Environmental Microplastic Particles vs. Engineered Plastic Microparticles—A Comparative Review
Abstract
1. Introduction
2. Categories of Microplastic Particles
3. Sampling, Separation, Identification and Characterisation of Collected Microplastic Particles
3.1. Sampling
3.2. Separation
3.3. Identification: Particle Polymer Type
3.4. Characterisation: Polymer Particle Sizes
3.5. Characterisation: Particle Shape
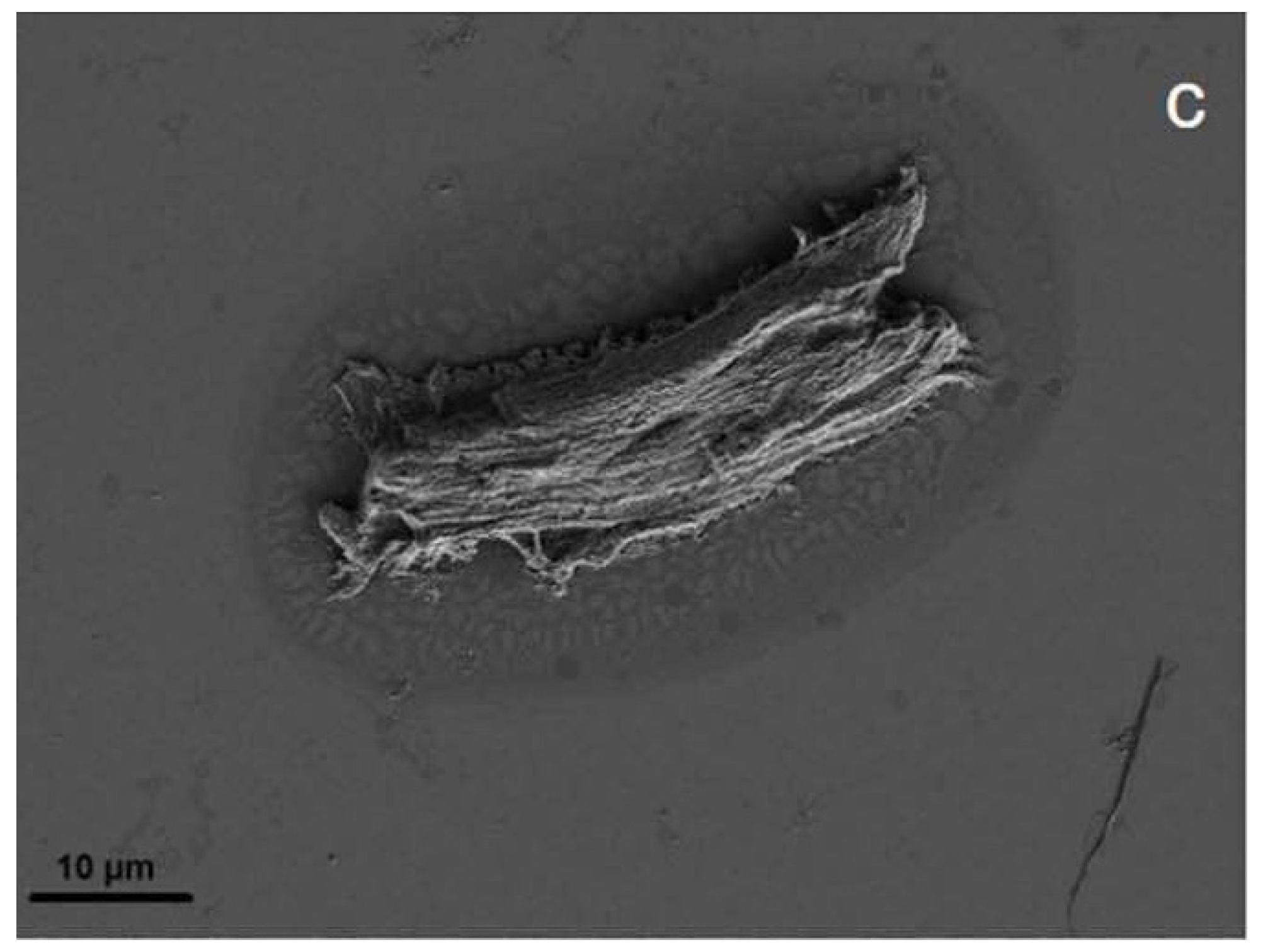
3.6. Characterisation: Surface Morphology
4. Use of Microplastic Particles in Environmental Research
4.1. Reference Particles for Extraction Protocols and Recovery Experiments
4.2. Exposure Experiments
5. Production of Microplastic Particles
5.1. Bottom-Up Generation
5.2. Top-Down Generation
5.2.1. Milling
5.2.2. Ultra-Sonic Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchanan, J.B. Pollution by synthetic fibres. Mar. Pollut. Bull. 1971, 2, 23. [Google Scholar] [CrossRef]
- Colton, J.B.; Knapp, F.D.; Burns, B.R. Plastic Particles in Surface Waters of the Northwestern Atlantic. Science 1974, 185, 491–497. [Google Scholar] [CrossRef]
- Hays, H.; Cormons, G. Plastic particles found in tern pellets, on coastal beaches and at factory sites. Mar. Pollut. Bull. 1974, 5, 44–46. [Google Scholar] [CrossRef]
- Rothstein, S.I. Plastic particle pollution of the surface of the Atlantic Ocean: Evidence from a seabird. Condor 1973, 75, 344–345. [Google Scholar] [CrossRef]
- Akindele, E.O.; Ehlers, S.M.; Koop, J.H. First empirical study of freshwater microplastics in West Africa using gastropods from Nigeria as bioindicators. Limnologica 2019, 78, 125708. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef]
