Synthesis of a Compound Phosphorus-Nitrogen Intumescent Flame Retardant for Applications to Raw Lacquer
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterizations
2.3. Synthesis of FR-1 and FR-2
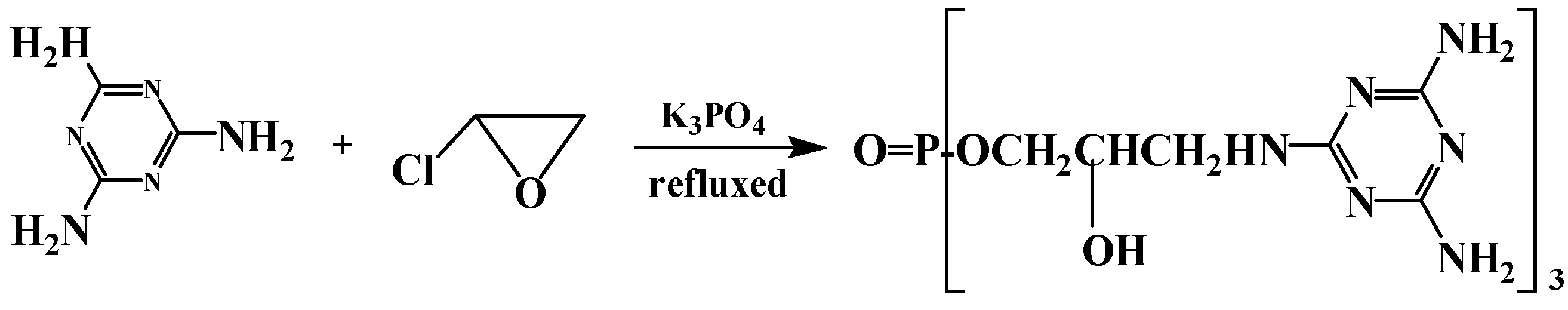
2.3.1. Synthesis of FR-1
2.3.2. Synthesis of FR-2
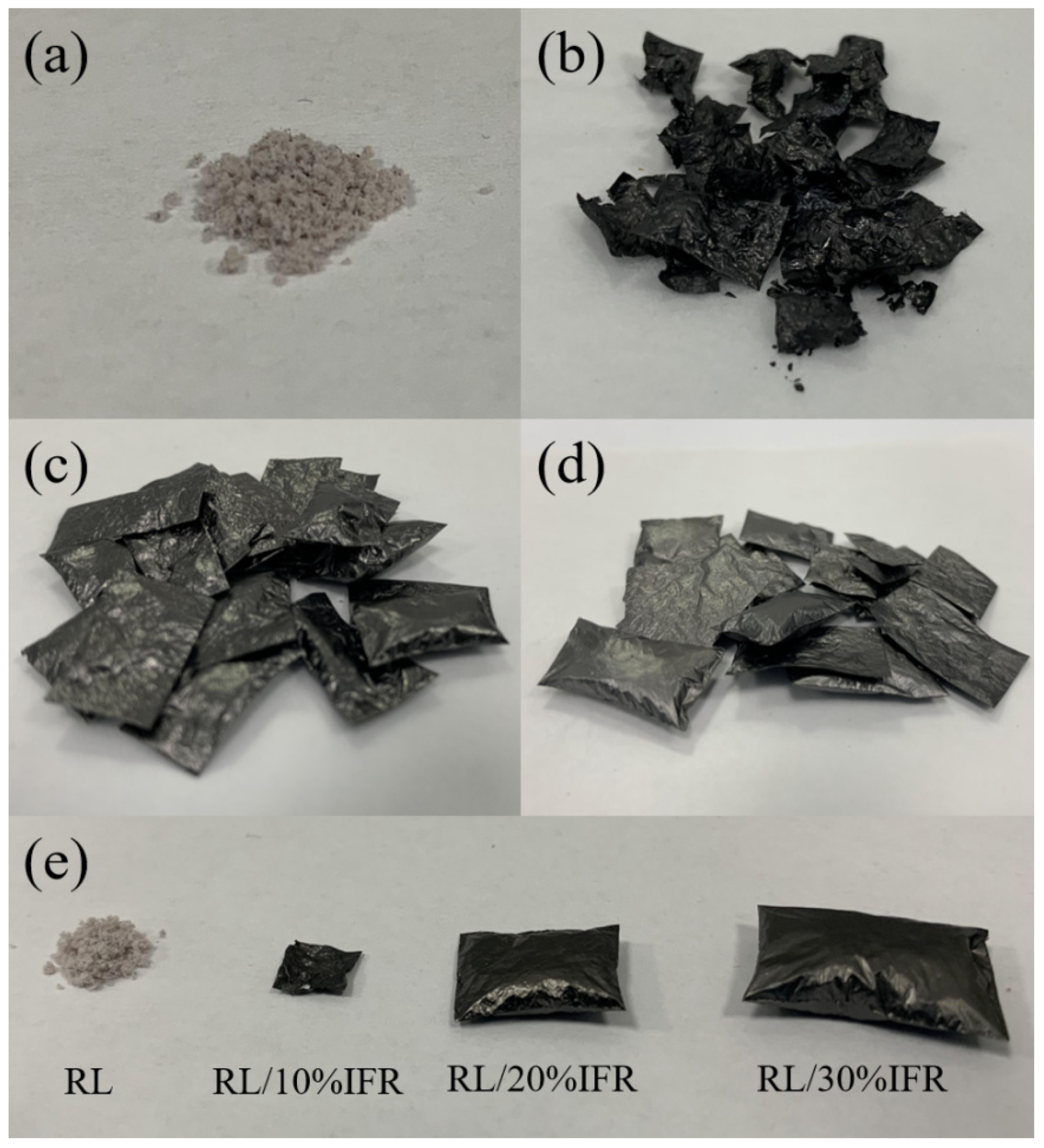
2.4. Preparation of RL/IFR Membranes
3. Results and Discussion
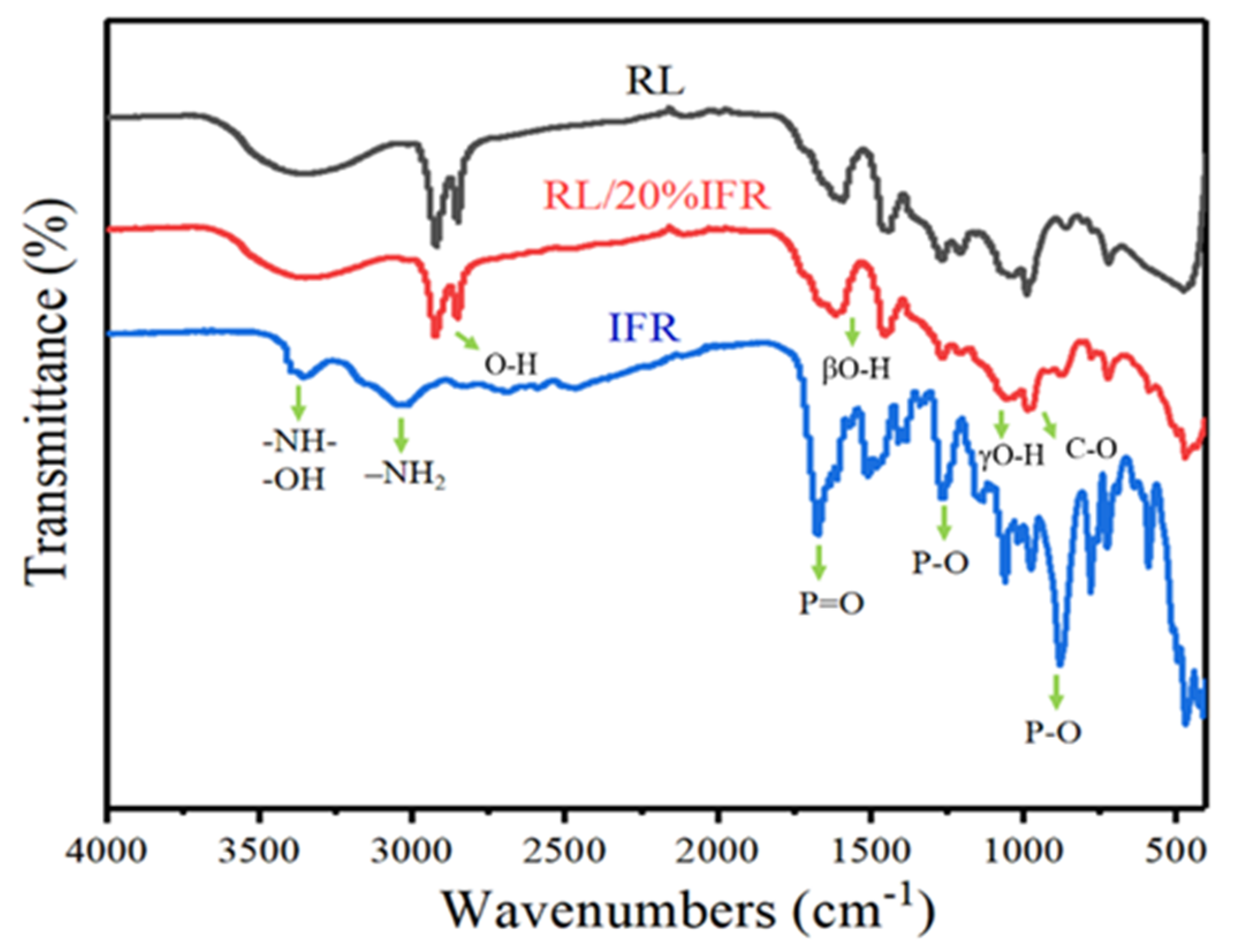
3.1. FT-IR Spectrum Analysis
3.2. Thermal Stability of Flame Retardant RL/IFR Membranes
3.3. Combustion Analysis of Flame Retardant RL/IFR Membranes
3.4. Micro-Scale Combustion Calorimetry (MCC)
3.5. Effect of IFR Content on Combustion Resistance of Raw Lacquer Membranes
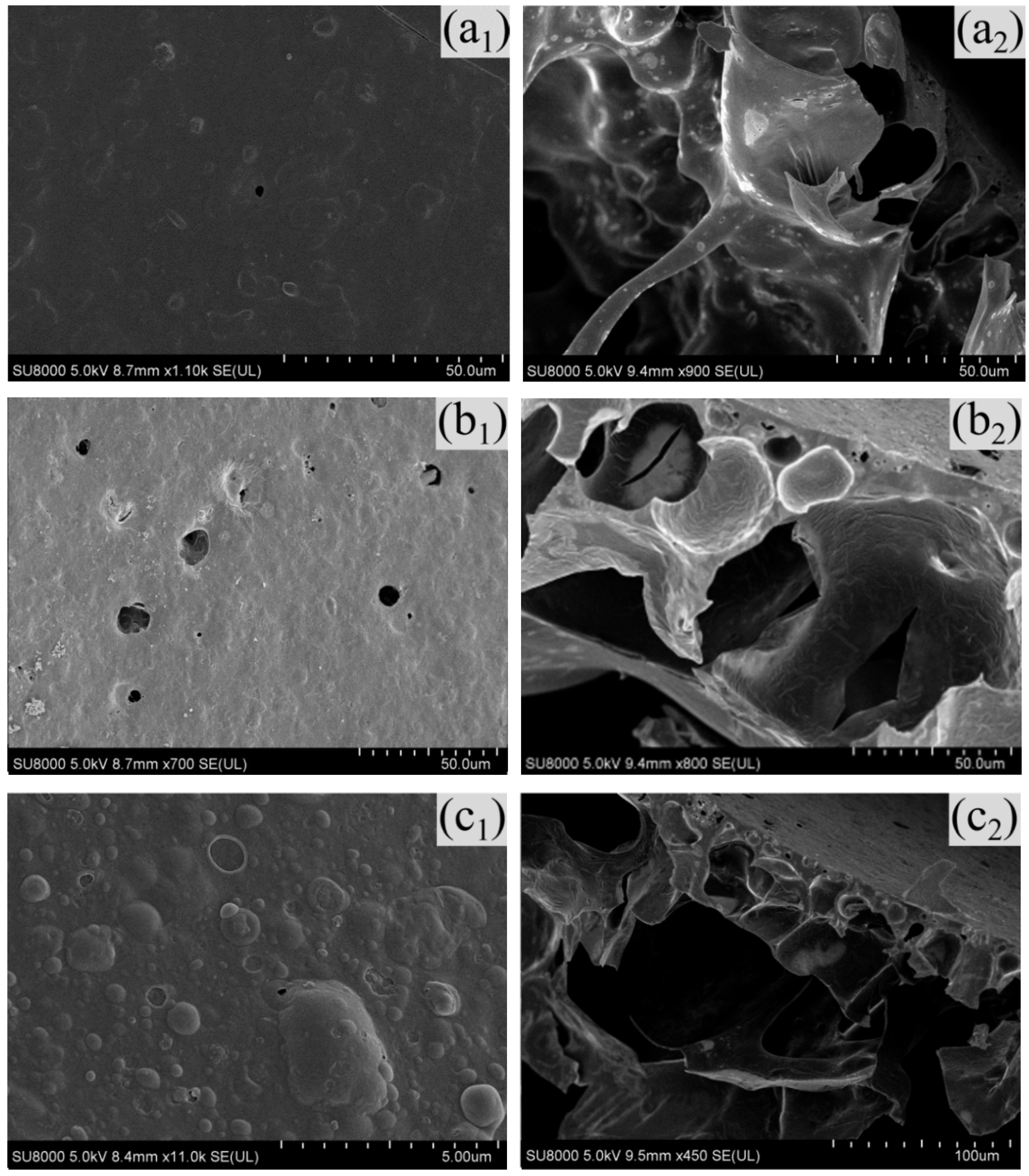
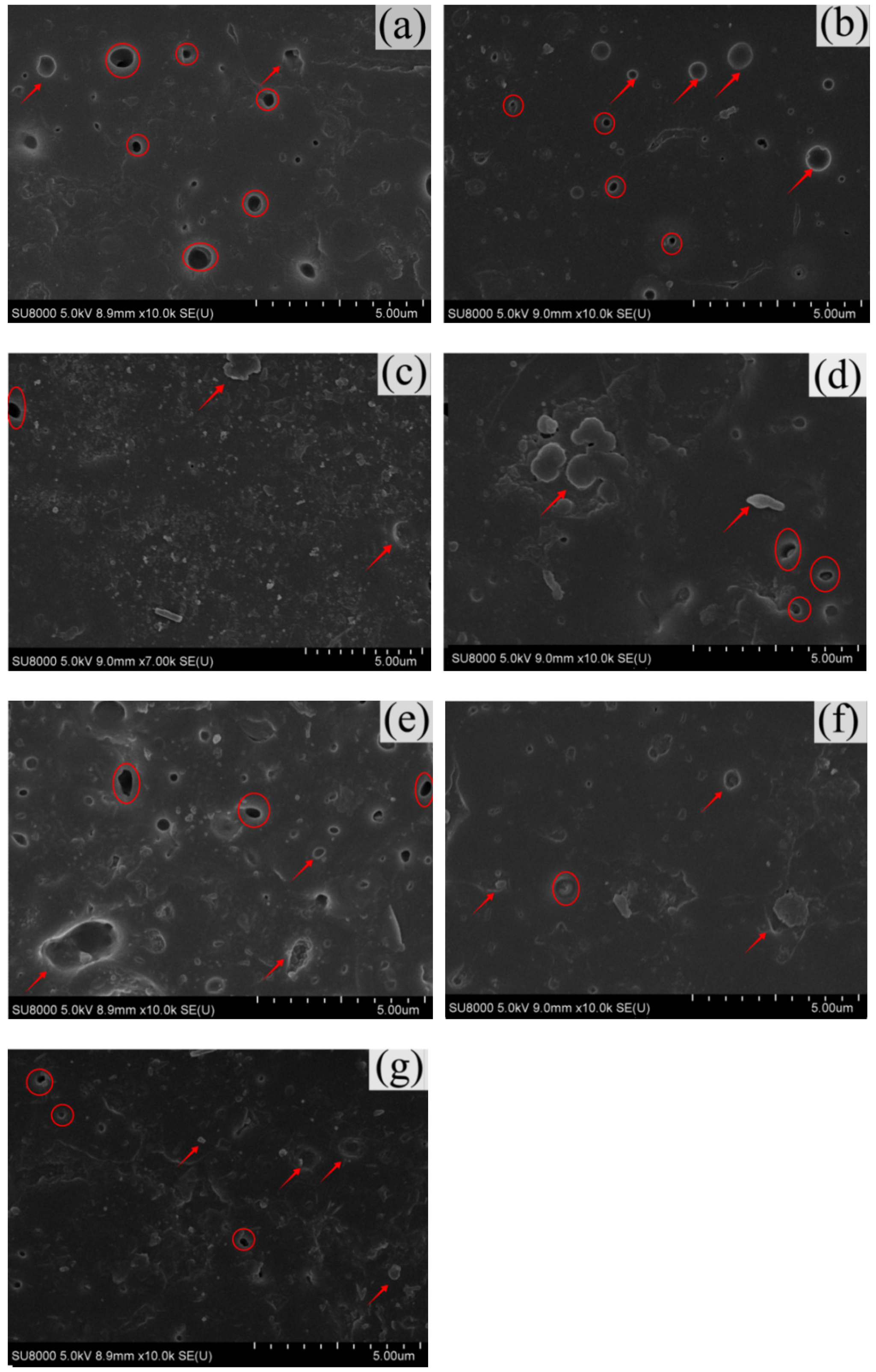
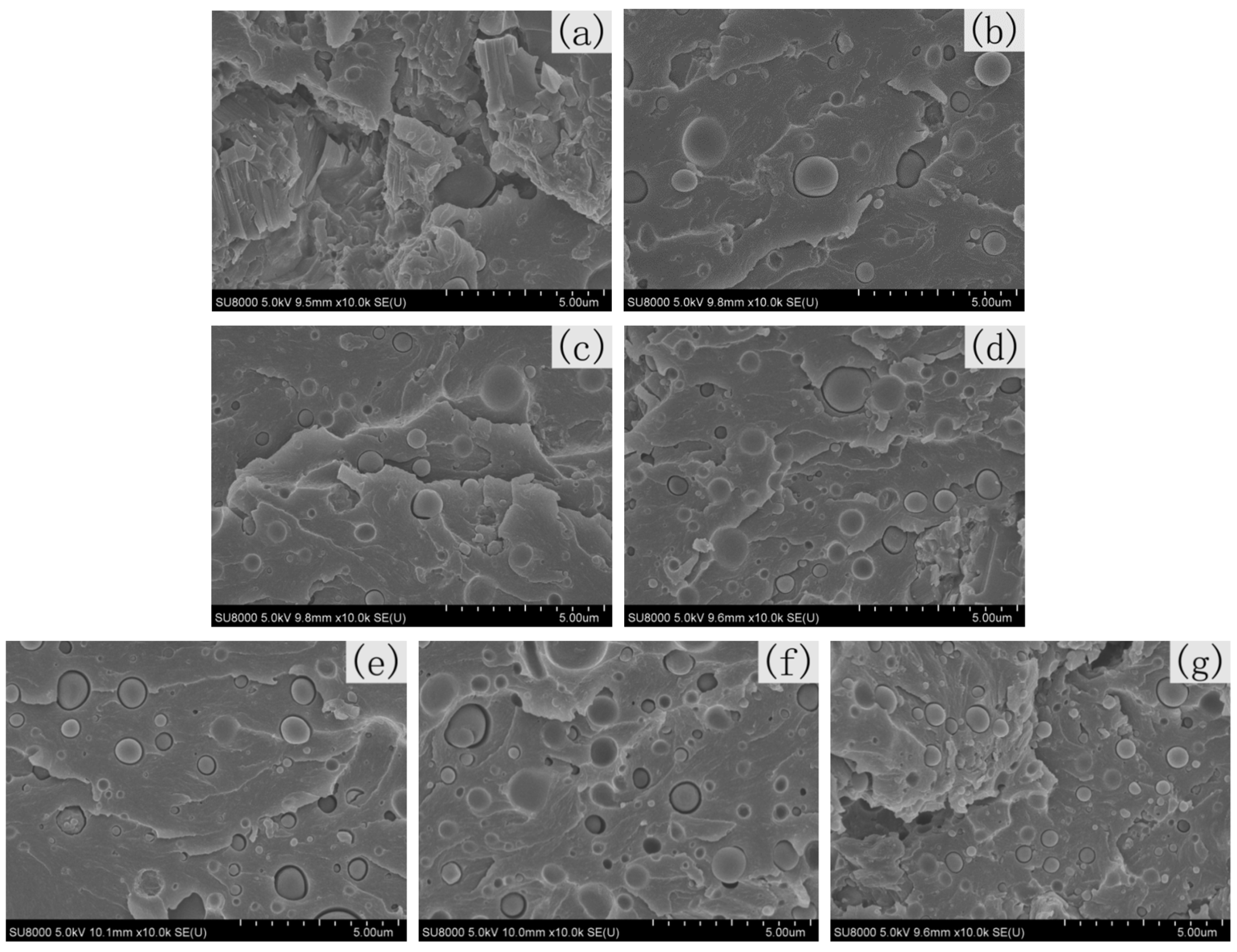
3.6. SEM Analysis
3.7. Mechanical Performances of Combustion Resistant Raw Lacquer Membranes
3.8. Optics Properties of Flame Retardant RL/IFR Membranes
3.9. Chemical Corrosion Resistance of Combustion Resistant RL/IFR Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Chen, Q.; Bai, W.; Lin, J. Preparation and properties of raw lacquer/multihydroxyl polyacrylate/organophilic montmorillonite nanocomposites. Polym. Bull. 2012, 68, 983–992. [Google Scholar] [CrossRef]
