Ethylic Esters as Green Solvents for the Extraction of Intracellular Polyhydroxyalkanoates Produced by Mixed Microbial Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. PHA Production
2.2. Preliminary Dissolution Tests
2.3. Extraction Experiments
2.4. Characterization Methods
3. Results and Discussion
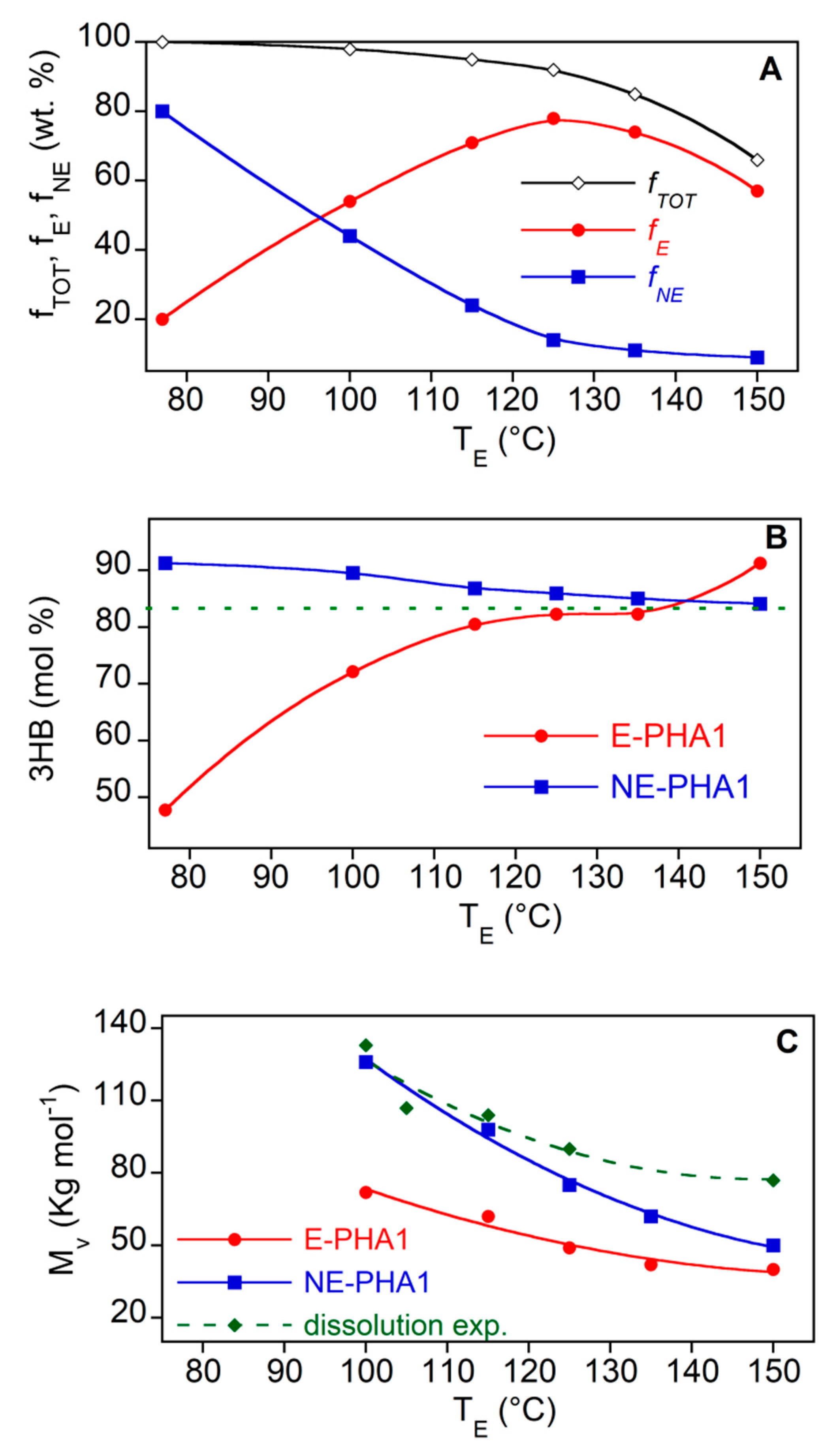
3.1. Screening of Different EEs as PHA-Extraction Solvents
3.2. Evaluation of PHA Extraction Performances with Ethyl Acetate (EA)
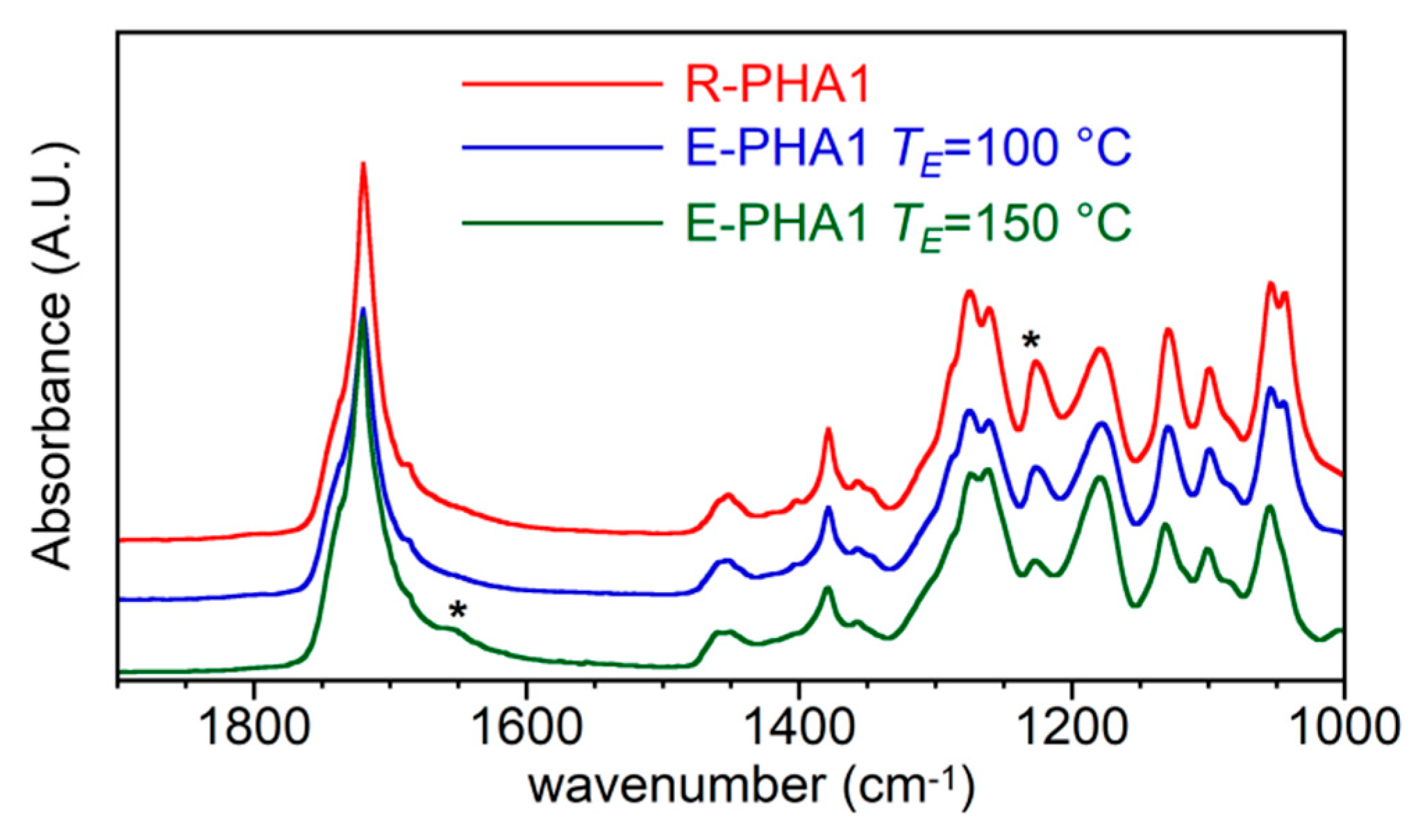
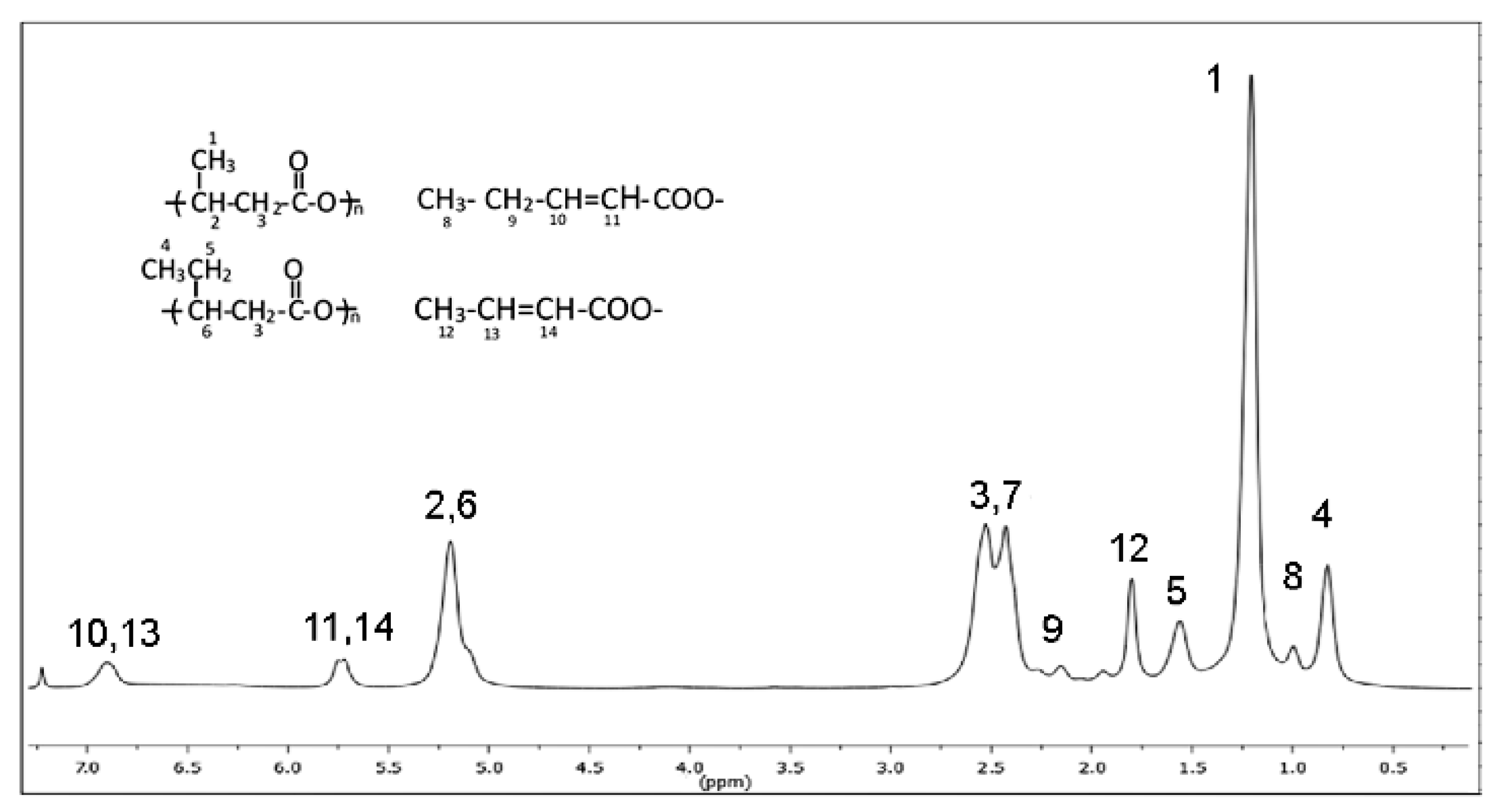
3.3. FT-IR and 1H-NMR Analysis
4. Conclusions
- -
- ethyl acetate is the best solvent because it dissolves the copolymer at a temperature lower than ethyl propionate and ethyl butyrate;
- -
- by increasing the temperature from 100 °C to 150 °C, the PHA dissolved in ethyl acetate underwent a progressive reduction of its molecular weight.
- -
- the higher the molecular weight of the polymer in the biomass, the lower the recovery yield;
- -
- at the minimum dissolution temperature, ethyl acetate gave recovery yields higher than the other ethylic esters, and that it preferentially extracts the copolymer fraction richer in 3HV comonomer and with the lower molecular weight;
- -
- by increasing the extraction temperature from 100 °C to 130 °C, the recovery yield increased from about 50 wt% to 80 wt% and the composition of the extracted polymer approached that of the reference sample;
- -
- by increasing the extraction temperature up to 150 °C, a progressive reduction of molecular weight of the extracted polymer and of the polymer fraction remaining in the biomass occurred;
- -
- the purity of the samples extracted with ethyl acetate was always very high, between 90 and 97 wt%, without the need for further purification by anti-solvent precipitation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Andreasi Bassi, S.; Boldrin, A.; Frenna, G.; Astrup, T.F. An Environmental and Economic Assessment of Bioplastic from Urban Biowaste. The Example of Polyhydroxyalkanoate. Bioresour. Technol. 2021, 327, 124813. [Google Scholar] [CrossRef]
