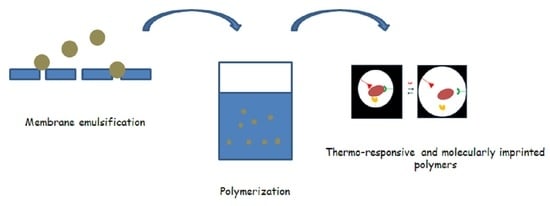
Membrane Emulsification Process as a Method for Obtaining Molecularly Imprinted Polymers
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Chemicals
2.2. Apparatus
2.3. Methods
2.3.1. Preparation of Polymers
2.3.2. Analysis of Materials
Scanning Electron Microscopy
Porous Structure Characterization
Nitrogen Content
Water Regain
Characterization of Size and Polydispersity of Microspheres
Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3.3. Evaluation of the Sorption Properties
Influence of Temperature
Adsorption Isotherms
Adsorption Kinetics
2.3.4. Analysis of BPA Concentration
3. Results and Discussion
3.1. Preparation of Polymers and Characterization of Materials
3.2. Evaluation of Sorption Properties
3.2.1. Influence of Temperature on the Adsorption of BPA
3.2.2. Adsorption Isotherms
3.2.3. Kinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIBN | Azoisobutyronitrile |
| ATR-FTIR | Attenuated Total Reflectance-Fourier-Transform Infrared Spectroscopy |
| BET | Brunauer–Emmett–Teller isotherm |
| BJH | Barret–Joyner–Halenda method |
| BPA | Bisphenol A |
| DLS | Dynamic Light Scattering |
| EDCs | Endocrine disrupting compounds |
| EGDMA | Ethylene glycol dimethacrylate |
| LCST | Lower critical solution temperature |
| M | Functional monomers |
| ME | Membrane emulsification |
| MIPs | Molecularly imprinted polymers |
| MIP-1 | Thermoresponsive molecularly imprinted polymer, 7 wt.% of BPA |
| MIP-2 | Thermoresponsive molecularly imprinted polymer, 5 wt.% of BPA |
| MMA | Methyl methacrylate |
| NIP | Thermoresponsive non-imprinted polymer, 0 wt.% of BPA |
| NIPAM | N-isopropylacrylamide |
| PNIPAM | Poly(N-isopropylacrylamide) |
| PSD | Pore size distribution |
| SEM | Scanning electron microscopy |
| SPAN | Characteristic polydispersity index |
| TSMIP | Thermoresponsive molecularly imprinted polymer |
References
- Piacentini, E.; Drioli, E.; Giorno, L. Membrane emulsification technology: Twenty-five years of inventions and research through patent survey. J. Membr. Sci. 2014, 468, 410–422. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M. Preparation of poly(styrene-co-divinylbenzene) microspheres by membrane emulsification. Desalination 2009, 241, 331–336. [Google Scholar] [CrossRef]
- Arkoumanis, P.G.; Norton, I.T.; Spyropoulos, F. Pickering particle and emulsifier co-stabilised emulsions produced via rotating membrane emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Joscelyne, S.M.; Trägårdh, G. Membrane emulsification—A literature review. J. Membr. Sci. 2000, 169, 107–117. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M. Molecularly Imprinted Polymers for Water Polishing. In Advanced Separations by Specialized Sorbents. Chromatographic Science Series; Dragan, E.S., Ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA; New York, NY, USA; London, UK, 2015; Volume 108, pp. 195–208. [Google Scholar]
- Omi, S.; Taguchi, T.; Nagai, M.; Ma, G.-H. Synthesis of 100 μm uniform porous spheres by SPG emulsification with subsequent swelling of the droplets. J. Appl. Polym. Sci. 1997, 63, 931–942. [Google Scholar] [CrossRef]
- Sugiura, S.; Nakajima, M.; Seki, M. Preparation of Monodispersed Polymeric Microspheres over 50 μm Employing Microchannel Emulsification. Ind. Eng. Chem. Res. 2002, 41, 4043–4047. [Google Scholar] [CrossRef]
- Cáceres, C.; Bravo, C.; Rivas, B.; Moczko, E.; Saez, P.; Garcia, Y.; Pereira, E. Molecularly Imprinted Polymers for the Selective Extraction of Bisphenol A and Progesterone from Aqueous Media. Polymers 2018, 10, 679. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, X.; Lu, W.; Wang, X.; Li, J.; You, H.; Xiong, H.; Chen, L. Water-compatible temperature and magnetic dual-responsive molecularly imprinted polymers for recognition and extraction of bisphenol A. J. Chromatogr. A 2016, 1435, 30–38. [Google Scholar] [CrossRef]