- Lu, R.; Harigaya, S.; Ishimura, T.; Nagase, K.; Miyakoshi, T. Development of a fast drying lacquer based on raw lacquer sap. Prog. Org. Coat. 2004, 51, 238–243. [Google Scholar] [CrossRef]
- Yang, J.; Deng, J.; Zhang, Q.; Shen, Q.; Li, D.; Xiao, Z. Effects of polysaccharides on the properties of Chinese lacquer sap. Prog. Org. Coat. 2015, 78, 176–182. [Google Scholar] [CrossRef]
- Lu, R.; Yoshida, T.; Miyakoshi, T. Oriental Lacquer: A Natural Polymer. Polym. Rev. 2013, 53, 153–191. [Google Scholar] [CrossRef]
- Vogl, O. Oriental lacquer, poison ivy, and drying oils. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4327–4335. [Google Scholar] [CrossRef]
- Xia, J.; Xu, Y.; Lin, J. UV-induced polymerization of urushiol. II: Effects of hydrogenation degree of urushiol on surface morphology. Prog. Org. Coat. 2010, 67, 365–369. [Google Scholar] [CrossRef]
- Kim, H.S.; Yeum, J.H.; Choi, S.W.; Lee, J.Y.; Cheong, I.W. Urushiol/polyurethane–urea dispersions and their film properties. Prog. Org. Coat. 2009, 65, 341–347. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, Y.; Yang, Z.; Yuan, T.; Huang, J.; Li, P.; Liu, Y.; Zhang, S.; Yang, Z. Facile synthesis and characterization of urushiol analogues from tung oil via ultraviolet photocatalysis. Prog. Org. Coat. 2018, 120, 240–251. [Google Scholar] [CrossRef]
