Charge Modification as a Mechanism for Tunable Properties in Polymer–Surfactant Complexes
Abstract
:1. Introduction
2. Materials and Methods
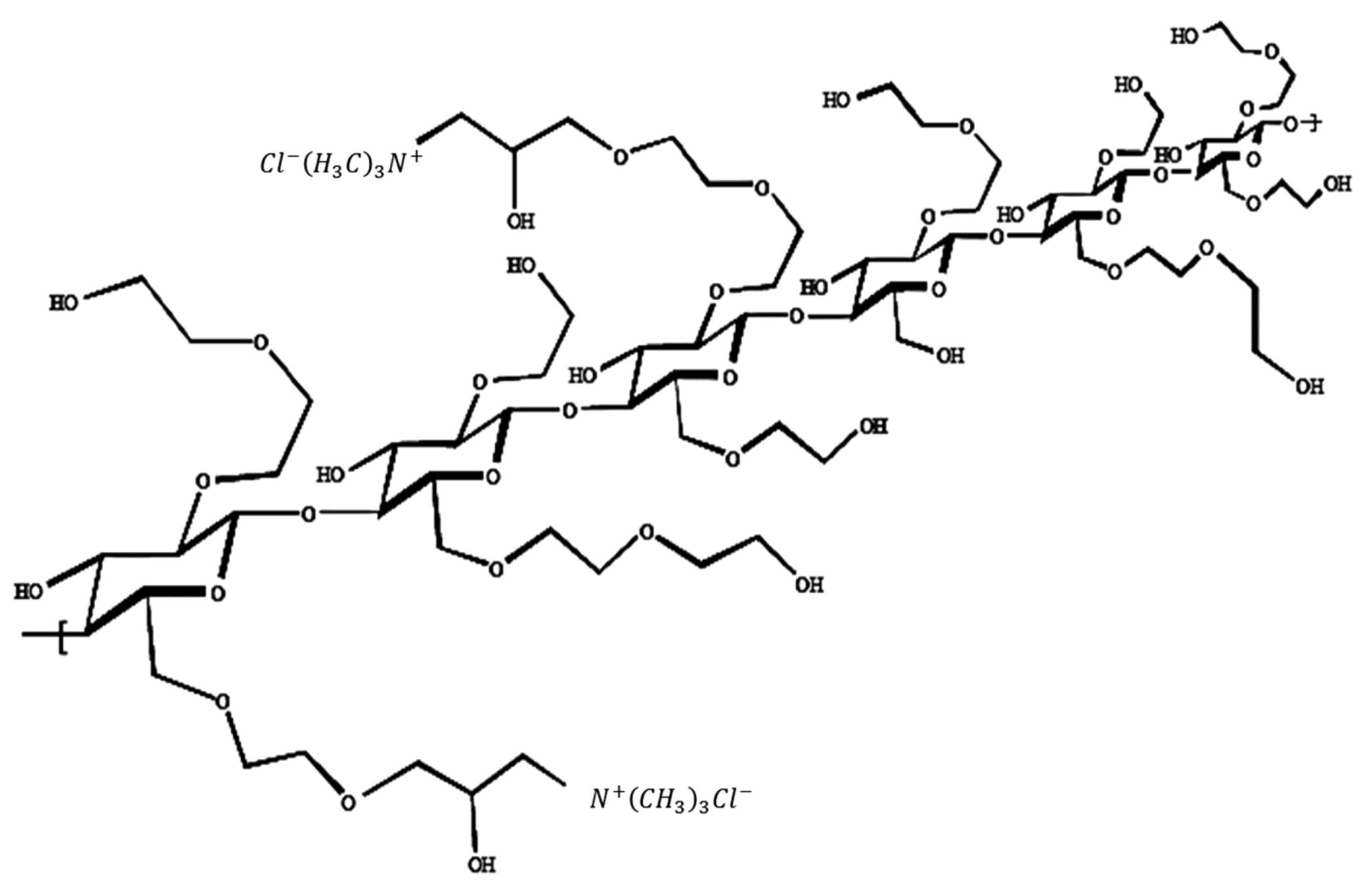
2.1. Materials
2.2. Methods
Small-Angle Neutron Scattering (SANS)
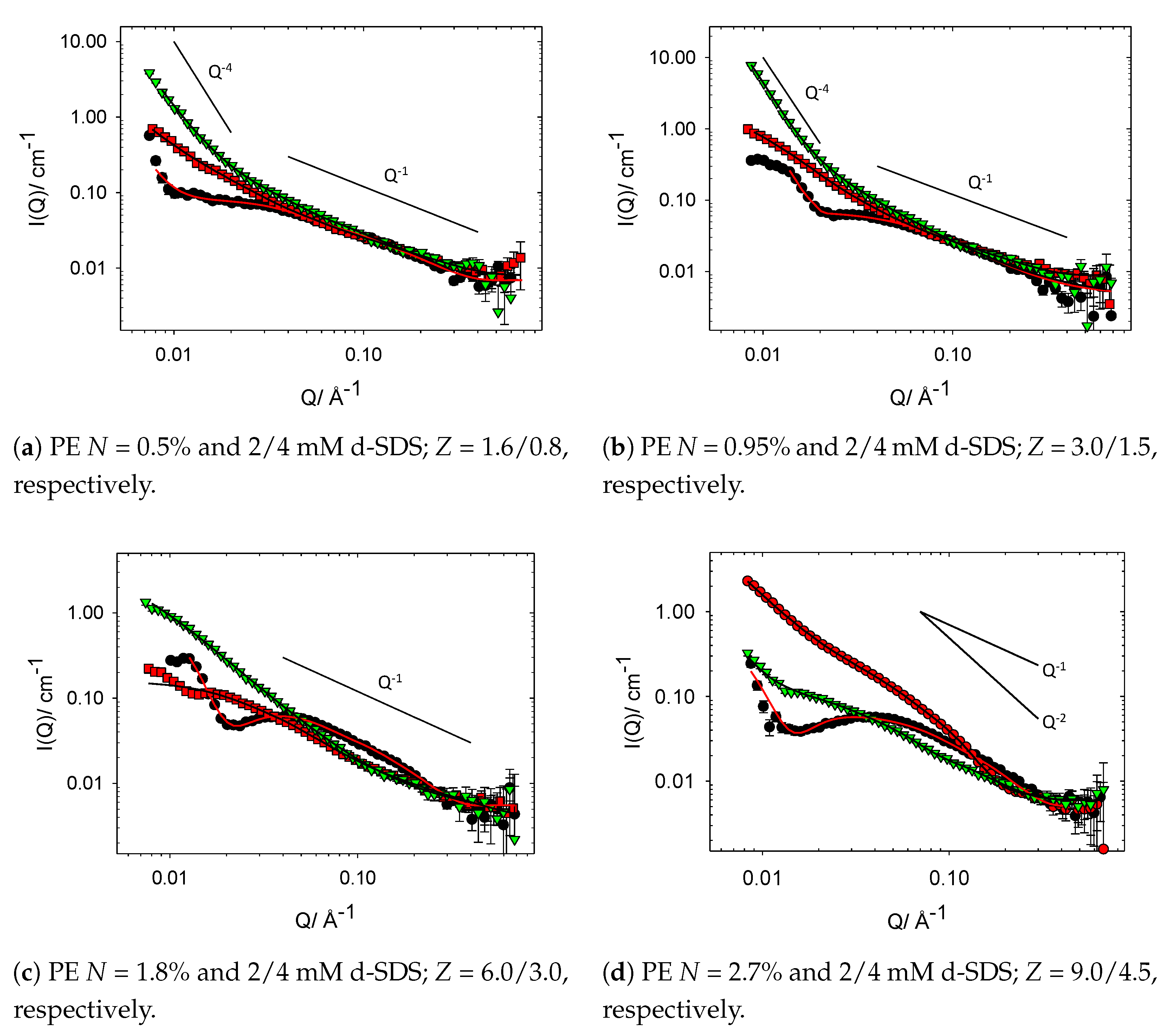
3. Results and Discussion
3.1. Polymer-Only Solutions
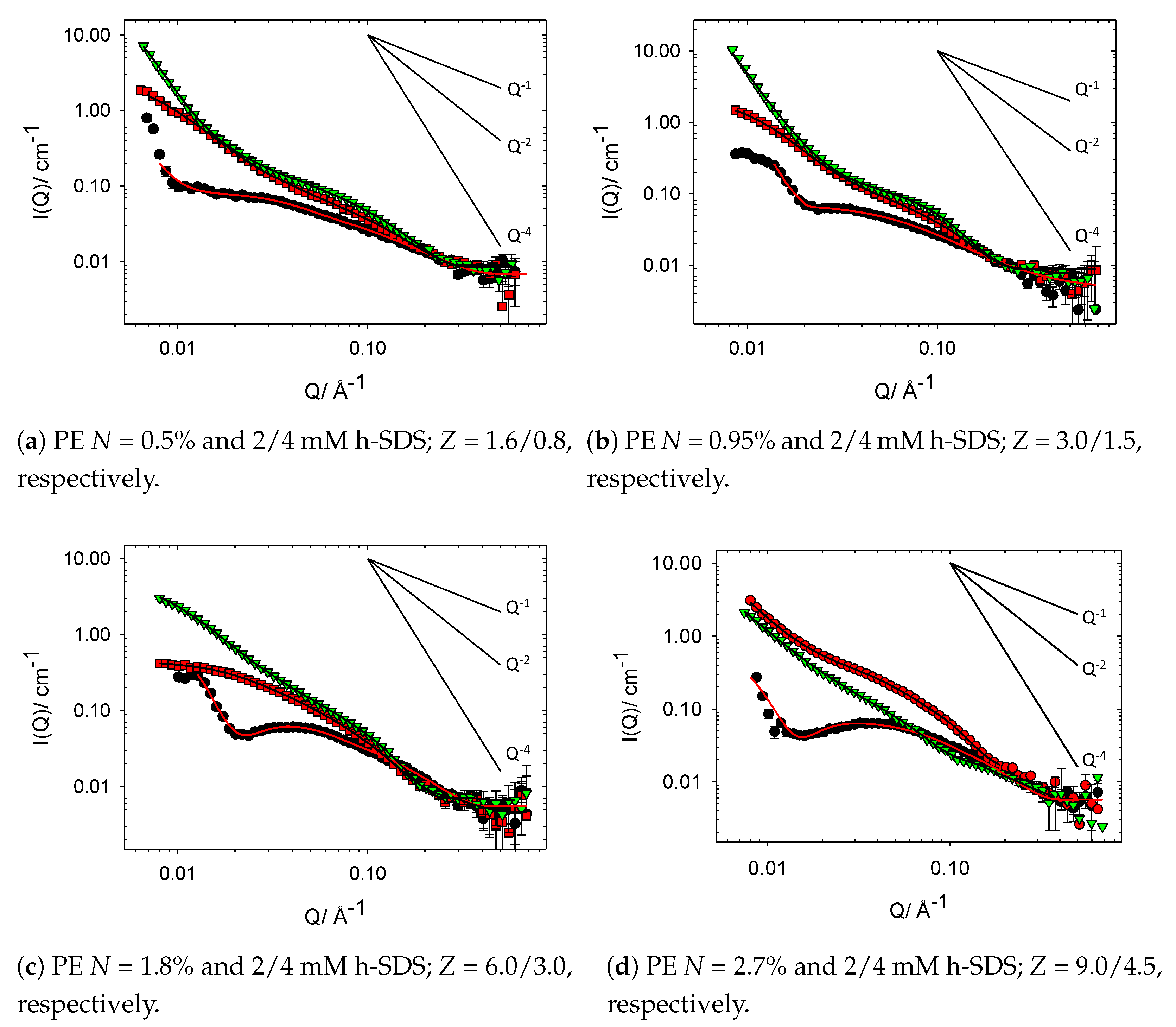
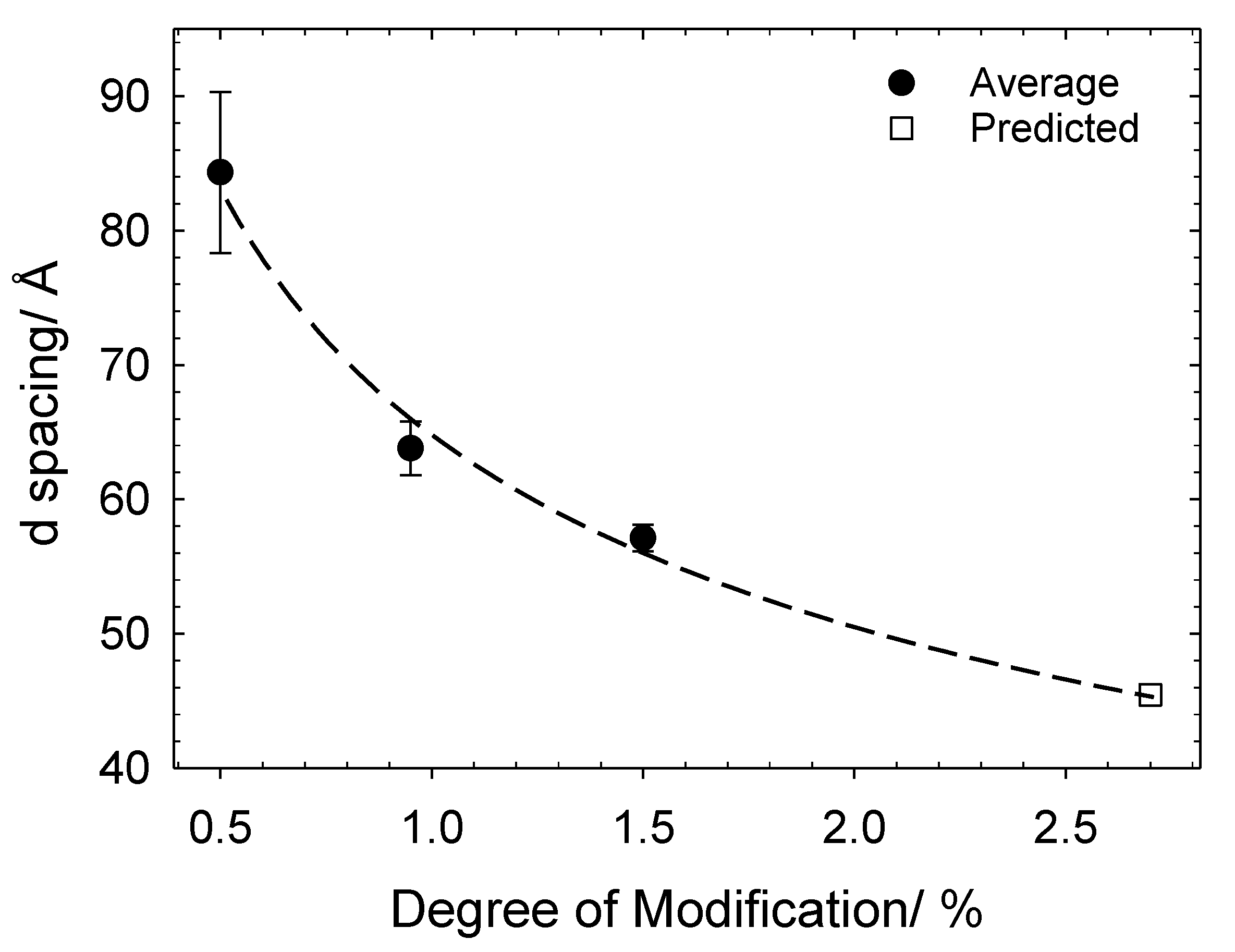
3.2. Cat-HEC/SDS Complexes in the Semidilute Regime
3.2.1. Unmodified HEC–SDS Complexes
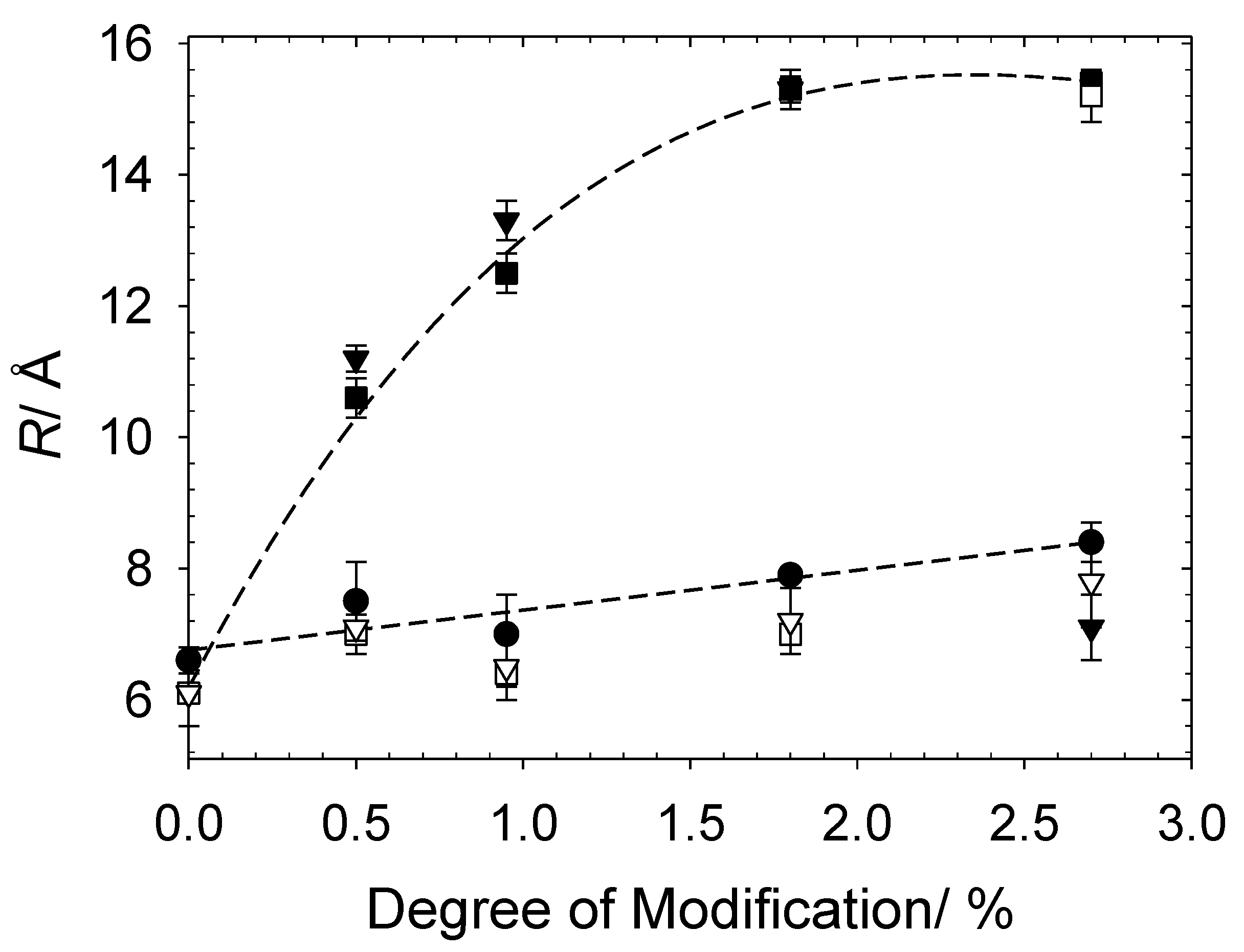
3.2.2. Charge Modified Cat-HEC/SDS Complexes (Full Contrast)
3.2.3. Charge Modified Cat-HEC/SDS Complexes (PE Contrast)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goddard, E. Polymer/surfactant interaction—Its relevance to detergent systems. J. Am. Oil Chem. Soc. 1994, 71, 1–16. [Google Scholar] [CrossRef]
- Chiappisi, L.; Hoffmann, I.; Gradzielski, M. Complexes of oppositely charged polyelectrolytes and surfactants–recent developments in the field of biologically derived polyelectrolytes. Soft Matter 2013, 9, 3896–3909. [Google Scholar] [CrossRef] [Green Version]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef]
- Oh, K.T.; Bronich, T.K.; Bromberg, L.; Hatton, T.A.; Kabanov, A.V. Block ionomer complexes as prospective nanocontainers for drug delivery. J. Control. Release 2006, 115, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tam, K. Interaction between polyelectrolyte and oppositely charged surfactant: Effect of charge density. J. Phys. Chem. B 2004, 108, 8976–8982. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Voets, I.K.; De Keizer, A.; Stuart, M.A.C. Complex coacervate core micelles. Adv. Colloid Interface Sci. 2009, 147, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Magny, B.; Iliopoulos, I.; Zana, R.; Audebert, R. Mixed micelles formed by cationic surfactants and anionic hydrophobically modified polyelectrolytes. Langmuir 1994, 10, 3180–3187. [Google Scholar] [CrossRef]
- Leung, P.; Goddard, E. Gels from dilute polymer/surfactant solutions. Langmuir 1991, 7, 608–609. [Google Scholar] [CrossRef]
- Patel, L.; Mansour, O.; Bryant, H.; Abdullahi, W.; Dalgliesh, R.M.; Griffiths, P.C. Interaction of Low Molecular Weight Poly (diallyldimethylammonium chloride) and Sodium Dodecyl Sulfate in Low Surfactant–Polyelectrolyte Ratio, Salt-Free Solutions. Langmuir 2020, 36, 8815–8825. [Google Scholar] [CrossRef]
- Lankalapalli, S.; Kolapalli, V. Polyelectrolyte complexes: A review of their applicability in drug delivery technology. Indian J. Pharm. Sci. 2009, 71, 481. [Google Scholar] [CrossRef] [Green Version]
- Meka, V.S.; Sing, M.K.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Muthukumar, M. 50th anniversary perspective: A perspective on polyelectrolyte solutions. Macromolecules 2017, 50, 9528–9560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, L.; Mansour, O.; Crossman, M.; Griffiths, P. Electrophoretic