implants in a white rat model. Eur. Surg. Res. 2013, 51, 146–155. [Google Scholar] [CrossRef]
- Ashcroft, G.S. Bidirectional regulation of macrophage function by TGF-beta. Microbes Infect. 1999, 1, 1275–1282. [Google Scholar] [CrossRef] [Green Version]
- Rappolee, D.A.; Mark, D.; Banda, M.J.; Werb, Z. Wound macrophages express TGF-alpha and other growth factors in vivo: Analysis by mRNA phenotyping. Science 1988, 241, 708–712. [Google Scholar] [CrossRef]
- Santibañez, J.F.; Quintanilla, M.; Bernabeu, C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin. Sci. 2011, 121, 2233–2251. [Google Scholar] [CrossRef] [Green Version]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, H.; Kikuchi, S.; Arai, N.; Koda, A. The mechanism involved in the inhibitory action of tranilast on collagen biosynthesis of keloid fibroblasts. Jpn. J. Pharmacol. 1992, 60, 91–96. [Google Scholar] [CrossRef]
- Bonnet, F.; Cao, Z.; Cooper, M.E.; Cox, A.J.; Kelly, D.J.; Gilbert, R.E. Tranilast attenuates vascular hypertrophy, matrix accumulation and growth factor overexpression in experimental diabetes. Diabetes Metab. 2003, 29, 386–392. [Google Scholar] [CrossRef]
- Tanaka, K.; Honda, M.; Kuramochi, T.; Morioka, S. Prominent inhibitory effects of tranilast on migration and proliferation of and collagen synthesis by vascular smooth muscle cells. Atherosclerosis 1994, 107, 179–185. [Google Scholar] [CrossRef]
- Yamada, H.; Tajima, S.; Nishikawa, T.; Murad, S.; Pinnell, S.R. Tranilast, a selective inhibitor of collagen synthesis in human skin fibroblasts. J. Biochem. 1994, 116, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Zhang, Y.; Gow, R.; Gilber, R.E. Transnilast attenuates structural and functional aspects of renal injury in the remnant kidney model. J. Am. Soc. Nephrol. 2004, 15, 2619–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Park, M.; Kim, B.H.; Lee, J.E.; Park, H.J.; Lee, S.H.; Park, C.G.; Kim, M.H.; Kim, R.; Kim, E.H.; et al. Acute suppression of TGF-ß with local, sustained releaseof tranilast against the formation of fibrous capsules around silicone implants. J. Control. Release 2015, 200, 125–137. [Google Scholar] [CrossRef]
- Kim, B.H.; Huh, B.K.; Lee, W.S.; Kim, C.R.; Lee, K.S.; Nam, S.-Y.; Lee, M.; Heo, C.Y.; Choy, Y.B. Silicone Implant Coated with Tranilast-Loaded Polymer in a Pattern for Fibrosis Suppression. Polymers 2019, 11, 223. [Google Scholar] [CrossRef] [Green Version]
- Amano, Y.; Lee, S.W.; Allison, A.C. Inhibition by glucocorticoids of the formation of interleukin-1 alpha, interleukin-1 beta, and interleukin-6: Mediation by decreased mRNA stability. Mol. Pharmacol. 1993, 43, 176–182. [Google Scholar]
- Joyce, D.A.; Steer, J.H.; Abraham, L.J. Glucocorticoid modulation of human monocyte/macrophage function: Control of TNF-alpha secretion. Inflamm. Res. 1997, 46, 447–451. [Google Scholar] [CrossRef]
- Schleimer, R.P. An overview of glucocorticoid anti-inflammatory actions. Eur. J. Clin. Pharmacol. 1993, 45, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Sahare, P.; Ayala, M.; Vazquez-Duhalt, R.; Agrawal, V. Immobilization of peroxidase enzyme onto the porous silicon structure for enhancing its activity and stability. Nanoscale Res. Lett. 2014, 9, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pápa, Z.; Ramakrishnan, S.K.; Marin, M.; Cloitre, T.; Zimányi, L.; Márquez, J.; Budai, J.; Tóth, Z.; Gergely, C. Interactions at the peptide/silicon surfaces: Evidence of peptide multilayer assembly. Langmuir 2016, 32, 7250–7258. [Google Scholar] [CrossRef] [Green Version]
- Thiessen, A.N.; Ha, M.; Hooper, R.W.; Yu, H.; Oliynyk, A.O.; Veinot, J.G.C.; Michaelis, V.K. Silicon nanoparticles: Are they crystalline from the core to the surface. Chem. Mater. 2019, 31, 678–688. [Google Scholar] [CrossRef]