- Choi, J.S.; Jung, Y.-J.; Hong, N.-H.; Hong, S.H.; Park, J.-W. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 2018, 129, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Moore, F.; Akhbarizadeh, R. Microplastic pollution in deposited urban dust, Tehran metropolis, Iran. Environ. Sci. Pollut. Res. 2017, 24, 20360–20371. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Uchiyama-Matsumoto, K.; Uchida, K.; Tokai, T. Microplastics in the Southern Ocean. Mar. Pollut. Bull. 2017, 114, 623–626. [Google Scholar] [CrossRef]
- Firdaus, M.; Trihadiningrum, Y.; Lestari, P. Microplastic pollution in the sediment of Jagir Estuary, Surabaya City, Indonesia. Mar. Pollut. Bull. 2020, 150, 110790. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.-P.W.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.-P.W.; Galbraith, M.; Ross, P.S. Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size matters more than shape: Ingestion of primary and secondary microplastics by small predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut. 2018, 236, 425–431. [Google Scholar] [CrossRef]
- Kwak, J.I.; An, Y.-J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard. Mater. 2021, 402, 124034. [Google Scholar] [CrossRef]
- Varnamkhasti, A. Method for Producing Microplastic Fragments. Australia Patent Application No. AU2015101459A4, 12 October 2015. [Google Scholar]
- Joint Research Center. Stakeholder Needs for Microplastic Test Materials. Available online: https://ec.europa.eu/eusurvey/runner/MicroplasticStakeholderSurvey (accessed on 15 January 2021).
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef] [PubMed]
- NOAA. What Are Microplastics? Available online: https://oceanservice.noaa.gov/facts/microplastics.html (accessed on 14 August 2021).