- Jalal, N.; Surendranath, A.R.; Pathak, J.L.; Yu, S.; Chung, C.Y. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 2018, 5, 76–84. [Google Scholar] [CrossRef]
- Duan, F.; Chen, C.; Chen, L.; Sun, Y.; Wang, Y.; Yang, Y.; Liu, X.; Qin, Y. Preparation and Evaluation of Water-Compatible Surface Molecularly Imprinted Polymers for Selective Adsorption of Bisphenol A from Aqueous Solution. Ind. Eng. Chem. Res. 2014, 53, 14291–14300. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Yan, R.; Fu, X.; Wang, G.; Wang, Y.; Li, Z.; Zhang, X.; Hou, J. Facile fabrication of snowman-like magnetic molecularly imprinted polymer microspheres for bisphenol A via one-step Pickering emulsion polymerization. React. Funct. Polym. 2021, 164. [Google Scholar] [CrossRef]
- Dar, K.K.; Shao, S.; Tan, T.; Lv, Y. Molecularly imprinted polymers for the selective recognition of microorganisms. Biotechnol. Adv. 2020, 45. [Google Scholar] [CrossRef]
- Becskereki, G.; Horvai, G.; Tóth, B. The Selectivity of Molecularly Imprinted Polymers. Polymers 2021, 13, 1781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, Y.; Lu, H.; Shi, L.; Wang, P.; Ali, Z.; Li, J. Electrochemical detection of bisphenols in food: A review. Food Chem. 2021, 346. [Google Scholar] [CrossRef] [PubMed]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Janczura, M.; Luliński, P.; Sobiech, M. Imprinting Technology for Effective Sorbent Fabrication: Current State-of-Art and Future Prospects. Materials 2021, 14, 1850. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145. [Google Scholar] [CrossRef]
- Olcer, Y.A.; Demirkurt, M.; Demir, M.M.; Eroglu, A.E. Development of molecularly imprinted polymers (MIPs) as a solid phase extraction (SPE) sorbent for the determination of ibuprofen in water. RSC Adv. 2017, 7, 31441–31447. [Google Scholar] [CrossRef] [Green Version]
- Poliwoda, A.; Mościpan, M.; Wieczorek, P.P. Application of molecular imprinted polymers for selective solid phase extraction of bisphenol A. Ecol. Chem. Eng. S 2016, 23, 651–664. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, A.; Ciric, A.; Biesaga, M. Dummy molecularly imprinted polymer (DMIP) as a sorbent for bisphenol S and bisphenol F extraction from food samples. Microchem. J. 2020, 156. [Google Scholar] [CrossRef]
- Jakubiak-Marcinkowska, A.; Legan, M.; Jezierska, J. Molecularly imprinted polymeric Cu(II) catalysts with modified active centres mimicking oxidation enzymes. J. Polym. Res. 2013, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325. [Google Scholar] [CrossRef]
- Marć, M.; Wieczorek, P.P. Application potential of dummy molecularly imprinted polymers as solid-phase extraction sorbents for determination of low-mass polybrominated diphenyl ethers in soil and sediment samples. Microchem. J. 2019, 144, 461–468. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wu, W.; Wang, Z.; Chu, Y.; Chen, X. Hollow porous dummy molecularly imprinted polymer as a sorbent of solid-phase extraction combined with accelerated solvent extraction for determination of eight bisphenols in plastic products. Microchem. J. 2019, 145, 1176–1184. [Google Scholar] [CrossRef]
