Recycled Porcine Bone Powder as Filler in Thermoplastic Composite Materials Enriched with Chitosan for a Bone Scaffold Application
Abstract
:1. Introduction
2. Materials and Methods
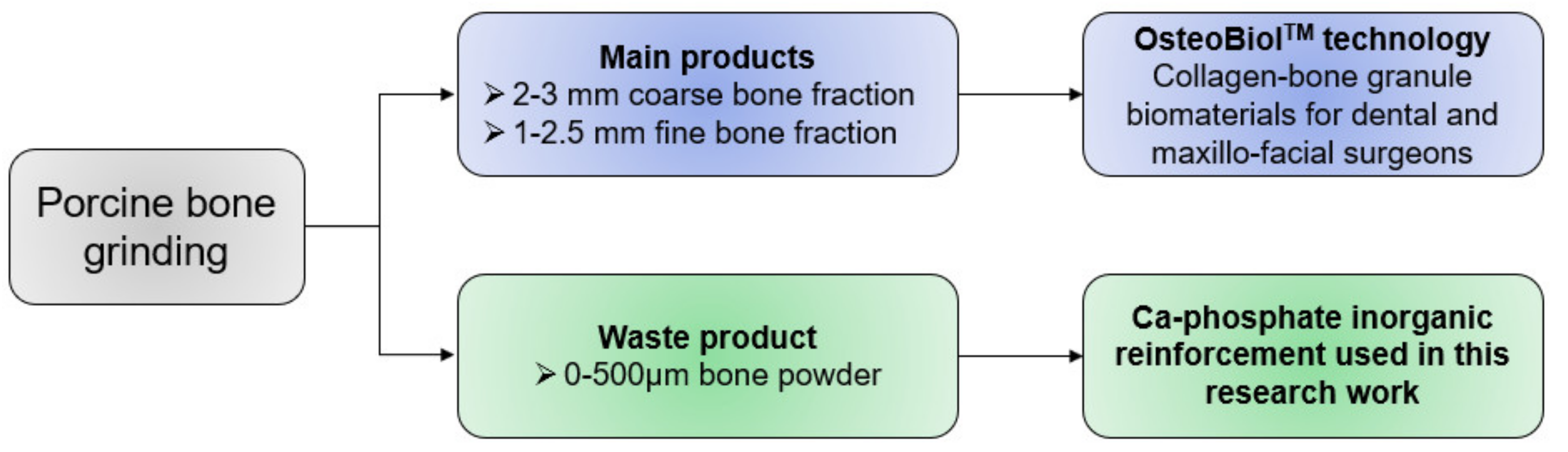
2.1. Materials
2.2. Experimental
2.2.1. Solvent Casting
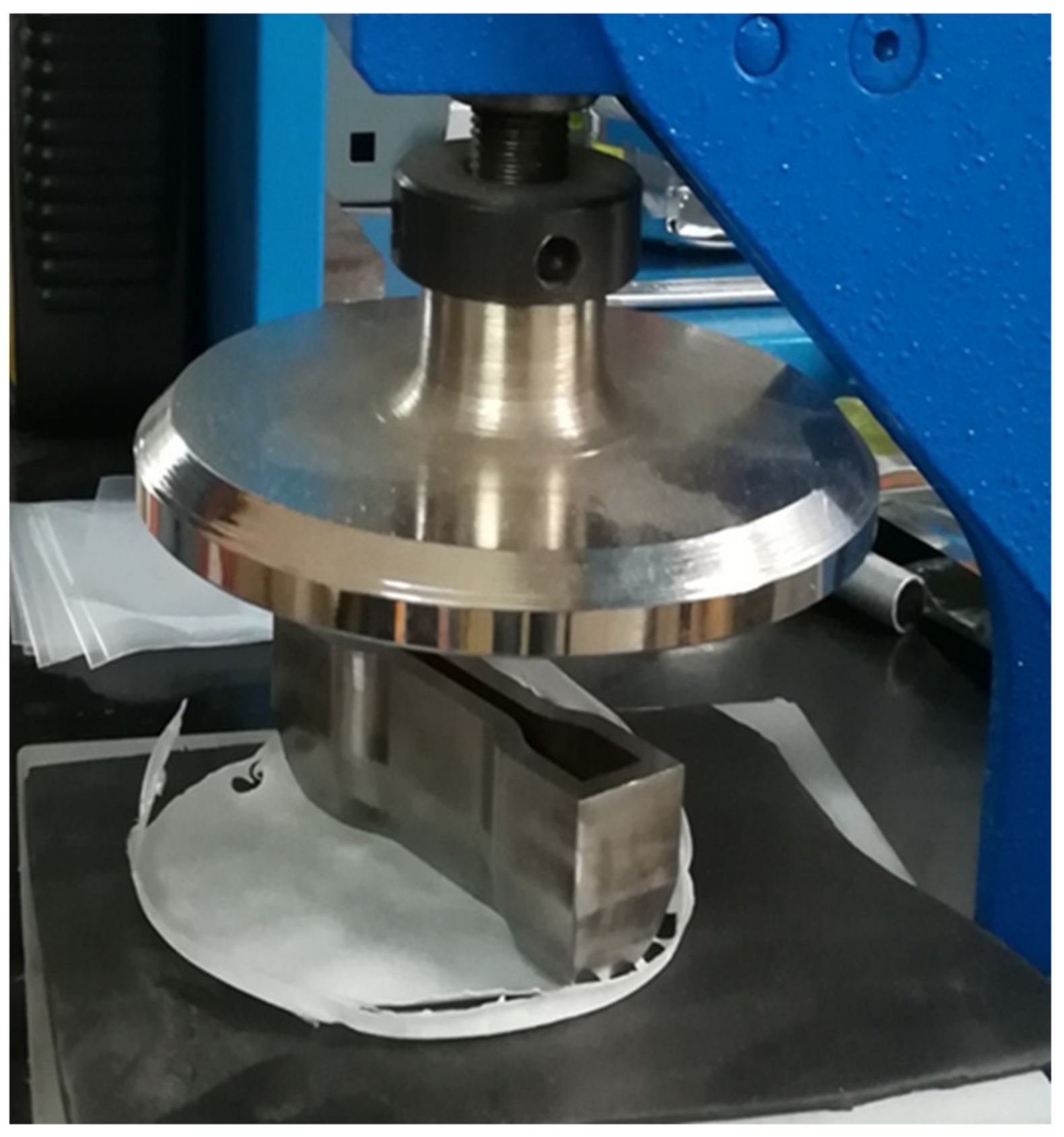
2.2.2. Samples Preparation for Mechanical Tests
2.3. Characterization
2.3.1. SEM
2.3.2. Mechanical Tests
2.3.3. Assay of Bacterial Growth and Adhesion
3. Results and Discussions
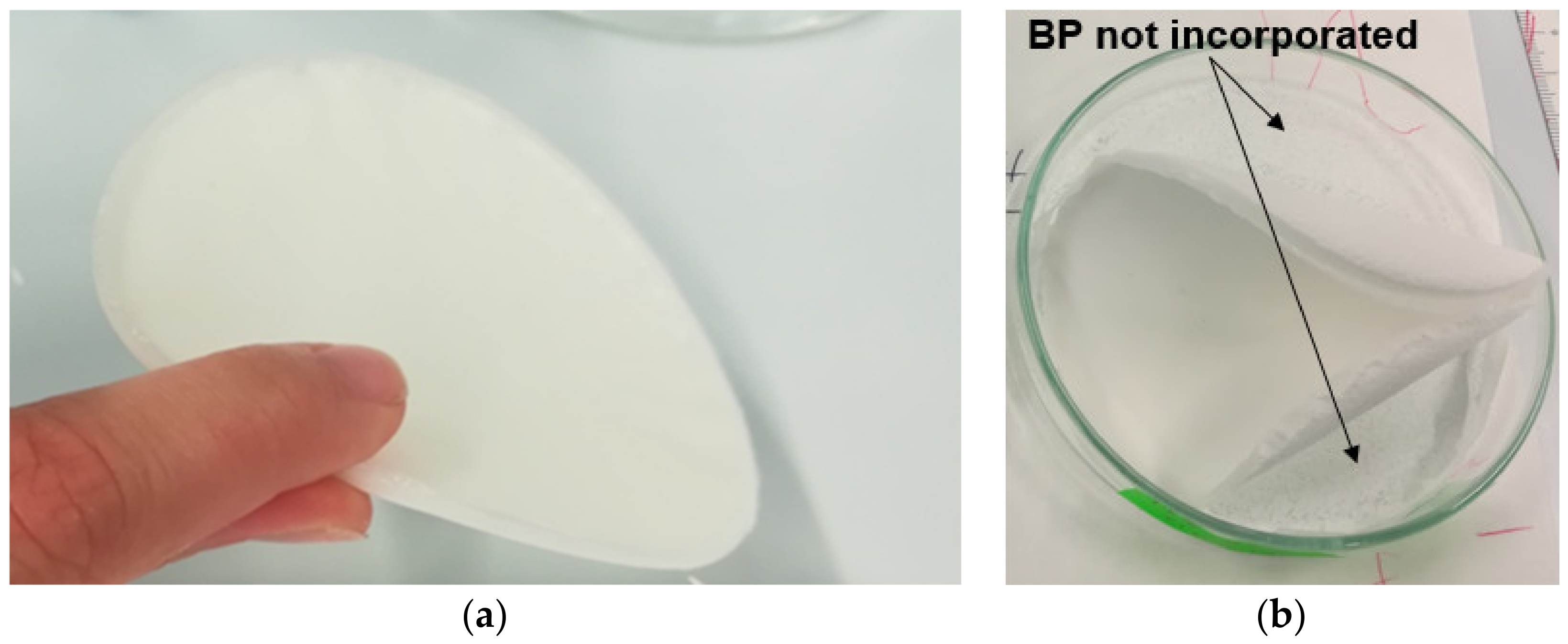
3.1. Quality Check of Post-Solvent-Casted Specimens
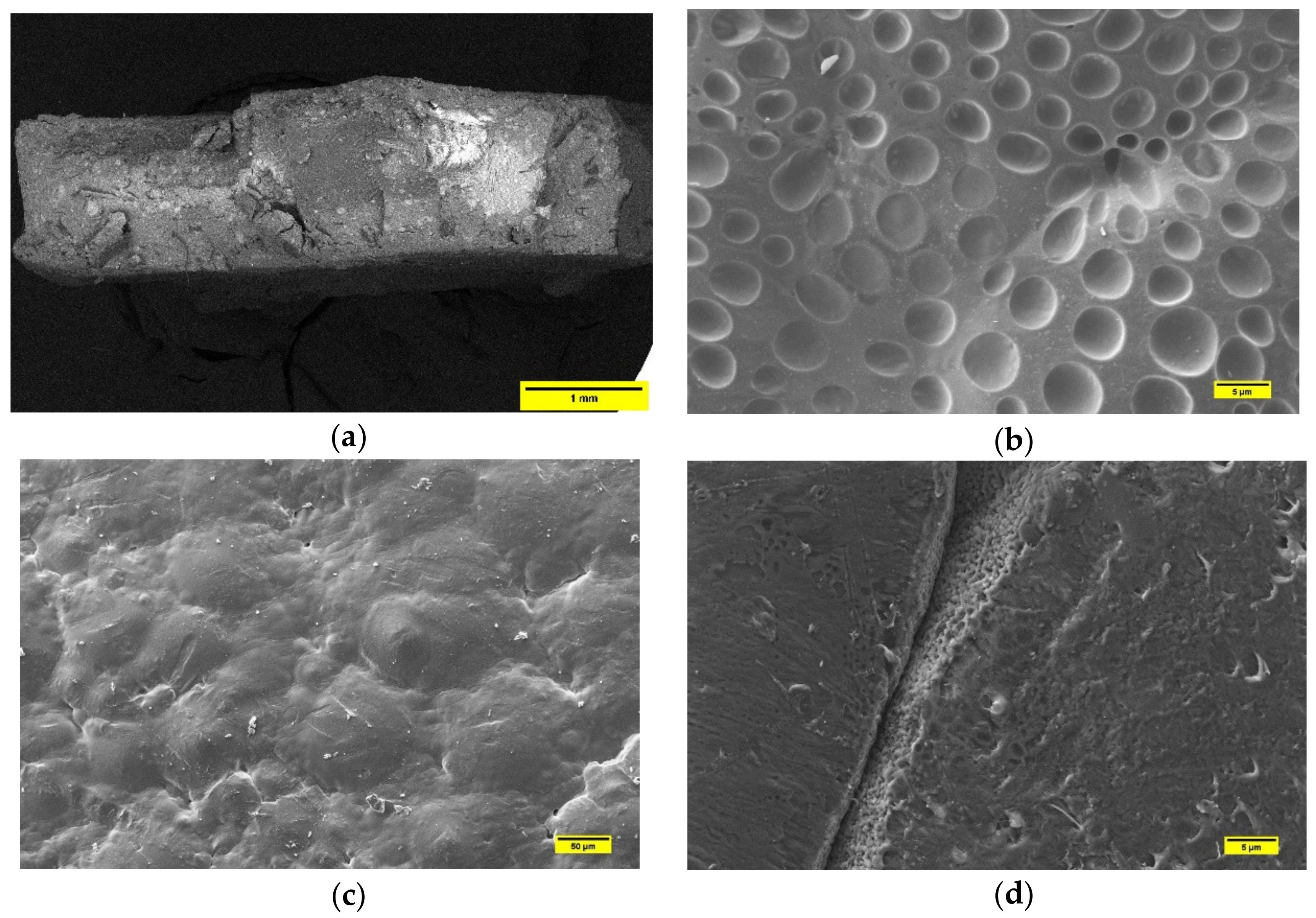
3.2. Microstructural Characterization by SEM
3.2.1. PLA-Based Samples Prepared by Solvent Casting