NMR Characterization of Charged Side Chain Cationic Polyelectrolytes and Their Interaction with the Anionic Surfactant, Sodium Dodecyl Sulfate. Langmuir 2019, 35, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, I.; Farago, B.; Schweins, R.; Falus, P.; Sharp, M.; Prévost, S.; Gradzielski, M. On the mesoscopic origins of high viscosities in some polyelectrolyte-surfactant mixtures. J. Chem. Phys. 2015, 143, 074902. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, G.R.; Cristiglio, V.; Clemens, D.; Hoffmann, I.; Schneck, E. Influence of the Surfactant Tail Length on the Viscosity of Oppositely Charged Polyelectrolyte/Surfactant Complexes. Macromolecules 2021, 54, 2529–2540. [Google Scholar] [CrossRef]
- Hoffmann, I.; Heunemann, P.; Prévost, S.; Schweins, R.; Wagner, N.J.; Gradzielski, M. Self-aggregation of mixtures of oppositely charged polyelectrolytes and surfactants studied by rheology, dynamic light scattering and small-angle neutron scattering. Langmuir 2011, 27, 4386–4396. [Google Scholar] [CrossRef]
- Hoffmann, I.; Farago, B.; Schweins, R.; Falus, P.; Sharp, M.; Gradzielski, M. Structure and dynamics of polyelectrolytes in viscous polyelectrolyte-surfactant complexes at the mesoscale. EPL (Europhys. Lett.) 2013, 104, 28001. [Google Scholar] [CrossRef]