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Plastics and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 0471095206. [Google Scholar]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for Plastics to Transport Hydrophobic Contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Higgins, R.J.; Goldsmith, R.L. Process and system for production of inorganic nanoparticles. U.S. Patent Application No. 921670, 2 September 1997. [Google Scholar]
- Tsuzuki, T. Commercial scale production of inorganic nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Klaine, S.J.; Koelmans, A.A.; Horne, N.; Carley, S.; Handy, R.D.; Kapustka, L.; Nowack, B.; Kammer, F. von der Kammer, F. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem. 2012, 31, 3–14. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the aquatic environment. Critical review. In Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015; pp. 325–340. [Google Scholar]
- Reverchon, E. Supercritical-Assisted Atomization to Produce Micro- and/or Nanoparticles of Controlled Size and Distribution. Ind. Eng. Chem. Res. 2002, 41, 2405–2411. [Google Scholar] [CrossRef]
- da Costa, J.P.; Santos, P.S.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total. Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef]
- Hartmann, N.; Nolte, T.; Sørensen, M.; Jensen, P.; Baun, A. Aquatic ecotoxicity testing of nanoplastics: Lessons Learned from Nanoecotoxicology. In Proceedings of the ASLO Aquatic Sciences Meeting, Granada, Spanien, 27 February 2015. [Google Scholar]
- Klabunde, K.J.; Richards, R.M. Nanoscale Materials in Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 0470523662. [Google Scholar]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Bertling, J.; Hamann, L.; Bertling, R. Kunststoffe in der Umwelt. 2018. Available online: https://www.umweltbundesamt.de/presse/pressemitteilungen/kunststoffe-in-der-umwelt (accessed on 5 October 2019).
- ter Halle, A.; Ladirat, L.; Gendre, X.; Goudouneche, D.; Pusineri, C.; Routaboul, C.; Tenailleau, C.; Duployer, B.; Perez, E. Understanding the Fragmentation Pattern of Marine Plastic Debris. Environ. Sci. Technol. 2016, 50, 5668–5675. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.-P.; Faisant, N.; Venier-Julienne, M.-C.; Menei, P. Development of microspheres for neurological disorders: From basics to clinical applications. J. Control. Release 2000, 65, 285–296. [Google Scholar] [CrossRef]
- Finishing Systems Inc. Sandblasting Media Guide. Available online: https://www.finishingsystems.com/blog/sandblasting-material-guide/ (accessed on 16 April 2021).
- Blaustein, M. Cosmetic Powder Compositions Containing Polyethylene. U.S. Patent Application No. 844212, 5 October 1959. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Plastic ‘scrubbers’ in hand cleansers: A further (and minor) source for marine pollution identified. Mar. Pollut. Bull. 1996, 32, 867–871. [Google Scholar] [CrossRef]
- Zitko, V.; Hanlon, M. Another source of pollution by plastics: Skin cleaners with plastic scrubbers. Mar. Pollut. Bull. 1991, 22, 41–42. [Google Scholar] [CrossRef]
- van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Ogonowski, M.; Schür, C.; Jarsén, Å.; Gorokhova, E. The Effects of Natural and Anthropogenic Microparticles on Individual Fitness in Daphnia magna. PLoS ONE 2016, 11, e0155063. [Google Scholar] [CrossRef]
- Bayo, J.; Rojo, D.; Olmos, S. Abundance, morphology and chemical composition of microplastics in sand and sediments from a protected coastal area: The Mar Menor lagoon (SE Spain). Environ. Pollut. 2019, 252, 1357–1366. [Google Scholar] [CrossRef]