- Je, H.; Won, J. Natural urushiol as a novel under-water adhesive. Chem. Eng. J. 2021, 404, 126424. [Google Scholar] [CrossRef]
- Zheng, L.; Lin, Y.; Wang, D.; Chen, J.; Yang, K.; Zheng, B.; Bai, W.; Jian, R.; Xu, Y. Facile one-pot synthesis of silver nanoparticles encapsulated in natural polymeric urushiol for marine antifouling. RSC Adv. 2020, 10, 13936–13943. [Google Scholar] [CrossRef] [Green Version]
- Lone, N.; Cheong, I.W.; Cho, M.; Hong, Y.-K.; Choi, Y.S.; Perumal, S.; Oh, B.-T.; Joo, J. Preparation of urushiol-containing poly(methyl methacrylate) copolymers for antibacterial and antifouling coatings. J. Coat. Technol. Res. 2017, 14, 621–630. [Google Scholar] [CrossRef]
- Xia, J.; Xu, Y.; Lin, J.; Hu, B. UV-induced polymerization of urushiol without photoinitiator. Prog. Org. Coat. 2008, 61, 7–10. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, J.; Shen, F.; Cai, J.; Zhou, M. Promotion by copper (II)-modified montmorillonite of the drying property of oriental lacquer sap. Prog. Org. Coat. 2018, 118, 72–81. [Google Scholar] [CrossRef]