- Vermeer, C.M.; Tamis, J.; Jonkers, H.M.; Kleerebezem, R. From Waste to Self-Healing Concrete: A Proof-of-Concept of a New Application for Polyhydroxyalkanoate. Resour. Conserv. Recy. 2021, 164, 105206. [Google Scholar] [CrossRef]
- Keskin, G.; Klzll, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of Polyhydroxyalkanoate (PHA) Polymers Family as Substitutes of Petroleum Based Polymers for Packaging Applications and Solutions Brought by Their Composites to Form Barrier Materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Kulkarni, S.O.; Kanekar, P.P.; Jog, J.P.; Patil, P.A.; Nilegaonkar, S.S.; Sarnaik, S.S.; Kshirsagar, P.R. Characterisation of Copolymer, Poly (Hydroxybutyrate-Co-Hydroxyvalerate) (PHB-Co-PHV) Produced by Halomonas Campisalis (MCM B-1027), Its Biodegradability and Potential Application. Bioresour. Technol. 2011, 102, 6625–6628. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable Polymers with a Range of Applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Valentino, F.; Gottardo, M.; Micolucci, F.; Pavan, P.; Bolzonella, D.; Rossetti, S.; Majone, M. Organic Fraction of Municipal Solid Waste Recovery by Conversion into Added-Value Polyhydroxyalkanoates and Biogas. ACS Sustain. Chem. Eng. 2018, 6, 16375–16385. [Google Scholar] [CrossRef]
- Madkour, M.H.; Heinrich, D.; Alghamdi, M.A.; Shabbaj, I.I.; Steinbüchel, A. PHA Recovery from Biomass. Biomacromolecules 2013, 14, 2963–2972. [Google Scholar] [CrossRef]
- Rodriguez-Perez, S.; Serrano, A.; Pantión, A.A.; Alonso-Fariñas, B. Challenges of Scaling-up PHA Production from Waste Streams. A Review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.; Braunegg, G. Advanced Approaches to Produce Polyhydroxyalkanoate (PHA) Biopolyesters in a Sustainable and Economic Fashion. EuroBiotech J. 2018, 2, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Valentino, F.; Moretto, G.; Lorini, L.; Bolzonella, D.; Pavan, P.; Majone, M. Pilot-Scale Polyhydroxyalkanoate Production from Combined Treatment of Organic Fraction of Municipal Solid Waste and Sewage Sludge. Ind. Eng. Chem. Res. 2019, 58, 12149–12158. [Google Scholar] [CrossRef]