- Amrutha, K.; Warrier, A.K. The first report on the source-to-sink characterization of microplastic pollution from a riverine environment in tropical India. Sci. Total Environ. 2020, 739, 140377. [Google Scholar] [CrossRef]
- Covernton, G.A.; Pearce, C.M.; Gurney-Smith, H.J.; Chastain, S.G.; Ross, P.S.; Dower, J.F.; Dudas, S.E. Size and shape matter: A preliminary analysis of microplastic sampling technique in seawater studies with implications for ecological risk assessment. Sci. Total Environ. 2019, 667, 124–132. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, Q.; Sun, Y.; Sun, X.; Lin, H. Environmental implications of microplastic pollution in the Northwestern Pacific Ocean. Mar. Pollut. Bull. 2019, 146, 215–224. [Google Scholar] [CrossRef]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the sediments of a UK urban lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef]
- Grbić, J.; Helm, P.; Athey, S.; Rochman, C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020, 174, 115623. [Google Scholar] [CrossRef]
- Mao, R.; Hu, Y.; Zhang, S.; Wu, R.; Guo, X. Microplastics in the surface water of Wuliangsuhai Lake, northern China. Sci. Total Environ. 2020, 723, 137820. [Google Scholar] [CrossRef]
- Dong, M.; Luo, Z.; Jiang, Q.; Xing, X.; Zhang, Q.; Sun, Y. The rapid increases in microplastics in urban lake sediments. Sci. Rep. 2020, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.; Hecq, J.-H.; Glagani, F.; Voisin, P.; Collard, F.; Goffart, A. Neustonic microplastic and zooplankton in the North Western Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J.; Moore, S.L.; Weisberg, S.B.; Lattin, G.L.; Zellers, A.F. A comparison of neustonic plastic and zooplankton abundance in southern California’s coastal waters. Mar. Pollut. Bull. 2002, 44, 1035–1038. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Chai, B.; Wei, Q.; She, Y.; Lu, G.; Dang, Z.; Yin, H. Soil microplastic pollution in an e-waste dismantling zone of China. Waste Manag. 2020, 118, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhang, K.; Chen, X.; Shi, H.; Luo, Z.; Wu, C. Sources and distribution of microplastics in China’s largest inland lake-Qinghai Lake. Environ. Pollut. 2018, 235, 899–906. [Google Scholar] [CrossRef]
- Simon-Sánchez, L.; Grelaud, M.; Garcia-Orellana, J.; Ziveri, P. River Deltas as hotspots of microplastic accumulation: The case study of the Ebro River (NW Mediterranean). Sci. Total Environ. 2019, 687, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Pervez, R.; Wang, Y.; Mahmood, Q.; Jattak, Z. Stereomicroscopic and Fourier Transform Infrared (FTIR) Spectroscopic Characterization of the Abundance, Distribution and Composition of Microplastics in the Beaches of Qingdao, China. Anal. Lett. 2020, 53, 2960–2977. [Google Scholar] [CrossRef]
- Tran Nguyen, Q.A.; Nguyen, H.N.Y.; Strady, E.; Nguyen, Q.T.; Trinh-Dang, M.; van Vo, M. Characteristics of microplastics in shoreline sediments from a tropical and urbanized beach (Da Nang, Vietnam). Mar. Pollut. Bull. 2020, 161, 111768. [Google Scholar] [CrossRef]
- Patchaiyappan, A.; Ahmed, S.Z.; Dowarah, K.; Jayakumar, S.; Devipriya, S.P. Occurrence, distribution and composition of microplastics in the sediments of South Andaman beaches. Mar. Pollut. Bull. 2020, 156, 111227. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar] [CrossRef]
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Uurasjärvi, E.; Hartikainen, S.; Setälä, O.; Lehtiniemi, M.; Koistinen, A. Microplastic concentrations, size distribution, and polymer types in the surface waters of a northern European lake. Water Environ. Res. 2020, 92, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Migwi, F.K.; Ogunah, J.A.; Kiratu, J.M. Occurrence and Spatial Distribution of Microplastics in the Surface Waters of Lake Naivasha, Kenya. Environ. Toxicol. Chem. 2020, 39, 765–774. [Google Scholar] [CrossRef]