- O’Farrell, N.; Houlton, A.; Horrocks, B.R. Silicon nanoparticles: Applications in cell biology and medicine. Int. J. Nanomed. 2006, 1, 451–472. [Google Scholar] [CrossRef]
- Malcolm, R.K.; McCullagh, S.D.; Woolfson, A.D.; Gorman, S.P.; Jones, D.S.; Cuddy, J. Controlled release of a model antibacterial drug from a novel self-lubricating silicone biomaterial. J. Control. Release 2004, 97, 313–320. [Google Scholar] [CrossRef]
- Beom, S.J.; Shin, B.H.; Huh, B.K.; Kim, B.H.; Kim, S.N.; J, H.B.; Lee, S.H.; Kang, S.I.; Shim, J.H.; Kang, S.M.; et al. Silicone implants capable of the local, controlled delivery of triamcinolone for the prevention of fibrosis with minimized drug side effects. J. Ind. Eng. Chem. 2018, 63, 168–180. [Google Scholar]
- Patel, T.R.; McFadden, B.A. Caenorhabditis elegans and Ascaris suum: Inhibition of isocitrate lyase by itaconate. Exp. Parasitol. 1978, 44, 262–268. [Google Scholar] [CrossRef]
- Cordes, T.; Michelucci, A.; Hiller, K. Itaconic Acid: The Surprising Role of an Industrial Compound as a Mammalian. Antimicrob. Metab. Annu. Rev. Nutr. 2015, 35, 451–473. [Google Scholar] [CrossRef]
- Birajdar, M.S.; Kim, B.H.; Sutthiwanjampa, C.; Kang, S.H.; Heo, C.H.; Park, H. Inhibition of Capsular Contracture of Poly (Dimethyl Siloxane) Medical Implants by Surface Modification with Itaconic Acid Conjugated Gelatin. J. Ind. Eng. Chem. 2020, 89, 128–138. [Google Scholar] [CrossRef]
- McGaha, T.L.; Phelps, R.G.; Spiera, H.; Bona, C. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J. Investig. Dermatol. 2002, 118, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Spira, G.; Mawasi, N.; Paizi, M.; Anbinder, N.; Genina, O.; Alexiev, R.; Pines, M. Halofuginone, a collagen type I inhibitor improves liver regeneration in cirrhotic rats. J. Hepatol. 2002, 37, 331–339. [Google Scholar] [CrossRef]
- Zeplin, P.H.; Larena-Avellaneda, A.; Schmidt, K. Surface modification of silicone breast implants by binding the antifibrotic drug halofuginone reduces capsular fibrosis. Plast. Reconstr. Surg. 2010, 126, 266–274. [Google Scholar] [CrossRef]
- Spiess, K.; Lammel, A.; Scheibel, T. Recombinant spider silk proteins for applications in biomaterials. Macromol. Biosci. 2010, 10, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Zeplin, P.H.; Maksimovikj, N.C.; Jordan, M.C.; Nickel, J.; Lang, G.; Leimer, A.H.; Römer, L.; Scheibel, T. Spider Silk Coatings as a Bioshield to Reduce Periprosthetic Fibrous Capsule Formation. Adv. Funct. Mater. 2014, 24, 2658–2666. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, S.; Shin, B.-H.; Heo, C.-Y.; Faruq, O.; Van Anh, L.T.; Dönmez, N.; Chien, P.N.; Shin, D.-S.; Nam, S.-Y.; et al. Silicone Implants Immobilized with Interleukin-4 Promote the M2 Polarization of Macrophages and Inhibit the Formation of Fibrous Capsules. Polymers 2021, 13, 2630. [Google Scholar] [CrossRef]
- Nicoud, G.R.; Donno, R.; Cadman, C.J.; Alexander, M.R.; Tirelli, N. Surface modification of silicone via colloidal deposition of amphiphilic block copolymers. Polym. Chem. 2014, 5, 6687–6701. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.J.; Han, H.M.; Wang, J.; Xiao, S.J.; Dai, Z.D. Surface-hydrophilic and protein-resistant silicone elastomers prepared by hydrosilylation of vinyl poly(ethylene glycol) on hydrosilanes-poly(dimethylsiloxane) surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2007, 308, 129–135. [Google Scholar] [CrossRef]
- Jin, Y.J.; Kang, S.; Park, P.; Choi, D.; Kim, D.W.; Jung, D.; Koh, J.; Jeon, J.; Lee, M.; Ham, J.; et al. Anti-inflammatory and Antibacterial Effects of Covalently Attached Biomembrane-Mimic Polymer Grafts on Gore-Tex Implants. ACS Appl. Mater. Interfaces 2017, 9, 19161–19175. [Google Scholar] [CrossRef]
- Ishihara, K. Blood-Compatible Surfaces with Phosphorylcholine-Based Polymers for Cardiovascular Medical Devices. Langmuir 2019, 35, 1778–1787. [Google Scholar] [CrossRef]