- Gao, R.; Wang, L.; Lin, Q. Effect of hexamethylenetetramine on the property of Chinese lacquer film. Prog. Org. Coat. 2019, 133, 169–173. [Google Scholar] [CrossRef]
- Zhang, D.; Xia, J.; Xue, H.; Zhang, Y.; Lin, Q. Improvement on properties of Chinese lacquer by polyamidoamine. Polym. Eng. Sci. 2020, 60, 1177–1185. [Google Scholar] [CrossRef]
- Vothi, H.; Nguyen, C.; Pham, L.H.; Hoang, D.; Kim, J. Novel Nitrogen–Phosphorus Flame Retardant Based on Phosphonamidate: Thermal Stability and Flame Retardancy. ACS Omega 2019, 4, 17791–17797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, M.-J.; Cha, S.-H. Synthesis and Characterization of Reactive-Phosphorus/Nitrogen Flame Retardant for Epoxy Resin. Polym. Korea 2019, 43, 204–210. [Google Scholar] [CrossRef]
- Gu, J.-W.; Zhang, G.-C.; Dong, S.-L.; Zhang, Q.-Y.; Kong, J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, F.L.; Park, S.J. Synthesis of a novel phosphorus-nitrogen-containing intumescent flame retardant and its application to fabrics. J. Ind. Eng. Chem. 2015, 27, 40–43. [Google Scholar] [CrossRef]
- Kang, N.; Du, Z.; Li, H.; Zhang, C. Synthesis of polysiloxane-type multifunctional flame retardant and its application in epoxy systems. J. Appl. Polym. Sci. 2011, 124, 4915–4919. [Google Scholar] [CrossRef]