- Sruthy, S.; Ramasamy, E.V. Microplastic pollution in Vembanad Lake, Kerala, India: The first report of microplastics in lake and estuarine sediments in India. Environ. Pollut. 2017, 222, 315–322. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef]
- Lin, L.; Zuo, L.-Z.; Peng, J.-P.; Cai, L.-Q.; Fok, L.; Yan, Y.; Li, H.-X.; Xu, X.-R. Occurrence and distribution of microplastics in an urban river: A case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Su, L.; Craig, N.J.; Du, F.; Wu, C.; Shi, H. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ. Pollut. 2019, 246, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Zheng, Y.; He, C. Detection of microplastics in local marine organisms using a multi-technology system. Anal. Methods 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Zhang, Y.; Yang, W.; Zhou, L.; Liu, S.; Xu, E.G. Occurrence and distribution of microplastics in China’s largest freshwater lake system. Chemosphere 2020, 261, 128186. [Google Scholar] [CrossRef]
- Matsuguma, Y.; Takada, H.; Kumata, H.; Kanke, H.; Sakurai, S.; Suzuki, T.; Itoh, M.; Okazaki, Y.; Boonyatumanond, R.; Zakaria, M.P.; et al. Microplastics in Sediment Cores from Asia and Africa as Indicators of Temporal Trends in Plastic Pollution. Arch. Environ. Contam. Toxicol. 2017, 73, 230–239. [Google Scholar] [CrossRef]
- Sun, D.; Wang, J.; Xie, S.; Tang, H.; Zhang, C.; Xu, G.; Zou, J.; Zhou, A. Characterization and spatial distribution of microplastics in two wild captured economic freshwater fish from north and west rivers of Guangdong province. Ecotoxicol. Environ. Saf. 2021, 207, 111555. [Google Scholar] [CrossRef]
- Pan, Z.; Guo, H.; Chen, H.; Wang, S.; Sun, X.; Zou, Q.; Zhang, Y.; Lin, H.; Cai, S.; Huang, J. Microplastics in the Northwestern Pacific: Abundance, distribution, and characteristics. Sci. Total Environ. 2019, 650, 1913–1922. [Google Scholar] [CrossRef]
- Gewert, B.; Ogonowski, M.; Barth, A.; MacLeod, M. Abundance and composition of near surface microplastics and plastic debris in the Stockholm Archipelago, Baltic Sea. Mar. Pollut. Bull. 2017, 120, 292–302. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, Q.; Jiang, R.; Li, W.; Sun, X.; Lin, H.; Jiang, S.; Huang, H. Microplastic pollution and ecological risk assessment in an estuarine environment: The Dongshan Bay of China. Chemosphere 2021, 262, 127876. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Llorca, M.; Seró, R.; Moyano, E.; Barceló, D.; Abad, E.; Farré, M. Trace analysis of polystyrene microplastics in natural waters. Chemosphere 2019, 236, 124321. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, M.; Song, L.; Yu, J.; Liu, R.; Wang, S.; Wang, Z.; Sokolova, I.M.; Huang, W.; Wang, Y. Coastal zone use influences the spatial distribution of microplastics in Hangzhou Bay, China. Environ. Pollut. 2020, 266, 115137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Coffin, S.; Sun, C.; Schlenk, D.; Gan, J. Negligible effects of microplastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environ. Pollut. 2019, 249, 776–784. [Google Scholar] [CrossRef]
- Naji, A.; Nuri, M.; Amiri, P.; Niyogi, S. Small microplastic particles (S-MPPs) in sediments of mangrove ecosystem on the northern coast of the Persian Gulf. Mar. Pollut. Bull. 2019, 146, 305–311. [Google Scholar] [CrossRef]
