Preparation and Characterization of Eco-Friendly Spent Coffee/ENR50 Biocomposite in Comparison to Carbon Black
Abstract
:1. Introduction
2. Materials and Method
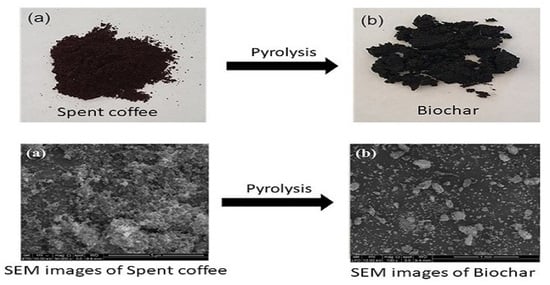
2.1. Preparation of Spent Coffee Ground and Its Derivatives
2.2. Preparation of Spent Coffee Ground and Its Derivatives—ENR Biocomposites
2.3. Chemical and Physical Material Properties
2.4. Tensile Testing
3. Results and Discussion
3.1. Particle Size Analysis
3.2. Cure Characteristics and Tensile Properties
3.3. Fourier Transmitted Infrared (FT-IR) Analysis of ENR Biocomposites
3.4. X-ray Diffraction Analyses of ENR Biocomposites
3.5. Thermal Analysis of ENR Biocomposites
3.6. Morphological Analysis of ENR Biocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azammi, A.M.N.; Sapuan, S.M.; Ishak, M.R.; Sultan, M.T.H. Physical and damping properties of kenaf fibre filled natural rubber/thermoplastic polyurethane composites. Def. Technol. 2020. [Google Scholar] [CrossRef]
- Magida, M.M.; Ibrahim, S.M.; Elnahas, H.H. Effect of gamma radiation on the characterization of chitosan/natural rubber latex polymer blends. Int. J. Polym. Anal. Charact. 2020. [Google Scholar] [CrossRef]
- Lay, M.; Samat, S.N.A.; Hwa, K.T.; Rashid, A.A. Ecofriendly latex films from cassava starch-filled radiation pre-vulcanized natural rubber latex. Radiat. Eff. Defects Solids 2019. [Google Scholar] [CrossRef]
- Chantawee, K.; Riyajan, S.A. Effect of Glycerol on the Physical Properties of Carboxylated Styrene-Butadiene Rubber/Cassava Starch Blend Films. J. Polym. Environ. 2019. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Pan, X.; Meng, F.; You, J.; Wang, Z. Preparation and properties of modified porous starch/carbon black/natural rubber composites. Compos. Part B Eng. 2019. [Google Scholar] [CrossRef]
- Jong, L. Reinforcement effect of soy protein nanoparticles in amine-modified natural rubber latex. Ind. Crop Prod. 2017. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Pongwisuthiruchte, A.; Potiyaraj, P. Recent advances of natural fibers based green rubber composites: Properties, current status, and future perspectives. J. Appl. Polym. Sci. 2021, 138, 50866. [Google Scholar] [CrossRef]
- Shen, Z.; Song, W.; Li, X.; Yang, L.; Wang, C.; Hao, Z.; Luo, Z. Enhancing performances of hemp fiber/natural rubber composites via polyhydric hyperbranched polyester. J. Polym. Eng. 2021. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Tzounis, L.; Pongwisuthiruchte, A.; Potiyaraj, P. Effect of various surface treatments on the performance of jute fibers filled natural rubber (NR) composites. Polymers 2020. [Google Scholar] [CrossRef] [Green Version]
- Anandan, S.; Lim, C.Y.; Tan, B.T.; Anggraini, V.; Raghunandan, M.E. Numerical and experimental investigation of oil palm shell reinforced rubber composites. Polymers 2020. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, M.S.; Syed Ismail, S.N.; Mohd Shaipul Amini, S.; Abdul Wahab, N.M.; Majid, N.A.; Sarip, M.N. Pineapple Leaf Fibre Filled Natural Rubber Composite: The Effect of Filler Loading. In Charting the Sustainable Future of ASEAN in Science and Technology; Springer Nature: Singapore, 2020. [Google Scholar]
- Abdelsalam, A.A.; Araby, S.; El-Sabbagh, S.H.; Abdelmoneim, A.; Hassan, M.A. Effect of carbon black loading on mechanical and rheological properties of natural rubber/styrene-butadiene rubber/nitrile butadiene rubber blends. J. Thermoplast. Compos. Mater. 2021. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Yu, H.; Xiao, T.; Li, H.; Yang, J. Analysis of effect of modification of silica and carbon black co-filled rubber composite on mechanical properties. e-Polymers 2021. [Google Scholar] [CrossRef]
- Peterson, S.C.; Kim, S. Reducing Biochar Particle Size with Nanosilica and Its Effect on Rubber Composite Reinforcement. J. Polym. Environ. 2020, 28, 317–322. [Google Scholar] [CrossRef]
- Peterson, S.C.; Chandrasekaran, S.R.; Sharma, B.K. Birchwood biochar as partial carbon black replacement in styrene-butadiene rubber composites. J. Elastomers Plast. 2016. [Google Scholar] [CrossRef]
- Jong, L.; Peterson, S.C.; Jackson, M.A. Utilization of Porous Carbons Derived from Coconut Shell and Wood in Natural Rubber. J. Polym. Environ. 2014. [Google Scholar] [CrossRef]
- Taleb, F.; Ammar, M.; ben Mosbah, M.; ben Salem, R.; Moussaoui, Y. Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Sci. Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020. [Google Scholar] [CrossRef]
- Jiang, C.; Bo, J.; Xiao, X.; Zhang, S.; Wang, Z.; Yan, G.; Wu, Y.; Wong, C.; He, H. Converting waste lignin into nano-biochar as a renewable substitute of carbon black for reinforcing styrene-butadiene rubber. Waste Manag. 2020. [Google Scholar] [CrossRef]