- Moretto, G.; Russo, I.; Bolzonella, D.; Pavan, P.; Majone, M.; Valentino, F. An Urban Biorefinery for Food Waste and Biological Sludge Conversion into Polyhydroxyalkanoates and Biogas. Water Res. 2020, 170, 115371. [Google Scholar] [CrossRef]
- Werker, A.; Bengtsson, S.; Korving, L.; Hjort, M.; Anterrieu, S.; Alexandersson, T.; Johansson, P.; Karlsson, A.; Karabegovic, L.; Magnusson, P. Consistent Production of high quality PHa using activated sludge harvested from full scale municipal wastewaterreatment- PHARIO. Water Sci. Technol. 2018, 11, 2256–2269. [Google Scholar] [CrossRef]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon Recovery from Wastewater through Bioconversion into Biodegradable Polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, M.; Albuquerque, M.; Villano, M.; Majone, M. Mixed culture processes for polyhydroxyalkanoate production from agro-industrial surplus/wastes as feedstocks. In Comprehensive Biotechnology; Fava, F., Agathos, S., Young, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 669–683. [Google Scholar]
- Majone, M.; Chronopoulou, L.; Lorini, L.; Martinelli, A.; Palocci, C.; Rossetti, S.; Valentino, F.; Villano, M. PHA Copolymers from Microbial Mixed Coltures: Synthesis, Extraction and Related Properties. In Current Advantages in Biopolymer Processing and Characterization; Koller, M., Ed.; Nova Science Publisher: New York, NY, USA, 2017; pp. 223–277. [Google Scholar]
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of Polyhydroxyalkanoates From Single and Mixed Microbial Cultures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 624021. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rivero, C.; López-Gómez, J.P.; Roy, I. A Sustainable Approach for the Downstream Processing of Bacterial Polyhydroxyalkanoates: State-of-the-Art and Latest Developments. Biochem. Eng. J. 2019, 150, 107283. [Google Scholar] [CrossRef]
- Koller, M. Established and Advanced Approaches for Recovery of Microbial Polyhydroxyalkanoate (PHA) Biopolyesters from Surrounding Microbial Biomass. EuroBiotech J. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Kurdikar, D.L.; Strauser, F.E.; Solodar, A.J.; Paster, M.D.; Asrar, J. Methods of PHA Extraction and Recovery Using Non-Halogenated Solvents. U.S. Patent US6043063A, 14 April 1998. [Google Scholar]