- Park, J.U.; Ham, J.; Kim, S.; Seo, J.H.; Kim, S.H.; Lee, S.; Min, H.J.; Choi, S.; Choi, R.M.; Kim, H.; et al. Alleviation of capsular formations on silicone implants in rats using biomembrane-mimicking coatings. Acta Biomater. 2014, 10, 4217–4225. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.; Kim, S.; Wufuer, M.; Park, S.; Kim, Y.; Choi, D.; Jin, X.; Kim, Y.; Huang, Y.; et al. Efficient reduction of fibrous capsule formation around silicone breast implantdensely grafted with 2-methacryloyloxyethyl phosphorylcholine (MPC) polymers by heat-induced polymerization. Biomater. Sci. 2020, 8, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Leigh, B.L.; Cheng, E.; Xu, L.; Derk, A.; Hansen, M.R.; Guymon, C.A. Antifouling Photograftable Zwitterionic Coatings on PDMS Substrates. Langmuir 2019, 35, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, D.R.; Miranda, R.N.; Kaura, A.; Francis, A.M.; Campanale, A.; Boldrini, R.; Alexander, J.; Deva, A.K.; Gravina, P.R.; Medeiros, L.J. Global adverse event reports of breast implant-associated ALCL: An international review of 40 government authority databases. Plast. Reconstr. Surg. 2017, 139, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.Y.; Kim, B.H.; Lee, J.S.; Shin, B.H.; Kwon, H.; Koh, W.G.; Heo, C.Y. Dual surface modification of PDMS-based silicone implants to suppress capsular contracture. Acta Biomater. 2018, 76, 56–70. [Google Scholar] [CrossRef]
- Hahn, S.K.; Hoffman, A.S. Characterization of biocompatible polyelectrolyte complex multilayer of hyaluronic acid and poly-L-lysine. Biotechnol. Bioprocess. Eng. 2004, 9, 179–183. [Google Scholar] [CrossRef]
- Szarpak, A.; Cui, D.; Dubreuil, F.; De Geest, B.G.; De Cock, L.J.; Picart, C.; Auzély-Velty, R. Designing Hyaluronic Acid-Based Layer-by-Layer Capsules as a Carrier for Intracellular Drug Delivery. Biomacromolecules 2010, 11, 713–720. [Google Scholar] [CrossRef]
- Jones, K.S. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin. Immunol. 2008, 20, 130–136. [Google Scholar] [CrossRef]
- Lei, Z.Y.; Liu, T.; Li, W.J.; Shi, X.H.; Fan, D.L. Biofunctionalization of silicone rubber with microgroove-patterned surface and carbon-ion implantation to enhance biocompatibility and reduce capsule formation. Int. J. Nanomed. 2016, 11, 5563–5572. [Google Scholar] [CrossRef] [Green Version]
- Fanous, N.; Tawilé, C.; Brousseau, V.J. Minimal inframammary incision for breast augmentation. Can. J. Plast. Surg. 2008, 16, 14–17. [Google Scholar] [CrossRef]
- Hidalgo, D.A. Breast augmentation: Choosing the optimal incision, implant, and pocket plane. Plast. Reconstr. Surg. 2000, 105, 2202–2216. [Google Scholar] [CrossRef] [PubMed]
- Huh, B.K.; Kim, B.H.; Kim, C.R.; Kim, S.N.; Shin, B.H.; Ji, H.B.; Lee, S.H.; Kim, M.J.; Heo, C.Y.; Choy, Y.B. Elastic net of polyurethane strands for sustained delivery of triamcinolone around silicone implants of various sizes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110565. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faruq, O.; Chien, P.N.; Dönmez, N.; Nam, S.-Y.; Heo, C.-Y. Functionalization of Silicone Surface with Drugs and Polymers for Regulation of Capsular Contracture. Polymers 2021, 13, 2731. https://doi.org/10.3390/polym13162731
Faruq O, Chien PN, Dönmez N, Nam S-Y, Heo C-Y. Functionalization of Silicone Surface with Drugs and Polymers for Regulation of Capsular Contracture. Polymers. 2021; 13(16):2731. https://doi.org/10.3390/polym13162731
Chicago/Turabian StyleFaruq, Omar, Pham Ngoc Chien, Nilsu Dönmez, Sun-Young Nam, and Chan-Yeong Heo. 2021. "Functionalization of Silicone Surface with Drugs and Polymers for Regulation of Capsular Contracture" Polymers 13, no. 16: 2731. https://doi.org/10.3390/polym13162731