- Peterson, S.C. Coppiced biochars as partial replacement of carbon black filler in polybutadiene/natural rubber composites. J. Compos. Sci. 2020. [Google Scholar] [CrossRef]
- Xue, B.; Wang, X.; Sui, J.; Xu, D.; Zhu, Y.; Liu, X. A facile ball milling method to produce sustainable pyrolytic rice husk bio-filler for reinforcement of rubber mechanical property. Ind. Crops Prod. 2019. [Google Scholar] [CrossRef]
- Peterson, S.C.; Kim, S. Using heat-treated starch to modify the surface of biochar and improve the tensile properties of biochar-filled styrene–butadiene rubber composites. J. Elastomers Plast. 2019. [Google Scholar] [CrossRef]
- Masek, A.; Cichosz, S.; Piotrowska, M. Biocomposites of Epoxidized Natural Rubber/Poly(lactic acid) Modified with Natural Fillers (Part I). Int. J. Mol. Sci. 2021, 22, 3150. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.F.; Ismail, H.; Ishak, K.M.K. Poly(lactic acid)/natural rubber/kenaf biocomposites production using poly(methyl methacrylate) and epoxidized natural rubber as co-compatibilizers. Iran. Polym. J. 2021, 30, 737–749. [Google Scholar] [CrossRef]
- Boonmahitthisud, A.; Mongkolvai, A.; Chuayjuljit, S. Toughness Improvement in Bio-based Poly(Lactic Acid)/Epoxidized Natural Rubber Blend Reinforced with Nanosized Silica. J. Polym. Environ. 2021, 29, 2530–2545. [Google Scholar] [CrossRef]
- Aswathy, T.R.; Dash, B.; Dey, P.; Nair, S.; Naskar, K. Synergistic effect of graphene with graphene oxide, nanoclay, and nanosilica in enhancing the mechanical and barrier properties of bromobutyl rubber/epoxidized natural rubber composites. J. Appl. Polym. Sci. 2021, 138, 50746. [Google Scholar] [CrossRef]
- Block, I.; Günter, C.; Duarte Rodrigues, A.; Paasch, S.; Hesemann, P.; Taubert, A. Carbon Adsorbents from Spent Coffee for Removal of Methylene Blue and Methyl Orange from Water. Materials 2021, 14, 3996. [Google Scholar] [CrossRef] [PubMed]
- Masłowski, M.; Miedzianowska, J.; Delekta, M.; Czylkowska, A.; Strzelec, K. Natural rubber biocomposites filled with phyto-ashes rich in biogenic silica obtained from wheat straw and field horsetail. Polymers 2021, 13, 1177. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A review on natural fiber reinforced polymer composite for bullet proof and ballistic applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef]
- Mohan Kumar, H.K.; Subramaniam, S.; Rathanasamy, R.; Pal, S.K.; Palaniappan, S.K. Substantial reduction of carbon black and balancing the technical properties of styrene butadiene rubber compounds using nanoclay. J. Rubber Res. 2020, 23, 79–87. [Google Scholar] [CrossRef]
- Raju, G.; Mas Haris, M.R.H. Preparation and characterization of acidified chitosan immobilized in epoxidized natural rubber. Polym. Test. 2016. [Google Scholar] [CrossRef]
- Yangthong, H.; Wisunthorn, S.; Pichaiyut, S.; Nakason, C. Novel epoxidized natural rubber composites with geopolymers from fly ash waste. Waste Manag. 2019, 87, 148–160. [Google Scholar] [CrossRef] [PubMed]









| Materials | Parts per Hundred of Rubber (phr) | |
|---|---|---|
| ENR50 | 100 | 100 |
| Zinc Oxide | 3 | 3 |
| Stearic Acid | 2 | 2 |
| Biochar | 0, 2.5, 5, 10, 15, 20 | - |
| Carbon Black (N330) | - | 0, 2.5, 5, 10, 15, 20 |
| CBS | 1 | 1 |
| Sulfur | 1.5 | 1.5 |
| Sample | Particle Size (µm) | Standard Deviation (µm) | % | ||
|---|---|---|---|---|---|
| C | H | N | |||
| SC | 0.211 | 0.171 | 39.06 | 1.63 | 3.21 |
| BioChar | 0.081 | 0.034 | 59.56 | 6.61 | 2.13 |
| CB | 0.036 | 0.012 | 62.25 | 0.5 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, G.; Khalid, M.; Shaban, M.M.; Azahari, B. Preparation and Characterization of Eco-Friendly Spent Coffee/ENR50 Biocomposite in Comparison to Carbon Black. Polymers 2021, 13, 2796. https://doi.org/10.3390/polym13162796
Raju G, Khalid M, Shaban MM, Azahari B. Preparation and Characterization of Eco-Friendly Spent Coffee/ENR50 Biocomposite in Comparison to Carbon Black. Polymers. 2021; 13(16):2796. https://doi.org/10.3390/polym13162796
Chicago/Turabian StyleRaju, Gunasunderi, Mohammad Khalid, Mahmoud M. Shaban, and Baharin Azahari. 2021. "Preparation and Characterization of Eco-Friendly Spent Coffee/ENR50 Biocomposite in Comparison to Carbon Black" Polymers 13, no. 16: 2796. https://doi.org/10.3390/polym13162796
APA StyleRaju, G., Khalid, M., Shaban, M. M., & Azahari, B. (2021). Preparation and Characterization of Eco-Friendly Spent Coffee/ENR50 Biocomposite in Comparison to Carbon Black. Polymers, 13(16), 2796. https://doi.org/10.3390/polym13162796