- Kurdikar, D.L.; Strauser, F.E.; Solodar, A.J.; Paster, M.D. High Temperature PHA Extraction Using PHA-Poor. Solvents. Patent WO/1998/046783, 22 October 1998. [Google Scholar]
- Macagnan, K.L.; Alves, M.I.; Moreira, A. Approaches for Enhancing Extraction of Bacterial Polyhydroxyalkanoates for Industrial Applications. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019. [Google Scholar]
- Dubey, S.; Bharmoria, P.; Gehlot, P.S.; Agrawal, V.; Kumar, A.; Mishra, S. 1-Ethyl-3-methylimidazolium Diethylphosphate Based Extraction of Bioplastic “Polyhydroxyalkanoates” from Bacteria: Green and Sustainable Approach. ACS Sustain. Chem. Eng. 2018, 6, 766–773. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.W.; Wei, Y.H.; Wu, H.S.; Wang, S.S. Isolation and Purification of Bacterial Poly(3-Hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Jiang, G.; Johnston, B.; Townrow, D.E.; Radecka, I.; Radecka, I.; Chaber, P.; Adamus, Z.; Kowalczuk, M. Biomass Extraction Using Non-Chlorinated Solvents for Biocompatibility Improvement of Polyhydroxyalkanoates. Polymers 2018, 10, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosengart, A.; Cesário, M.T.; de Almeida, M.T.; Raposo, R.S.; Espert, A.; Díazde Apodaca, E.; da Fonseca, M.M. Efficient P(3HB) extraction from Burkholderia sacchari cells using non-chlorinated solvents. Biochem. Eng. J. 2015, 103, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Yabueng, N.; Napathorn, S.C. Toward non-toxic and simple recovery process of poly (3-hydroxybutyrate) using the green solvent 1, 3-dioxolane. Process Biochem. 2018, 69, 197–207. [Google Scholar] [CrossRef]
- Riedel, S.L.; Brigham, C.J.; Budde, C.F.; Bader, J.; Rha, C.; Stahl, U.; Sinskey, A.J. Recovery of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) from Ralstonia eutropha Cultures with Non-Halogenated Solvents. Biotechnol. Bioeng. 2013, 110, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Dimethyl Carbonate and Switchable Anionic Surfactants: Two Effective Tools for the Extraction of Polyhydroxyalkanoates from Microbial Biomass. Green Chem. 2015, 17, 1047–1056. [Google Scholar] [CrossRef]