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kelkar, M.S.; Wagner, N.J. Phase behavior and molecular thermodynamics of coacervation in oppositely charged polyelectrolyte/surfactant systems: A cationic polymer JR 400 and anionic surfactant SDS mixture. Langmuir 2012, 28, 10348–10362. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wagner, N.J. Universal binding behavior for ionic alkyl surfactants with oppositely charged polyelectrolytes. J. Am. Chem. Soc. 2013, 135, 17547–17555. [Google Scholar] [CrossRef]
- Lapitsky, Y.; Parikh, M.; Kaler, E.W. Calorimetric determination of surfactant/polyelectrolyte binding isotherms. J. Phys. Chem. B 2007, 111, 8379–8387. [Google Scholar] [CrossRef]
- Del Sorbo, G.R.; Prévost, S.; Schneck, E.; Gradzielski, M.; Hoffmann, I. On the Mechanism of Shear-Thinning in Viscous Oppositely Charged Polyelectrolyte Surfactant Complexes (PESCs). J. Phys. Chem. B 2020, 124, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, N.; Nguyen, G.T.; Kjøniksen, A.L.; Knudsen, K.D.; Nyström, B. Structure and dynamics of aqueous mixtures of an anionic cellulose derivative and anionic or cationic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 40–49. [Google Scholar] [CrossRef]
- Hoffmann, I.; Simon, M.; Farago, B.; Schweins, R.; Falus, P.; Holderer, O.; Gradzielski, M. Structure and dynamics of polyelectrolyte surfactant mixtures under conditions of surfactant excess. J. Chem. Phys. 2016, 145, 124901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivia, D.S. Elementary Scattering Theory: For X-ray and Neutron