- Mehdinia, A.; Dehbandi, R.; Hamzehpour, A.; Rahnama, R. Identification of microplastics in the sediments of southern coasts of the Caspian Sea, north of Iran. Environ. Pollut. 2020, 258, 113738. [Google Scholar] [CrossRef]
- Falahudin, D.; Cordova, M.R.; Sun, X.; Yogaswara, D.; Wulandari, I.; Hindarti, D.; Arifin, Z. The first occurrence, spatial distribution and characteristics of microplastic particles in sediments from Banten Bay, Indonesia. Sci. Total Environ. 2020, 705, 135304. [Google Scholar] [CrossRef]
- Chouchene, K.; da Costa, J.P.; Wali, A.; Girão, A.V.; Hentati, O.; Duarte, A.C.; Rocha-Santos, T.; Ksibi, M. Microplastic pollution in the sediments of Sidi Mansour Harbor in Southeast Tunisia. Mar. Pollut. Bull. 2019, 146, 92–99. [Google Scholar] [CrossRef]
- Chouchene, K.; Rocha-Santos, T.; Ksibi, M. Types, occurrence, and distribution of microplastics and metals contamination in sediments from south west of Kerkennah archipelago, Tunisia. Environ. Sci. Pollut. Res. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bretas Alvim, C.; Bes-Piá, M.A.; Mendoza-Roca, J.A. Separation and identification of microplastics from primary and secondary effluents and activated sludge from wastewater treatment plants. Chem. Eng. J. 2020, 402, 126293. [Google Scholar] [CrossRef]
- Wolff, S.; Kerpen, J.; Prediger, J.; Barkmann, L.; Müller, L. Determination of the microplastics emission in the effluent of a municipal waste water treatment plant using Raman microspectroscopy. Water Res. X 2019, 2, 100014. [Google Scholar] [CrossRef] [PubMed]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Laglbauer, B.J.; Franco-Santos, R.M.; Andreu-Cazenave, M.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and microplastics from beaches in Slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
- Woodall, L.C.; Gwinnett, C.; Packer, M.; Thompson, R.C.; Robinson, L.F.; Paterson, G.L. Using a forensic science approach to minimize environmental contamination and to identify microfibres in marine sediments. Mar. Pollut. Bull. 2015, 95, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Frère, L.; Paul-Pont, I.; Moreau, J.; Soudant, P.; Lambert, C.; Huvet, A.; Rinnert, E. A semi-automated Raman micro-spectroscopy method for morphological and chemical characterizations of microplastic litter. Mar. Pollut. Bull. 2016, 113, 461–468. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Li, D. Microplastic in three urban estuaries, China. Environ. Pollut. 2015, 206, 597–604. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Rathod, T.D.; Ajmal, P.Y.; Bhangare, R.C.; Sahu, S.K. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019, 140, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Zhao, J.; Zhang, C.; Cheng, B.; Koelmans, A.A.; Wu, D.; Gao, M.; Sun, X.; Liu, Y.; Wang, Q. A systems analysis of microplastic pollution in Laizhou Bay, China. Sci. Total Environ. 2020, 745, 140815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Danley, M.; Ward, J.E.; Li, D.; Mincer, T.J. An approach for extraction, characterization and quantitation of microplastic in natural marine snow using Raman microscopy. Anal. Methods 2017, 9, 1470–1478. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.N.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Ru, S.; Liu, X. High levels of microplastic pollution in the sediments and benthic organisms of the South Yellow Sea, China. Sci. Total Environ. 2019, 651, 1661–1669. [Google Scholar] [CrossRef]
- van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Mees, J.; Janssen, C.R. Assessment of marine debris on the Belgian Continental Shelf. Mar. Pollut. Bull. 2013, 73, 161–169. [Google Scholar] [CrossRef]