3.2.2. PCL-Based Samples Prepared by Solvent Casting
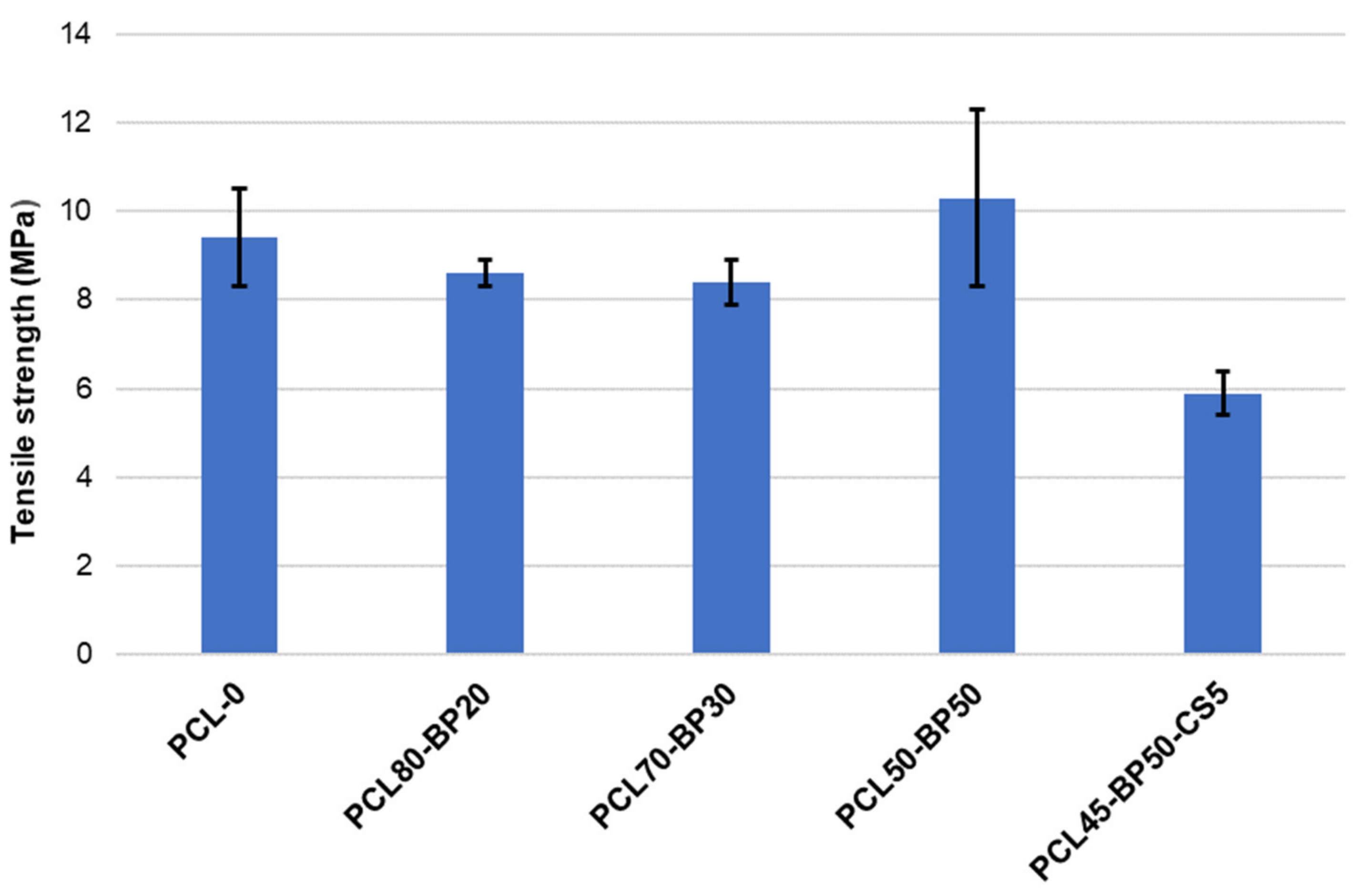
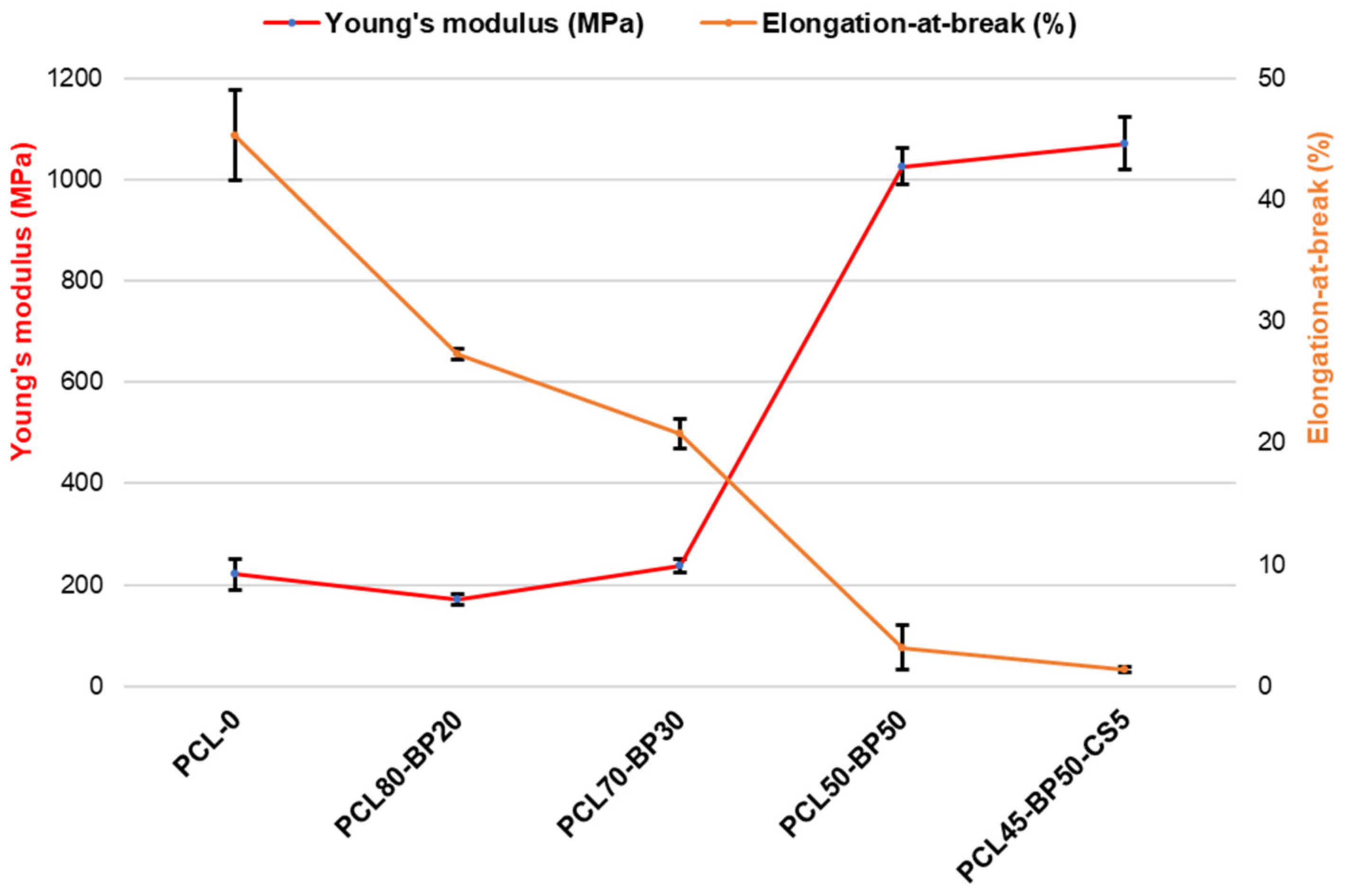
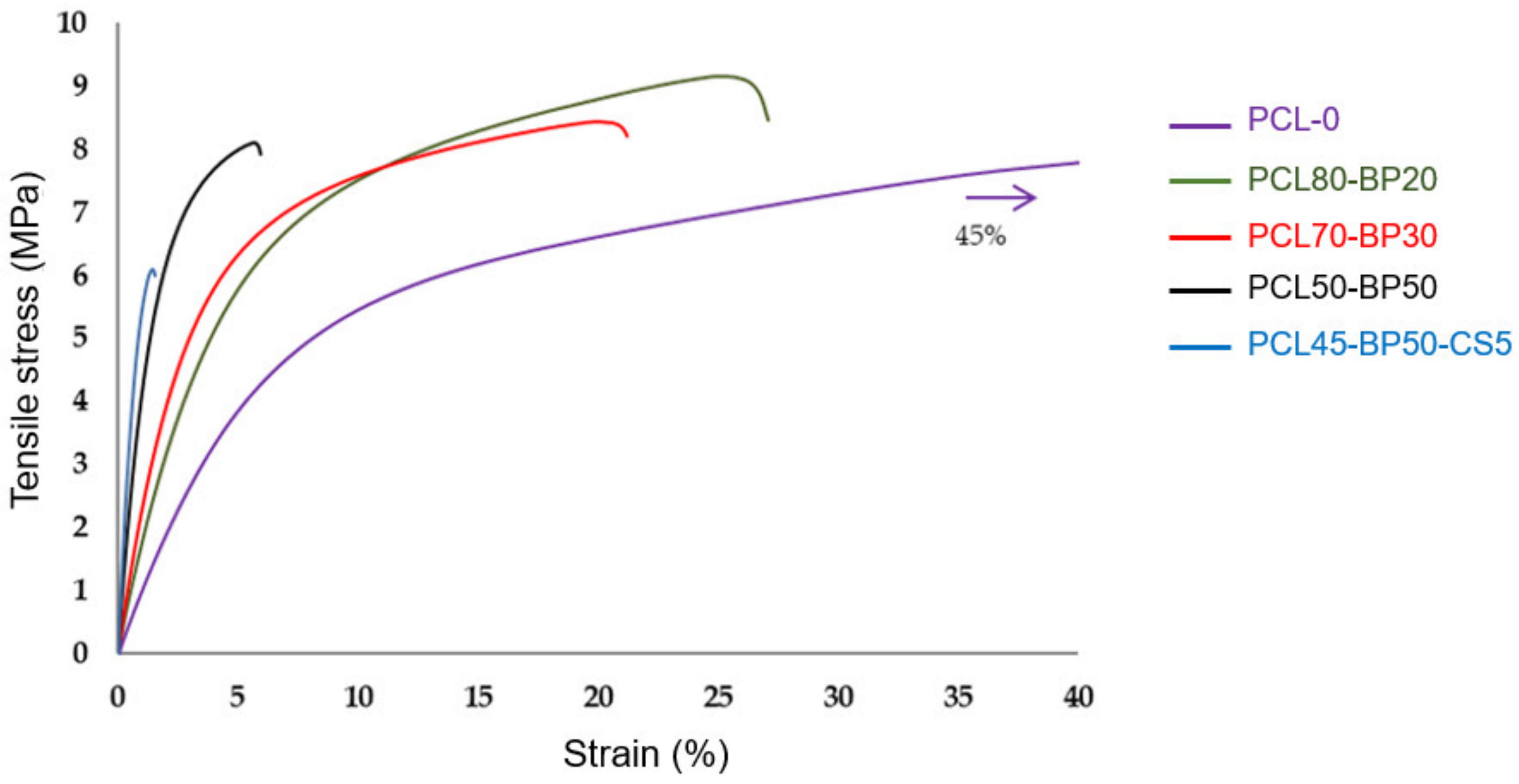
3.3. Mechanical Characterization by Tensile Tests
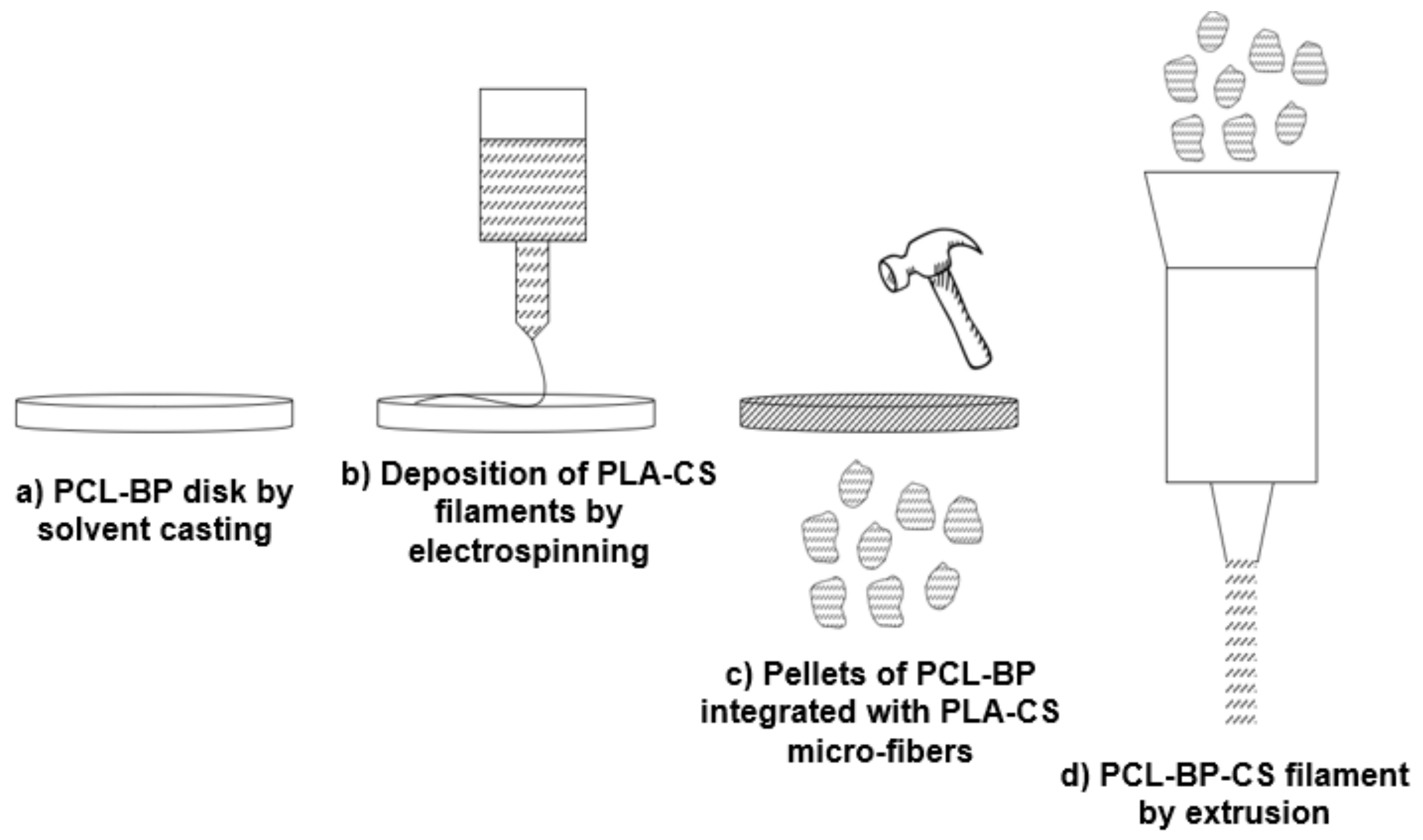
3.4. Process Upgrade
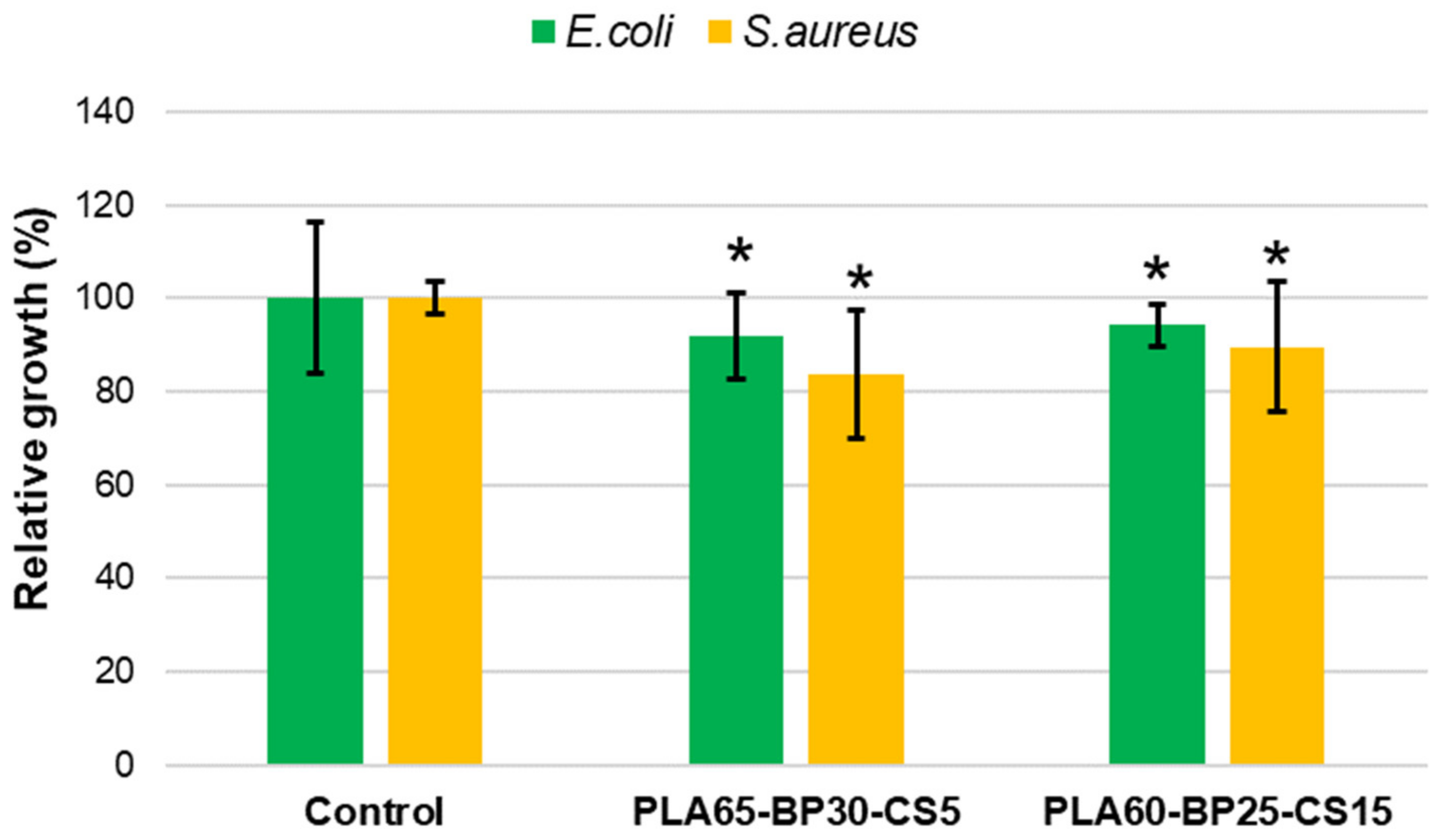
3.5. Antibacterial Activity of PLA-BP-CS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Bello, C. Biomateriali Introduzione allo Studio dei Materiali per uso Biomedico, 1st ed.; Patron: Bologna, Italy, 2004. [Google Scholar]
- Haleem, A.; Javaid, M.; Khan, R.H.; Suman, R. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma 2020, 11, S118–S124. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2020, 100721. [Google Scholar] [CrossRef]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L.S. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, G.; de Groot, K. Biomimetic coatings for bone tissue engineering of critical-sized defects. J. R. Soc. Interface 2010, 7, S631–S647. [Google Scholar] [CrossRef]
- Pérez-González, F.; Molinero-Mourelle, P.; Sánchez-Labrador, L.; Sáez-Alcaide, L.M.; Limones, A.; Brinkmann, J.C.B.; López-Quiles, J. Assessment of clinical outcomes and histomorphometric findings in alveolar ridge augmentation procedures with allogeneic bone block grafts: A systematic review and meta-analysis. Med. Oral. Patol. Oral. Cir. Bucal. 2020, 25, e291. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, M.; Dorkoosh, F. Cardiac tissue engineering, biomaterial scaffolds, and their fabrication techniques. Polym. Adv. Technol. 2021, 32, 2290–2305. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Vogel, C.; Siesler, H.W. Thermal Degradation of Poly(ɛ-caprolactone), Poly(L-lactic acid) and their Blends with Poly(3-hydroxy-butyrate) Studied by TGA/FT-IR Spectroscopy. Macromol. Symp. 2008, 265, 183–194. [Google Scholar] [CrossRef]
- Pang, X.; Zhuang, X.; Tang, Z.; Chen, X. Polylactic acid (PLA): Research, development and industrialization. Biotechnol. J. 2010, 5, 1125–1136. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Onder, O.C.; Sevgili, I.; Yilgor, E.; Kavakli, I.H.; Yilgor, I. 3D printed poly (lactic acid) scaffolds modified with chitosan and hydroxyapatite for bone repair applications. Mater. Today Commun. 2020, 25, 101515. [Google Scholar] [CrossRef]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef]