- Karrat, A.; Amine, A. Solid-phase extraction combined with a spectrophotometric method for determination of Bisphenol-A in water samples using magnetic molecularly imprinted polymer. Microchem. J. 2021, 168. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Athira, V.S.; Sekhar, V.C. Electrochemical sensing and nano molar level detection of Bisphenol-A with molecularly imprinted polymer tailored on multiwalled carbon nanotubes. Polymer 2018, 146, 312–320. [Google Scholar] [CrossRef]
- Viter, R.; Kunene, K.; Genys, P.; Jevdokimovs, D.; Erts, D.; Sutka, A.; Bisetty, K.; Viksna, A.; Ramanaviciene, A.; Ramanavicius, A. Photoelectrochemical Bisphenol S Sensor Based on ZnO-Nanoroads Modified by Molecularly Imprinted Polypyrrole. Macromol. Chem. Phys. 2020, 221. [Google Scholar] [CrossRef]
- Ali, H.; Mukhopadhyay, S.; Jana, N.R. Selective electrochemical detection of bisphenol A using a molecularly imprinted polymer nanocomposite. New J. Chem. 2019, 43, 1536–1543. [Google Scholar] [CrossRef]
- BEN Messaoud, N.; Lahcen, A.A.; Dridi, C.; Amine, A. Ultrasound assisted magnetic imprinted polymer combined sensor based on carbon black and gold nanoparticles for selective and sensitive electrochemical detection of Bisphenol A. Sens. Actuators B Chem. 2018, 276, 304–312. [Google Scholar] [CrossRef]
- Stillwell, M.T.; Holdich, R.G.; Kosvintsev, S.R.; Gasparini, G.; Cumming, I.W. Stirred Cell Membrane Emulsification and Factors Influencing Dispersion Drop Size and Uniformity. Ind. Eng. Chem. Res. 2007, 46, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Pstrowska, K.; Szyja, B.M.; Czapor-Irzabek, H.; Kiersnowski, A.; Walendziewski, J. The Properties and Activity of TiO2/beta-SiC Nanocomposites in Organic Dyes Photodegradation. Photochem. Photobiol. 2017, 93, 558–568. [Google Scholar] [CrossRef]
- Kujawska, M.; Zhou, T.; Trochimczuk, A.W.; Ye, L. Synthesis of naproxen-imprinted polymer using Pickering emulsion polymerization. J. Mol. Recognit. 2018, 31. [Google Scholar] [CrossRef] [Green Version]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D.; Chęcmanowski, J.; Kujawska, M.; Bryjak, M. New core-shell type polymeric supports based on the Amberlite XAD-4 adsorbent: A novel synthesis procedure. Chin. J. Chem. 2015, 33. [Google Scholar] [CrossRef]
- Wolska, J.; Kujawska, M.; Cyganowski, P. Selective sorption of diethyl phthalate on pH-responsive, molecularly imprinted polymeric adsorbents. Sep. Sci. Technol. 2020, 55, 2137–2148. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M. Preparation of polymeric microspheres for removal of boron by means of sorption-membrane filtration hybrid. Desalination 2011, 283, 193–197. [Google Scholar] [CrossRef]
- Kujawska, M.; Trochimczuk, A.W. Molecularly imprinted polymeric adsorbent for β-blockers removal synthesized using functionalized MSU-F silica as a sacrificial template. Sep. Sci. Technol. 2016, 51, 2565–2575. [Google Scholar] [CrossRef]
- Wolska, J. Chitosan and chitosan-polyethylenimine microspheres prepared by membrane emulsification and their application for drug delivery systems. Prog. Chem. Appl. Chitin Deriv. 2017, 22, 220–235. [Google Scholar] [CrossRef] [Green Version]
- Wolska, J.; Bryjak, M. Removal of bisphenol a from aqueous solution by molecularly imprinted polymers. Sep. Sci. Technol. 2014, 49, 1643–1653. [Google Scholar] [CrossRef]
- Polowczyk, I.; Bastrzyk, A.; Ulatowska, J.; Szczałba, E.; Koźlecki, T.; Sadowski, Z. Influence of pH on arsenic(III) removal by fly ash. Sep. Sci. Technol. 2016, 51, 2612–2619. [Google Scholar] [CrossRef]