- Claessens, M.; van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Imhof, H.K.; Schmid, J.; Niessner, R.; Ivleva, N.P.; Laforsch, C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Tsang, Y.Y.; Mak, C.W.; Liebich, C.; Lam, S.W.; Sze, E.T.-P.; Chan, K.M. Microplastic pollution in the marine waters and sediments of Hong Kong. Mar. Pollut. Bull. 2017, 115, 20–28. [Google Scholar] [CrossRef]
- Li, R.; Yu, L.; Chai, M.; Wu, H.; Zhu, X. The distribution, characteristics and ecological risks of microplastics in the mangroves of Southern China. Sci. Total Environ. 2020, 708, 135025. [Google Scholar] [CrossRef]
- Mani, T.; Primpke, S.; Lorenz, C.; Gerdts, G.; Burkhardt-Holm, P. Microplastic Pollution in Benthic Midstream Sediments of the Rhine River. Environ. Sci. Technol. 2019, 53, 6053–6062. [Google Scholar] [CrossRef]
- Vinay Kumar, B.N.; Löschel, L.A.; Imhof, H.K.; Löder, M.G.J.; Laforsch, C. Analysis of microplastics of a broad size range in commercially important mussels by combining FTIR and Raman spectroscopy approaches. Environ. Pollut. 2021, 269, 116147. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhang, K.; Yang, R.; Li, R.; Li, Y. Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Mar. Pollut. Bull. 2018, 136, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.N.; Jeyasanta, I.; Patterson, J. Occurrence of microplastics in epipelagic and mesopelagic fishes from Tuticorin, Southeast coast of India. Sci. Total Environ. 2020, 720, 137614. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Paço, A.; Reis, V.; Da Costa, J.P.; Fernandes, A.J.S.; Da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of microplastics in white wines capped with polyethylene stoppers using micro-Raman spectroscopy. Food Chem. 2020, 331, 127323. [Google Scholar] [CrossRef]
- Ehlers, S.M.; Manz, W.; Koop, J.H. Microplastics of different characteristics are incorporated into the larval cases of the freshwater caddisfly Lepidostoma basale. Aquat. Biol. 2019, 28, 67–77. [Google Scholar] [CrossRef]
- Fortin, S.; Song, B.; Burbage, C. Quantifying and identifying microplastics in the effluent of advanced wastewater treatment systems using Raman microspectroscopy. Mar. Pollut. Bull. 2019, 149, 110579. [Google Scholar] [CrossRef] [PubMed]
- González-Pleiter, M.; Velázquez, D.; Edo, C.; Carretero, O.; Gago, J.; Barón-Sola, Á.; Hernández, L.E.; Yousef, I.; Quesada, A.; Leganés, F.; et al. Fibers spreading worldwide: Microplastics and other anthropogenic litter in an Arctic freshwater lake. Sci. Total Environ. 2020, 722, 137904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Marson, R.L.; Ge, Z.; Glotzer, S.C.; Ma, P.X. Simultaneous Nano- and Microscale Control of Nanofibrous Microspheres Self-Assembled from Star-Shaped Polymers. Adv. Mater. 2015, 27, 3947–3952. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Pérez-Guevara, F.; Kutralam-Muniasamy, G. Metro station free drinking water fountain- A potential "microplastics hotspot" for human consumption. Environ. Pollut. 2020, 261, 114227. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Montesinos, F.; Oliva Ramírez, M.; González-Leal, J.M.; Carrizo, D.; Anfuso, G. Characterization of plastic beach litter by Raman spectroscopy in South-western Spain. Sci. Total Environ. 2020, 744, 140890. [Google Scholar] [CrossRef]
- Valente, T.; Sbrana, A.; Scacco, U.; Jacomini, C.; Bianchi, J.; Palazzo, L.; de Lucia, G.A.; Silvestri, C.; Matiddi, M. Exploring microplastic ingestion by three deep-water elasmobranch species: A case study from the Tyrrhenian Sea. Environ. Pollut. 2019, 253, 342–350. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. R. Soc. Chem. 2013, 15, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, R.G.; Harthcock, M.A. Infrared microspectroscopy. In Theory and Applications//Infrared Microspectroscopy: Theory and Applications; Dekker: New York, NY, USA, 1988; ISBN 9780824780036. [Google Scholar]