- Fiorese, M.L.; Freitas, F.; Pais, J.; Ramos, A.M.; de Aragão, G.M.F.; Reis, M.A.M. Recovery of polyhydroxybutyrate (PHB) from Cupriavidus necator biomass by solvent extraction with 1,2-propylene carbonate. Eng. Life Sci. 2009, 9, 454–461. [Google Scholar] [CrossRef]
- de Souza Reis, G.A.; Michels, M.H.A.; Fajardo, G.L.; Lamot, I.; de Best, J.H. Optimization of Green Extraction and Purification of PHA Produced by Mixed Microbial Cultures from Sludge. Water 2020, 12, 1185. [Google Scholar] [CrossRef] [Green Version]
- Mongili, B.; Abdel Azim, A.; Fraterrigo Garofalo, S.; Batuecas, E.; Re, A.; Bocchini, S.; Fino, D. Novel insights in dimethyl carbonate-based extraction of polyhydroxybutyrate (PHB). Biotechnol. Biofuels 2021, 14, 13. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Chiellini, E.; Braunegg, G. Extraction of short-chain-length poly-[(R)-hydroxyalkanoates] (scl-PHA) by the “anti-solvent” acetone under elevated temperature and pressure. Biotechnol. Lett. 2013, 35, 1023–1028. [Google Scholar] [CrossRef]
- Chan, C.M.; Johansson, P.; Magnusson, P.; Vandi, L.J.; Arcos-Hernandez, M.; Halley, P.; Laycock, B.; Pratt, S.; Werker, A. Mixed culture polyhydroxyalkanoate-rich biomass assessment and quality control using thermogravimetric measurement methods. Polym. Degrad. Stab. 2017, 144, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Nonato, R.; Mantelatto, P.; Rossell, C. Integrated production of biodegradable plastic, sugar and ethanol. Appl. Microbiol. Biotechnol. 2001, 57, 1–5. [Google Scholar] [CrossRef]
- Righi, S.; Baioli, F.; Samorì, C.; Galletti, P.; Tagliavini, E.; Stramigioli, C.; Tugnoli, A.; Fantke, P. A life cycle assessment of poly-hydroxybutyrate extraction from microbial biomass using dimethyl carbonate. J. Clean. Prod. 2017, 168, 692–707. [Google Scholar] [CrossRef] [Green Version]