- Lopresti, F.; Pavia, F.C.; Vitrano, I.; Kersaudy-Kerhoas, M.; Brucato, V.; la Carrubba, V. Effect of hydroxyapatite concentration and size on morpho-mechanical properties of PLA-based randomly oriented and aligned electrospun nanofibrous mats. J. Mech. Behav. Biomed. Mater. 2020, 101, 103449. [Google Scholar] [CrossRef]
- Trakoolwannachai, V.; Kheolamai, P.; Ummartyotin, S. Characterization of hydroxyapatite from eggshell waste and polycaprolactone (PCL) composite for scaffold material. Compos. Part B Eng. 2019, 173, 106974. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D printing of PCL/nano-hydroxyapatite scaffolds derived from biogenic sources for bone tissue engineering. Sustain. Mater. Technol. 2021, e00318. [Google Scholar] [CrossRef]
- Miszuk, J.M.; Xu, T.; Yao, Q.; Fang, F.; Childs, J.D.; Hong, Z.; Tao, J.; Fong, H.; Sun, H. Functionalization of PCL-3D electrospun nanofibrous scaffolds for improved BMP2-induced bone formation. Appl. Mater. Today 2018, 10, 194–202. [Google Scholar] [CrossRef]
- Oliveira, H.L.; da Rosa, W.L.; Cuevas-Suárez, C.E.; Carreno, N.L.; da Silva, A.F.; Guim, T.N.; Odir, A.D.; Piva, E. Histological evaluation of bone repair with hydroxyapatite: A systematic review. Calcif. Tissue Int. 2017, 101, 341–354. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; LewisOscar, F.; Selvaraj, R.; Brindhadevi, K.; Pugazhendhi, A. Natural organic and inorganic–hydroxyapatite biopolymer composite for biomedical applications. Prog. Org. Coat. 2020, 147, 105858. [Google Scholar] [CrossRef]
- Tecu, C.; Antoniac, A.; Goller, G.; Gok, M.G.; Manole, M.; Mohan, A.; Moldovan, H.; Earar, K. The sintering behaviour and mechanical properties of hydroxyapatite—Based composites for bone tissue regeneration. Rev. Chim. 2018, 69, 1272–1275. [Google Scholar] [CrossRef]
- Zheng, T.; Guo, L.; Du, Z.; Leng, H.; Cai, Q.; Yang, X. Bioceramic fibrous scaffolds built with calcium silicate/hydroxyapatite nanofibers showing advantages for bone regeneration. Ceram. Int. 2021, 47, 18920–18930. [Google Scholar] [CrossRef]
- Osteobiol by Tecnoss. Available online: https://www.osteobiol.com/ (accessed on 28 June 2021).