Users; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Zhang, Y.; Douglas, J.F.; Ermi, B.D.; Amis, E.J. Influence of counterion valency on the scattering properties of highly charged polyelectrolyte solutions. J. Chem. Phys. 2001, 114, 3299–3313. [Google Scholar] [CrossRef] [Green Version]
- Spruijt, E.; Leermakers, F.A.; Fokkink, R.; Schweins, R.; van Well, A.A.; Cohen Stuart, M.A.; van der Gucht, J. Structure and dynamics of polyelectrolyte complex coacervates studied by scattering of neutrons, X-rays, and light. Macromolecules 2013, 46, 4596–4605. [Google Scholar] [CrossRef]
- Wei, Y.; Hore, M.J. Characterizing polymer structure with small-angle neutron scattering: A Tutorial. J. Appl. Phys. 2021, 129, 171101. [Google Scholar] [CrossRef]
- Thuresson, K.; Söderman, O.; Hansson, P.; Wang, G. Binding of SDS to ethyl (hydroxyethyl) cellulose. Effect of hydrophobic modification of the polymer. J. Phys. Chem. 1996, 100, 4909–4918. [Google Scholar] [CrossRef]
- Wittgren, B.; Stefansson, M.; Porsch, B. Interactions between sodium dodecyl sulphate and non-ionic cellulose derivatives studied by size exclusion chromatography with online multi-angle light scattering and refractometric detection. J. Chromatogr. A 2005, 1082, 166–175. [Google Scholar] [CrossRef]
- Persson, B.; Nilsson, S.; Sundelöf, L.O. On the characterization principles of some technically important water-soluble nonionic cellulose derivatives. Part II: Surface tension and interaction with a surfactant. Carbohydr. Polym. 1996, 29, 119–127. [Google Scholar] [CrossRef]