- Di Bitonto, L.; Menegatti, S.; Pastore, C. Process Intensification for the Production of the Ethyl Esters of Volatile Fatty Acids Using Aluminium Chloride Hexahydrate as a Catalyst. J. Clean. Prod. 2019, 239, 118122. [Google Scholar] [CrossRef] [Green Version]
- Prat, D.; Hayler, J.; Wells, A. A Survey of Solvent Selection Guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehadad, S. Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Lorini, L.; Martinelli, A.; Pavan, P.; Majone, M.; Valentino, F. Downstream Processing and Characterization of Polyhydroxyalkanoates (PHAs) Produced by Mixed Microbial Culture (MMC) and Organic Urban Waste as Substrate. Biomass Convers. Biorefinery 2020, 11, 693–703. [Google Scholar] [CrossRef]
- Braunegg, G.; Sonnleitner, B.; Lafferty, R. Rapid Gas-Chromatographic Method for Determination of Poly-Beta-Hydroxybutyric Acid in Microbial Biomass. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Okamura, K.; Su, C.J. Physical Properties of Poly (3-Hydroxy Butyrate). II. Conformational Aspects in Solution. Macromolecules 1970, 3, 735–740. [Google Scholar] [CrossRef]
- Hu, S.; McDonald, A.G.; Coats, E.R. Characterization of Polyhydroxybutyrate Biosynthesized from Crude Glycerol Waste Using Mixed Microbial Consortia. J. Appl. Polym. Sci. 2013, 129, 1314–1321. [Google Scholar] [CrossRef]
- Lorini, L.; Martinelli, A.; Capuani, C.; Frison, N.; Reis, M.A.M.; Sommer Ferreira, B.; Villano, M.; Majone, M.; Valentino, F. Characterization of Polyhydroxyalkanoates Produced at Pilot Scale from Different Organic Wastes. Front. Bioeng. Biotechnol. 2021, 9, 628719. [Google Scholar] [CrossRef] [PubMed]
- Kopinke, F.-D.; Remmler, M.; Mackenzie, K. Thermal decomposition of biodegradable polyesters—I: Poly (β-hydroxybutyric acid). Polym. Degrad. Stabil. 1996, 52, 25–38. [Google Scholar] [CrossRef]
- Xiang, H.; Wen, X.; Miu, X.; Li, Y.; Zhou, Z.; Zhu, M. Thermal depolymerization mechanisms of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci. 2016, 26, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Samorì, C.; Abbondanzi, F.; Galletti, P.; Giorgini, L.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Extraction of Polyhydroxyalkanoates from Mixed Microbial Cultures: Impact on Polymer Quality and Recovery. Bioresour. Technol. 2015, 189, 195–202. [Google Scholar] [CrossRef]