- Cela-Pérez, M.C.; Castro-López, M.M.; Lasagabáster-Latorre, A.; López-Vilariño, J.M.; González-Rodríguez, M.V.; Barral-Losada, L.F. Synthesis and characterization of bisphenol-A imprinted polymer as a selective recognition receptor. Anal. Chim. Acta 2011, 706, 275–284. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. Hindawi J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Polowczyk, I.; Urbano, B.F.; Rivas, B.L.; Bryjak, M.; Kabay, N. Equilibrium and kinetic study of chromium sorption on resins with quaternary ammonium and N-methyl-d-glucamine groups. Chem. Eng. J. 2016, 284, 395–404. [Google Scholar] [CrossRef]
- Kabay, N.; Sarp, S.; Yuksel, M.; Arar, O.; Bryjak, M. Removal of boron from seawater by selective ion exchange resins. React. Funct. Polym. 2007, 67, 1643–1650. [Google Scholar] [CrossRef]
- Juang, R.-S.; Linb, S.-H.; Tsaoa, K.-H. Mechanism of sorption of phenols from aqueous solutions onto surfactant-modified montmorillonite. J. Colloid Interface Sci. 2002, 254, 234–241. [Google Scholar] [CrossRef]
- Wolska, J. Thermoresponsive molecularly imprinted polymer for rapid sorption and desorption of diethyl phthalate. Sep. Sci. Technol. 2016, 51, 2547–2553. [Google Scholar] [CrossRef]
- Wolska, J.; Cyganowski, P.; Koźlecki, T. Fine polymer imprinted layers for the monitoring of bisphenol A in aqueous solutions. Sep. Sci. Technol. 2018, 53, 206–218. [Google Scholar] [CrossRef]
- Nabavi, S.A.; Vladisavljević, G.T.; Wicaksono, A.; Georgiadou, S.; Manovic, V. Production of molecularly imprinted polymer particles with amide-decorated cavities for CO 2 capture using membrane emulsification/suspension polymerisation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Moghadam, H.; Zakeri, M.; Samimi, A. Mono-Size Distribution Index (MSDI): A new criterion for the quantitative description of size distribution. J. Part. Sci. Technol. 2019, 5, 71–76. [Google Scholar] [CrossRef]
- Klejn, D.; Luliński, P.; Maciejewska, D. Molecularly imprinted solid phase extraction in an efficient analytical protocol for indole-3-methanol determination in artificial gastric juice. RSC Adv. 2016, 6. [Google Scholar] [CrossRef]
- Makhaeva, E.E.; Tenhu, H.; Khokhlov, A.R. Behavior of poly(N-vinylcaprolactam-co-methacrylic acid) macromolecules in aqueous solution: Interplay between coulombic and hydrophobic interaction. Macromolecules 2002, 35, 1870–1876. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Ap-plications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, T.; Guo, L.; Ye, J.; He, L.; Li, X. Polymerization induced shaping of Pickering emulsion droplets: From simple hollow microspheres to molecularly imprinted multicore microrattles. Chem. Eng. J. 2018, 332, 409–418. [Google Scholar] [CrossRef]
- Marć, M.; Panuszko, A.; Namieśnik, J.; Wieczorek, P.P. Preparation and characterization of dummy-template molecularly imprinted polymers as potential sorbents for the recognition of selected polybrominated diphenyl ethers. Anal. Chim. Acta 2018, 1030, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Janczura, M.; Sobiech, M.; Luliński, P. Insight into the morphology, pore structure and sorption properties of 4-hydroxy-3-nitrophenylacetic acid imprinted poly(acrylic acid-co-ethylene glycol dimethacrylate) sorbent. Polym. Test. 2021, 93. [Google Scholar] [CrossRef]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of micro- and mesoporous solids by physisorption methods and pore-size analysis. Appl. Catal. A Gen. 1998, 174, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, A.; Kannan, S.; Selvaraj, V.; Rajeswari, S. Development and Spectral Characterization of Poly(Methyl Methacrylate)/Hydroxyapatite Composite for Biomedical Applications. Trends Biomater. Artif. Organs. 