- Zheng, Z.; Qiang, L.; Yang, T.; Wang, B.; Cui, X.; Wang, H. Preparation of microencapsulated ammonium polyphosphate with carbon source- and blowing agent-containing shell and its flame retardance in polypropylene. J. Polym. Res. 2014, 21, 443. [Google Scholar] [CrossRef]
- Yang, J.; Shen, F.; Deng, J.; Cai, J.; Zhang, Q.; Liu, W. Laccase-catalyzed polymerization drying of Chinese lacquer sap with TiO2 nanoparticles. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stab. 2017, 137, 138–150. [Google Scholar] [CrossRef]
- Chang, P.R.; Yu, J.; Ma, X.; Anderson, D.P. Polysaccharides as stabilizers for the synthesis of magnetic nanoparticles. Carbohydr. Polym. 2011, 83, 640–644. [Google Scholar] [CrossRef]
- Xia, J.; Lin, J.; Xu, Y.; Chen, Q. On the UV-Induced Polymeric Behavior of Chinese Lacquer. ACS Appl. Mater. Interfaces 2010, 3, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, J.; Yang, R. The effects of phosphorus-based flame retardants and octaphenyl polyhedral oligomeric silsesquioxane on the ablative and flame-retardation properties of room temperature vulcanized silicone rubber insulating composites. Polym. Degrad. Stab. 2016, 125, 140–147. [Google Scholar] [CrossRef]