- Terada, M.; Marchessault, R.H. Determination of Solubility Parameters for Poly(3-Hydroxyalkanoates). Int. J. Biol. Macromol. 1999, 25, 207–215. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, G.; Park, S.; Lee, L. Industrial Scale Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). Appl. Microbiol. Biotechnol. 2001, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Manangan, T.; Shawaphun, S. Quantitative Extraction and Determination of Polyhydroxyalkanoate Accumulated in Alcaligenes Latus Dry Cells. Sci. Asia 2010, 36, 199–203. [Google Scholar] [CrossRef]
- Aramvash, A.; Gholami-Banadkuki, N.; Moazzeni-Zavareh, F.; Hajizadeh-Turchi, S. An Environmentally Friendly and Efficient Method for Extraction of PHB Biopolymer with Non-Halogenated Solvents. J. Microbiol. Biotechnol. 2015, 25, 1936–1943. [Google Scholar] [CrossRef]
- Gahlawat, G.; Soni, S.K. Study on Sustainable Recovery and Extraction of Polyhydroxyalkanoates (PHAs) Produced by Cupriavidus Necator Using Waste Glycerol for Medical Applications. Chem. Biochem. Eng. Q. 2019, 33, 99–110. [Google Scholar] [CrossRef]
- Werker, A.G.; Johansson, P.S.T.; Magnusson, O.G. Process for the Extraction of Polyhydroxyalkanoates from Biomass. U.S. Patent US20150368393A1, 24 December 2015. [Google Scholar]
- Grassie, N.; Murray, E.J.; Holmes, P.A. The Thermal Degradation of Poly(-(d)-β-Hydroxybutyric Acid): Part 1—Identification and Quantitative Analysis of Products. Polym. Degrad. Stab. 1984, 6, 47–61. [Google Scholar] [CrossRef]
- Nguyen, S.; Yu, G.; Marchessault, R.H. Thermal Degradation of Poly(3-Hydroxyalkanoates): Preparation of Well-Defined Oligomers. Biomacromolecules 2002, 3, 219–224. [Google Scholar] [CrossRef]
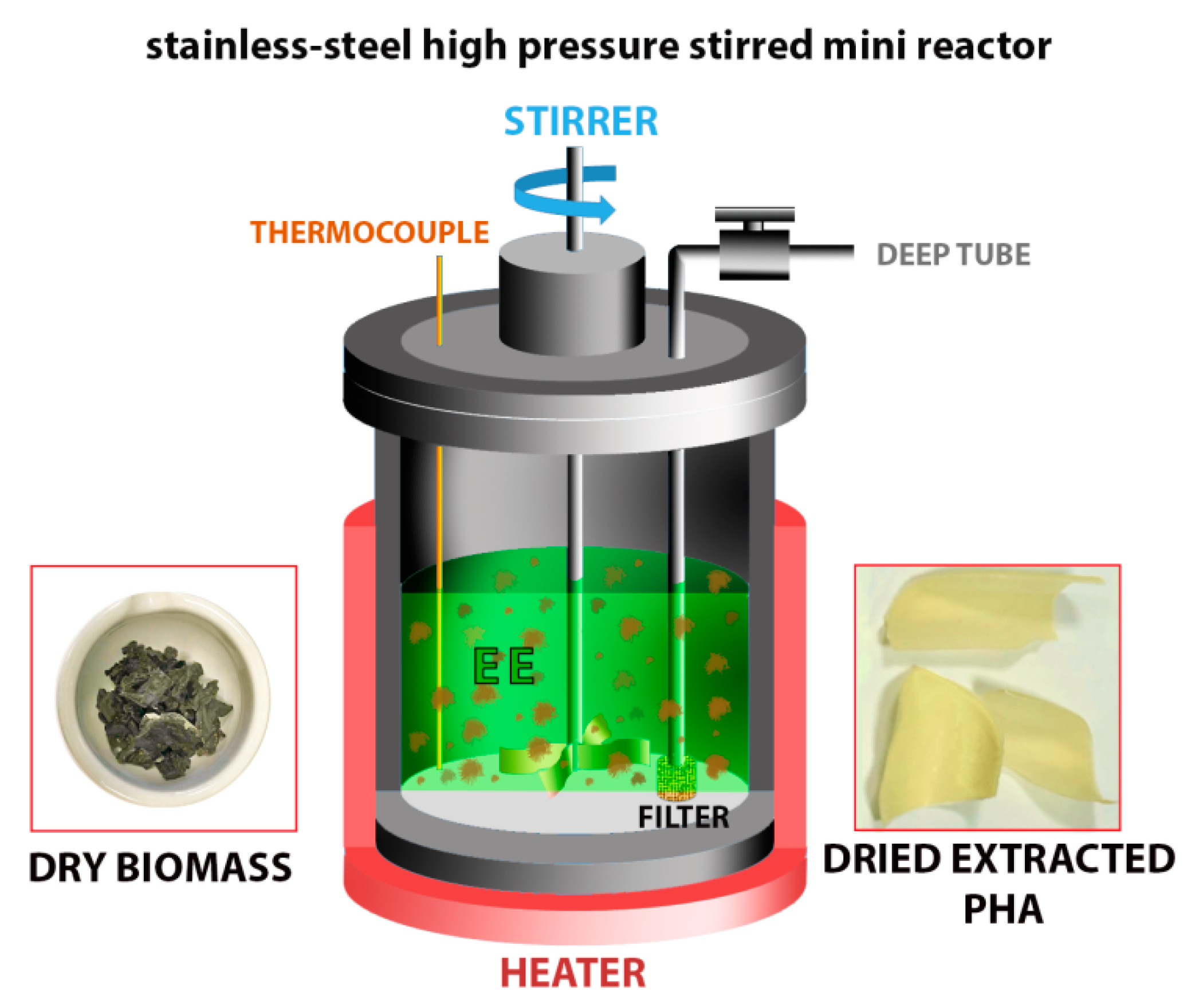
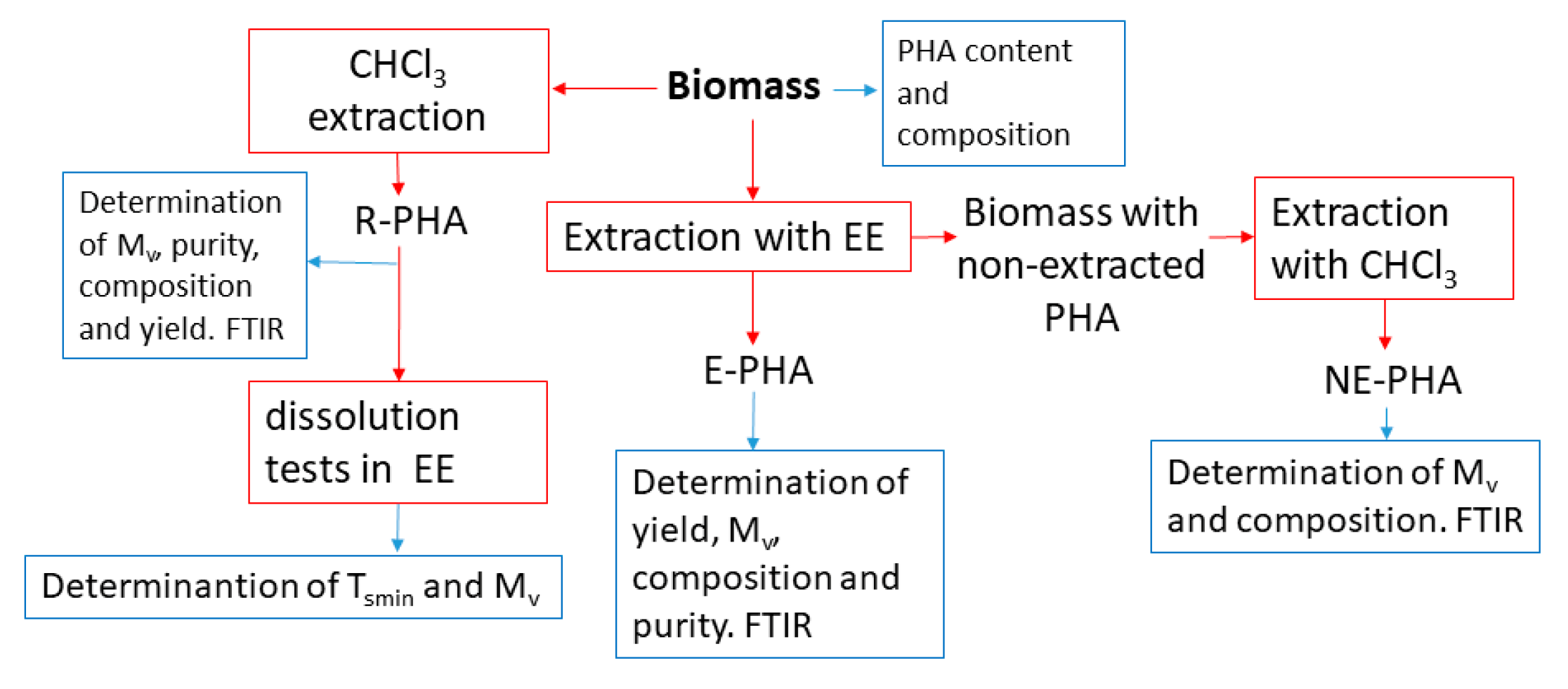

| Sample | PHA Content in Biomass (wt%) | Composition (3HB mol%) | Mv (kg mol−1) | Tsmin in EA (°C) | Tsmin in EP (°C) | Tsmin in EB (°C) |
|---|---|---|---|---|---|---|
| Biomass1 | 56 | 83 | – | – | – | – |