- Merta, J.; Garamus, V.M.; Willumeit, R.; Stenius, P. Structure of complexes formed by PDADMAC and sodium palmitate. Langmuir 2002, 18, 7272–7278. [Google Scholar] [CrossRef]
- Merta, J.; Garamus, V.M.; Kuklin, A.I.; Willumeit, R.; Stenius, P. Determination of the structure of complexes formed by a cationic polymer and mixed anionic surfactants by small-angle neutron scattering. Langmuir 2000, 16, 10061–10068. [Google Scholar] [CrossRef]
- Ermi, B.D.; Amis, E.J. Influence of backbone solvation on small angle neutron scattering from polyelectrolyte solutions. Macromolecules 1997, 30, 6937–6942. [Google Scholar] [CrossRef]
- Manning, G. Counterion condensation theory of attraction between like charges in the absence of multivalent counterions. Eur. Phys. J. E 2011, 34, 132. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Fernández-Peña, L.; Ortega, F.; Rubio, R.G. Equilibrium and kinetically trapped aggregates in polyelectrolyte–oppositely charged surfactant mixtures. Curr. Opin. Colloid Interface Sci. 2020, 48, 91–108. [Google Scholar] [CrossRef]
- Turro, N.J.; Yekta, A. Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J. Am. Chem. Soc. 1978, 100, 5951–5952. [Google Scholar] [CrossRef]
- Bergström, M.; Pedersen, J.S. Structure of pure SDS and DTAB micelles in brine determined by small-angle neutron scattering (SANS). Phys. Chem. Chem. Phys. 1999, 1, 4437–4446. [Google Scholar] [CrossRef]