- Li, C.; Qin, W.; Lakshmanan, S.; Ma, X.; Sun, X.; Xu, B. Hydroxyapatite based biocomposite scaffold: A highly biocompatible material for bone regeneration. Saudi J. Biol. Sci. 2020, 27, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lawrence, J.G.; Bhaduri, S.B. Fabrication aspects of PLA-CaP/PLGA-CaP composites for orthopedic applications: A review. Acta Biomater. 2012, 8, 1999–2016. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Copinet, A.; Tighzert, L.; Coma, V. Mechanical and barrier properties of biodegradable films made from chitosan and poly (lactic acid) blends. J. Polym. Environ. 2004, 12, 1–6. [Google Scholar] [CrossRef]
- Zakaria, Z.; Islam, M.; Hassan, A.; Mohamad Haafiz, M.K.; Arjmandi, R.; Inuwa, I.M.; Hasan, M. Mechanical properties and morphological characterization of PLA/chitosan/epoxidized natural rubber composites. Adv. Mater. Sci. Eng. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef]
- Fu, S.Z.; Wang, X.H.; Guo, G.; Shi, S.; Fan, M.; Liang, H.; Luo, F.; Qian, Z.Y. Preparation and properties of nano-hydroxyapatite/PCL-PEG-PCL composite membranes for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 74–83. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, R.; Huang, Q.; Ding, X. Preparation and characterization of hydroxyapatite/polycaprolactone–chitosan composites. J. Mater. Sci. Mater. Med. 2009, 20, 2375. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Mellegård, H.; Strand, S.P.; Christensen, B.E.; Granum, P.E.; Hardy, S.P. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.; Assis, O.B. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Revista Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Sarasam, A.R.; Krishnaswamy, R.K.; Madihally, S.V. Blending chitosan with polycaprolactone: Effects on physicochemical and antibacterial properties. Biomacromolecules 2006, 7, 1131–1138. [Google Scholar] [CrossRef]
- Li, K.; Guan, G.; Zhu, J.; Wu, H.; Sun, Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan–gallic acid derivative against Escherichia coli and Staphylococcus aureus. Food Control 2019, 96, 234–243. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Wootthikanokkhan, J.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Mechanical and antibacterial properties of the chitosan coated cellulose paper for packaging applications: Effects of molecular weight types and concentrations of chitosan. Int. J. Biol. Macromol. 2020, 155, 1510–1519. [Google Scholar] [CrossRef]
- Zivanovic, S.; Li, J.; Davidson, P.M.; Kit, K. Physical, mechanical, and antibacterial properties of chitosan/PEO blend films. Biomacromolecules 2007, 8, 1505–1510. [Google Scholar] [CrossRef]





| Method | Advantages | Drawbacks |
|---|---|---|
| Transplantation | ||
| Autografts | Availability of all necessary genetic elements (cells, tissue-inductive growth factors, substrates) for tissue regeneration [7] | Pain and morbidity of the donor site, prolonged surgery, limited available volume, and hardly manipulation to reproduce complex anatomical structures [7] |
| Allogeneic grafts | Virtually unlimited amount of material obtainable in various sizes and shapes from a human tissue bank. Shorter surgery compared to autografting because the tissue harvesting procedure is not necessary. Suitable healing capabilities [7,8] | Disease transmission, toxicity associated with the sterilization, high variability in host immune response, limited supplies. Lack of scientific evidence and standardized protocols [7,8] |
| Conventional Fabrication Technique | ||
| Solvent casting | Low-cost technique. High porosity (50–90%). Not need of sophisticated equipment [6,9] | Use of very toxic solvents. Residual solvent can defunctionalize the cell growth [6,9] |
| Freeze-drying | Possibility to manage the pore size distribution by changing the freezing method. Possibility to produce scaffold with suitable interconnectivity without implementing high working temperature [6,9] | Use of cytotoxic solvents. Generation of small and irregular pores (15–35 μm) [6,9] |
| Gas foaming | High porosity (up to 85%). No use of organic solvents [6,9] | Strict control of thermal operating conditions is necessary as the method is highly sensitive to the development of closed pore structures and non-porous skin layer [6,9] |
| Electrospinning | Possibility to produce highly porous scaffolds with small pore diameters (nano to micro scale). High tensile strength performance due the fibers-based structure [6,9] | Use of toxic solvents. Process depends on many variables (type of solvent, polymer concentration, voltage, flow rate, needle size, temperature, pressure, needle-to-collector distance). Problematic to obtain 3D structures [6,9] |
| Rapid Prototyping Methods | ||
| Stereolithography | High resolution. Uniformity in pores interconnectivity. Possibility to fabricate structures with anatomically shape [6,9] | Expensive equipment. Requiring massive amounts of monomers and post-treatment to improve the monomer conversion. Shrinkage during polymerization [6,9] |
| Selective laser sintering | Not using solvent. Rapid process. Excellent control over the scaffold microstructure by adapting the process parameters [6,9] | High operating temperature. High-cost equipment. Requiring many post-processing treatments to remove injected powder [6,9] |
| Fused deposition modelling | Low operating temperature. Solvent-free technique. Reaching of suitable strength properties. Low cost [6,9] | Medium precision. Several limitations in its application to biodegradable polymers [6,9] |
| Bioprinting | High accuracy and shape complexity. High cell viability (80–90%). Low costs [6] | Depends on existence of cells [6] |
| ID Sample | PCL (wt%) | BP (wt%) | CS (wt%) |
|---|---|---|---|
| PCL-0 | 100 | 0 | 0 |
| PCL80-BP20 | 80 | 20 | 0 |
| PCL70-BP30 | 70 | 30 | 0 |
| PCL50-BP50 | 50 | 50 | 0 |
| PCL45-BP50-CS5 | 45 | 50 | 5 |
| ID Sample | PLA (wt%) | BP (wt%) | CS (wt%) |
|---|---|---|---|
| PLA65-BP30-CS5 | 65 | 30 | 5 |
| PLA60-BP25-CS15 | 60 | 25 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, M.; Puiggalí, J.; del Valle, L.J.; Titolo, G.; Sambucci, M. Recycled Porcine Bone Powder as Filler in Thermoplastic Composite Materials Enriched with Chitosan for a Bone Scaffold Application. Polymers 2021, 13, 2751. https://doi.org/10.3390/polym13162751
Valente M, Puiggalí J, del Valle LJ, Titolo G, Sambucci M. Recycled Porcine Bone Powder as Filler in Thermoplastic Composite Materials Enriched with Chitosan for a Bone Scaffold Application. Polymers. 2021; 13(16):2751. https://doi.org/10.3390/polym13162751
Chicago/Turabian StyleValente, Marco, Jordi Puiggalí, Luis J. del Valle, Gioconda Titolo, and Matteo Sambucci. 2021. "Recycled Porcine Bone Powder as Filler in Thermoplastic Composite Materials Enriched with Chitosan for a Bone Scaffold Application" Polymers 13, no. 16: 2751. https://doi.org/10.3390/polym13162751
APA StyleValente, M., Puiggalí, J., del Valle, L. J., Titolo, G., & Sambucci, M. (2021). Recycled Porcine Bone Powder as Filler in Thermoplastic Composite Materials Enriched with Chitosan for a Bone Scaffold Application. Polymers, 13(16), 2751. https://doi.org/10.3390/polym13162751