- Araby, S.; Su, X.; Meng, Q.; Kuan, H.-C.; Wang, C.-H.; Mouritz, A.P.; Maged, A.; Ma, J. Graphene platelets versus phosphorus compounds for elastomeric composites: Flame retardancy, mechanical performance and mechanisms. Nanotechnology 2019, 30, 385703. [Google Scholar] [CrossRef]
- Ni, P.; Fang, Y.; Qian, L.; Qiu, Y. Flame-retardant behavior of a phosphorus/silicon compound on polycarbonate. J. Appl. Polym. Sci. 2018, 135, 45815. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, W.; Chen, S.; Xu, W.; Lin, J.; Liu, H.; Chen, Q. Urushiol-functionalized mesoporous silica nanoparticles and their self-assembly into a Janus membrane as a highly efficient hemostatic material. Nanoscale 2018, 10, 22818–22829. [Google Scholar] [CrossRef]
- Yang, J.; Deng, J.; Zhu, J.; Liu, W.; Zhou, M.; Li, D. Thermal polymerization of lacquer sap and its effects on the properties of lacquer film. Prog. Org. Coat. 2016, 94, 41–48. [Google Scholar] [CrossRef]



| Sample | Ante-Combustion (g) | Post-Combustion (g) | Char Formation Rate (%) |
|---|---|---|---|
| RL | 0.161 | 0 | 0 |
| RL/10%IFR | 0.186 | 0.026 | 13.98 |
| RL/20%IFR | 0.333 | 0.149 | 44.74 |
| RL/30%IFR | 0.350 | 0.185 | 52.86 |