- Zemb, T.; Charpin, P. Micellar structure from comparison of X-ray and neutron small-angle scattering. J. Phys. 1985, 46, 249–256. [Google Scholar] [CrossRef]
- Nierlich, M.; Williams, C.; Boué, F.; Cotton, J.; Daoud, M.; Famoux, B.; Jannink, G.; Picot, C.; Moan, M.; Wolff, C.; et al. Small angle neutron scattering by semi-dilute solutions of polyelectrolyte. J. Phys. 1979, 40, 701–704. [Google Scholar] [CrossRef]
- Sharratt, W.N.; O’Connell, R.; Rogers, S.E.; Lopez, C.G.; Cabral, J.T. Conformation and Phase Behavior of Sodium Carboxymethyl Cellulose in the Presence of Mono-and Divalent Salts. Macromolecules 2020, 53, 1451–1463. [Google Scholar] [CrossRef]
- Lopez, C.G.; Horkay, F.; Mussel, M.; Jones, R.L.; Richtering, W. Screening lengths and osmotic compressibility of flexible polyelectrolytes in excess salt solutions. Soft Matter 2020, 16, 7289–7298. [Google Scholar] [CrossRef] [PubMed]


| Degree of Modification (%) | R (Å) | L (Å) | f (±0.2) |
|---|---|---|---|
| 0 | 6.6 | 356 | - |
| 0.5 | 7.5 | 109 | 3.0 |
| 0.95 | 7.0 | 80 | 3.0 |
| 1.8 | 7.9 | 73 | 3.0 |
| 2.7 | 8.4 | 78 | 3.0 |
| Degree of Modification | Contrast | [SDS] | R | ± 0.5 | L |
|---|---|---|---|---|---|
| (%) | (mM) | (Å) | (Å) | ||
| 0 | Full | 2 | 6.3 | 345 | |
| 4 | 6.2 | 278 | |||
| PE | 2 | 6.1 | 300 | ||
| 4 | 6.1 | 310 | |||
| Degree of Modification (%) | Contrast | [SDS] (mM) | Z | R (Å) | |
|---|---|---|---|---|---|
| 0.5 | Full | 2 | 1.6 | 10.6 | 38 ± 7 |