| Biomass2 | 62 | 89 | – | – | – | – |
| R-PHA 1 | – | 83 | 139 ± 3 | 100 | 115 | 120 |
| R-PHA 2 | – | 89 | 405 ± 5 | 115 | – | – |
| BIOMER | – | 100 | 390 ± 10 | 115 | – | – |
| E-PHA1 | NE-PHA1 | |||||
|---|---|---|---|---|---|---|
| Solvent | fE (wt%) | Composition (3HB mol%) | Purity (wt%) | Mv (kg·mol−1) | fNE (wt%) | Composition (3HB mol%) |
| EA at 100 °C | 54 | 72 | 97 | 72 | 44 | 89 |
| EP at 115 °C | 45 | 74 | 100 | 38 | 52 | 88 |
| EB at 120 °C | 32 | 57 | 84 | 29 | 62 | 90 |
| Sample | Recovered Fractions (wt%) | Composition (3HB mol%) | Mv (kg·mol−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| fTOT | fE | fNE | R-PHA | E-PHA | NE-PHA | R-PHA | R-PHA TE = Ts | E-PHA | NE-PHA | |
| Biomass1 TE = 100 °C | 98 | 54 | 44 | 83 | 69 | 88 | 139 | 133 | 72 | 126 |
| Biomass1 TE = 115 °C | 94 | 71 | 23 | 83 | 78 | 85 | 139 | 104 | 62 | 98 |
| Biomass2 TE = 115 °C | 92 | 66 | 25 | 89 | 84 | 92 | 405 | 333 | 236 | 358 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfano, S.; Lorini, L.; Majone, M.; Sciubba, F.; Valentino, F.; Martinelli, A. Ethylic Esters as Green Solvents for the Extraction of Intracellular Polyhydroxyalkanoates Produced by Mixed Microbial Culture. Polymers 2021, 13, 2789. https://doi.org/10.3390/polym13162789
Alfano S, Lorini L, Majone M, Sciubba F, Valentino F, Martinelli A. Ethylic Esters as Green Solvents for the Extraction of Intracellular Polyhydroxyalkanoates Produced by Mixed Microbial Culture. Polymers. 2021; 13(16):2789. https://doi.org/10.3390/polym13162789
Chicago/Turabian StyleAlfano, Sara, Laura Lorini, Mauro Majone, Fabio Sciubba, Francesco Valentino, and Andrea Martinelli. 2021. "Ethylic Esters as Green Solvents for the Extraction of Intracellular Polyhydroxyalkanoates Produced by Mixed Microbial Culture" Polymers 13, no. 16: 2789. https://doi.org/10.3390/polym13162789
APA StyleAlfano, S., Lorini, L., Majone, M., Sciubba, F., Valentino, F., & Martinelli, A. (2021). Ethylic Esters as Green Solvents for the Extraction of Intracellular Polyhydroxyalkanoates Produced by Mixed Microbial Culture. Polymers, 13(16), 2789. https://doi.org/10.3390/polym13162789