| Sample | HRC (J·g−1·K−1) | PHRR (w/g) | THR (KJ/g) | T (°C) |
|---|---|---|---|---|
| RL | 211 | 210.8 | 15.8 | 449.5 |
| RL/10%IFR | 175 | 174.9 | 12.7 | 466.3 |
| RL/20%IFR | 173 | 172.2 | 11 | 480 |
| RL/30%IFR | 144 | 142.7 | 8.7 | 480.5 |
| Sample | RL (g) | FR (g) | UL-94 | LOI (%) | Dripping |
|---|---|---|---|---|---|
| RL | 10 | 0 | NR | 17.9 | YES |
| RL/5%IFR | 9.5 | 0.5 | V2 | 20.3 | YES |
| RL/10%IFR | 9 | 1 | V2 | 21.8 | NO |
| RL/15%IFR | 8.5 | 1.5 | V1 | 23.7 | NO |
| RL/20%IFR | 8 | 2 | V0 | 25.4 | NO |
| RL/25%IFR | 7.5 | 2.5 | V0 | 27.8 | NO |
| RL/30%IFR | 7 | 3 | V0 | 30.2 | NO |
| Sample | RL | RL/5% IFR | RL/10% IFR | RL/15% IFR | RL/20% IFR | RL/25% IFR | RL/30% IFR |
|---|---|---|---|---|---|---|---|
| Impact Resistance (cm) | 9 | 8 | 8 | 8 | 8 | 8 | 7 |
| Resilience (mm) | 10 | 10 | 15 | 15 | 15 | 15 | 15 |
| Rigidity | 3H | 3H | 4H | 4H | 5H | 6H | 5H |
| Adhesion Level | 4 | 1 | 0 | 0 | 0 | 0 | 1 |
| Sample | RL | RL/5% IFR | RL/10% IFR | RL/15% IFR | RL/20% IFR | RL/25% IFR | RL/30% IFR |
|---|---|---|---|---|---|---|---|
| Luster | 14.2 | 30.8 | 32.5 | 33.6 | 35.4 | 34.7 | 23.9 |
| Sample | Discoloration | Wrinkles | Blisters | Chips |
|---|---|---|---|---|