| 4 | 0.8 | 11.2 | 31 ± 6 | ||
| PE | 2 | 1.6 | 7.0 | - | |
| 4 | 0.8 | 7.1 | - | ||
| 0.95 | Full | 2 | 3.0 | 12.5 | 39 ± 5 |
| 4 | 1.5 | 13.3 | 40 ± 5 | ||
| PE | 2 | 3.0 | 6.4 | - | |
| 4 | 1.5 | 6.5 | - | ||
| 1.8 | Full | 2 | 5.0 | 15.3 | 42 ± 9 |
| 4 | 3.0 | 15.3 | 45 ± 7 | ||
| PE | 2 | 5.0 | 7.0 | - | |
| 4 | 3.0 | 7.2 | - | ||
| 2.7 | Full | 2 | 9.0 | 15.4 | - |
| 4 | 4.5 | 7.1 | - | ||
| PE | 2 | 9.0 | 15.2 | - | |
| 4 | 4.5 | 7.8 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, C.; Abdullahi, W.; Dalgliesh, R.; Crossman, M.; Griffiths, P.C. Charge Modification as a Mechanism for Tunable Properties in Polymer–Surfactant Complexes. Polymers 2021, 13, 2800. https://doi.org/10.3390/polym13162800
Hill C, Abdullahi W, Dalgliesh R, Crossman M, Griffiths PC. Charge Modification as a Mechanism for Tunable Properties in Polymer–Surfactant Complexes. Polymers. 2021; 13(16):2800. https://doi.org/10.3390/polym13162800
Chicago/Turabian StyleHill, Christopher, Wasiu Abdullahi, Robert Dalgliesh, Martin Crossman, and Peter Charles Griffiths. 2021. "Charge Modification as a Mechanism for Tunable Properties in Polymer–Surfactant Complexes" Polymers 13, no. 16: 2800. https://doi.org/10.3390/polym13162800
APA StyleHill, C., Abdullahi, W., Dalgliesh, R., Crossman, M., & Griffiths, P. C. (2021). Charge Modification as a Mechanism for Tunable Properties in Polymer–Surfactant Complexes. Polymers, 13(16), 2800. https://doi.org/10.3390/polym13162800