2004, 18, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Duan, G.; Zhang, C.; Li, A.; Yang, X.; Lu, L.; Wang, X. Preparation and Characterization of Mesoporous Zirconia Made by Using a Poly (methyl methacrylate) Template. Nanoscale Res. Lett. 2008, 3, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramelow, U.S.; Pingili, S. Synthesis of Ethylene Glycol Dimethacrylate-Methyl Methacrylate Copolymers, Determination of their Reactivity Ratios, and a Study of Dopant and Temperature Effects on their Conductivities. Polymers 2010, 2, 265–285. [Google Scholar] [CrossRef] [Green Version]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Grehan, L.; Kennedy, J.E.; Higginbotham, C.L. Modulating the mechanical properties of photopolymerised polyethylene glycol–polypropylene glycol hydrogels for bone regeneration. J. Mater. Sci. 2012, 47, 6577–6585. [Google Scholar] [CrossRef]
- Li, G.; Song, Y.; Yang, X.; Huang, W. Preparation of Poly(divinylbenzene-co-N-isopropylacrylamide) Microspheres and Their Hydrogen-Bonding Assembly Behavior for Raspberry-Like Core-Corona Polymer Composite. J. Appl. Polym. Sci. 2007, 104, 1350–1357. [Google Scholar] [CrossRef]
- Haq, A.U.; Saeed, M.; Usman, M.; Naqvi, S.A.R.; Bokhari, T.H.; Maqbool, T.; Ghaus, H.; Tahir, T.; Khalid, H. Sorption of chlorpyrifos onto zinc oxide nanoparticles impregnated Pea peels (Pisum sativum L): Equilibrium, kinetic and thermodynamic studies. Environ. Technol. Innov. 2020, 17, 100516. [Google Scholar] [CrossRef]
- Duan, F.; Chen, C.; Zhao, X.; Yang, Y.; Liu, X.; Qin, Y. Water-compatible surface molecularly imprinted polymers with synergy of bi-functional monomers for enhanced selective adsorption of bisphenol A from aqueous solution. Environ. Sci. Nano 2016, 3, 213–222. [Google Scholar] [CrossRef]







| Sample | NIPAM:MMA a | M:EGDMA b | BPA c | AIBN d | Toluene f:Monomers e |
|---|---|---|---|---|---|
| NIP | 3:7 | 4:6 | 0 | 1 | 50:50 |
| MIP-1 | 3:7 | 4:6 | 7 | 4 | 50:50 |
| MIP-2 | 3:7 | 4:6 | 5 | 4 | 50:50 |
| Sample | Average Diameter (µm) | Span | ZN (mmol g−1) | ZNteor. (mmol g−1) | Degree of Conversion [%] |
|---|---|---|---|---|---|
| NIP | 38 | 0.8 | 0.90 ± 0.05 | 1.21 | 74.4 |
| MIP-1 | 45 | 2.1 | 1.08 ± 0.05 | 1.21 | 89.2 |
| MIP-2 | 42 | 1.0 | 0.88 ± 0.05 | 1.21 | 72.7 |
| Sample | Average Pore Diameter (nm) | BET Specific Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) |
|---|---|---|---|
| NIP | 11.5 | 151.1 ± 2 | 0.41 |
| MIP-1 | 19.0 | 163.5 ± 2 | 0.77 |
| MIP-2 | 14.1 | 153.4 ± 2 | 0.52 |
| Sample | T (°C) | Langmuir | Freundlich | |||
|---|---|---|---|---|---|---|
| qm (mmol g−1) | RL2 | A | RF2 | |||
| NIP | 25 | 0.35 ± 0.01 | 0.990 | 0.806 | 0.576 | 0.896 |
| MIP-1 | 0.36 ± 0.02 | 0.986 | 0.544 | 0.420 | 0.902 | |
| MIP-2 | 0.39 ± 0.01 | 0.999 | 0.273 | 0.516 | 0.933 | |
| NIP | 35 | 0.40 ± 0.02 | 0.999 | 0.351 | 0.454 | 0.905 |
| MIP-1 | 0.40 ± 0.02 | 0.999 | 0.313 | 0.451 | 0.903 | |
| MIP-2 | 0.48 ± 0.02 | 0.998 | 0.304 | 0.455 | 0.949 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolska, J.; Jalilnejad Falizi, N. Membrane Emulsification Process as a Method for Obtaining Molecularly Imprinted Polymers. Polymers 2021, 13, 2830. https://doi.org/10.3390/polym13162830
Wolska J, Jalilnejad Falizi N. Membrane Emulsification Process as a Method for Obtaining Molecularly Imprinted Polymers. Polymers. 2021; 13(16):2830. https://doi.org/10.3390/polym13162830
Chicago/Turabian StyleWolska, Joanna, and Nasim Jalilnejad Falizi. 2021. "Membrane Emulsification Process as a Method for Obtaining Molecularly Imprinted Polymers" Polymers 13, no. 16: 2830. https://doi.org/10.3390/polym13162830
APA StyleWolska, J., & Jalilnejad Falizi, N. (2021). Membrane Emulsification Process as a Method for Obtaining Molecularly Imprinted Polymers. Polymers, 13(16), 2830. https://doi.org/10.3390/polym13162830