| RL | × | × | × | × |
| RL/5%IFR | × | × | × | × |
| RL/10%IFR | × | × | × | × |
| RL/15%IFR | × | × | × | × |
| RL/20%IFR | × | × | × | × |
| RL/25%IFR | × | × | × | × |
| RL/30%IFR | × | × | × | × |
| Sample | Discoloration | Wrinkle | Bubble | Chips |
|---|---|---|---|---|
| RL | × | √ | √ | √ |
| RL/5%IFR | × | √ | √ | √ |
| RL/10%IFR | × | √ | √ | × |
| RL/15%IFR | × | √ | √ | × |
| RL/20%IFR | × | √ | √ | × |
| RL/25%IFR | × | √ | √ | √ |
| RL/30%IFR | × | √ | √ | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiu, B.-C.; Wu, K.; Lou, C.-W.; Lin, Q.; Lin, J.-H. Synthesis of a Compound Phosphorus-Nitrogen Intumescent Flame Retardant for Applications to Raw Lacquer. Polymers 2021, 13, 2858. https://doi.org/10.3390/polym13172858
Shiu B-C, Wu K, Lou C-W, Lin Q, Lin J-H. Synthesis of a Compound Phosphorus-Nitrogen Intumescent Flame Retardant for Applications to Raw Lacquer. Polymers. 2021; 13(17):2858. https://doi.org/10.3390/polym13172858
Chicago/Turabian StyleShiu, Bing-Chiuan, Kunlin Wu, Ching-Wen Lou, Qi Lin, and Jia-Horng Lin. 2021. "Synthesis of a Compound Phosphorus-Nitrogen Intumescent Flame Retardant for Applications to Raw Lacquer" Polymers 13, no. 17: 2858. https://doi.org/10.3390/polym13172858