- Cabernard, L.; Roscher, L.; Lorenz, C.; Gerdts, G.; Primpke, S. Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2018, 52, 13279–13288. [Google Scholar] [CrossRef]
- von der Esch, E.; Kohles, A.J.; Anger, P.M.; Hoppe, R.; Niessner, R.; Elsner, M.; Ivleva, N.P. TUM-ParticleTyper: A detection and quantification tool for automated analysis of (Microplastic) particles and fibers. PLoS ONE 2020, 15, e0234766. [Google Scholar] [CrossRef]
- Brandt, J.; Bittrich, L.; Fischer, F.; Kanaki, E.; Tagg, A.; Lenz, R.; Labrenz, M.; Brandes, E.; Fischer, D.; Eichhorn, K.-J. High-Throughput Analyses of Microplastic Samples Using Fourier Transform Infrared and Raman Spectrometry. Appl. Spectrosc. 2020, 74, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Primpke, S.; Cross, R.K.; Mintenig, S.M.; Simon, M.; Vianello, A.; Gerdts, G.; Vollertsen, J. Toward the Systematic Identification of Microplastics in the Environment: Evaluation of a New Independent Software Tool (siMPle) for Spectroscopic Analysis. Appl. Spectrosc. 2020, 74, 1127–1138. [Google Scholar] [CrossRef]
- Primpke, S.; Lorenz, C.; Rascher-Friesenhausen, R.; Gerdts, G. An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Anal. Methods 2017, 9, 1499–1511. [Google Scholar] [CrossRef]
- Renner, G.; Schmidt, T.C.; Schram, J. A New Chemometric Approach for Automatic Identification of Microplastics from Environmental Compartments Based on FT-IR Spectroscopy. Anal. Chem. 2017, 89, 12045–12053. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Munno, K.; de Frond, H.; O’Donnell, B.; Rochman, C.M. Increasing the Accessibility for Characterizing Microplastics: Introducing New Application-Based and Spectral Libraries of Plastic Particles (SLoPP and SLoPP-E). Anal. Chem. 2020, 92, 2443–2451. [Google Scholar] [CrossRef]
- Klasios, N.; Frond, H.; Miller, E.; Sedlak, M.; Rochman, C.M. Microplastics and other anthropogenic particles are prevalent in mussels from San Francisco Bay, and show no correlation with PAHs. Environ. Pollut. 2020, 271, 116260. [Google Scholar] [CrossRef]
- Bishop, A.N.; Kearsley, A.T.; Patience, R.L. Analysis of sedimentary organic materials by scanning electron microscopy: The application of backscattered electron imagery and light element X-ray microanalysis. Org. Geochem. 1992, 18, 431–446. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Fiori, C.; Lifshin, E. X-ray Spectral Measurement: WDS and EDS. In Scanning Electron Microscopy and X-ray Microanalysis: A Text for Biologists, Materials Scientists, and Geologists; Goldstein, J.I., Newbury, D.E., Echlin, P., Joy, D.C., Fiori, C., Lifshin, E., Eds.; Springer: Berlin, Germany, 1981; pp. 205–273. ISBN 978-1-4613-3275-6. [Google Scholar]
- Shindo, D.; Oikawa, T. Energy Dispersive X-ray Spectroscopy. In Analytical Electron Microscopy for Materials Science; Shindo, D., Oikawa, T., Eds.; Springer: Japan, Tokyo, 2002; pp. 81–102. ISBN 978-4-431-66988-3. [Google Scholar]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A 2014, 31, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M. Kunststofftechnik: Grundlagen, Verarbeitung, Werkstoffauswahl und Fallbeispiele, 2. Auflage; Springer: Berlin, Germany, 2014; ISBN 978-3-658-03138-1. [Google Scholar]
- Kaiser, W. Kunststoffchemie für Ingenieure: Von der Synthese bis zur Anwendung, 3rd ed.; Hanser München: Munich, Germany, 2011; ISBN 9873446430471. [Google Scholar]
- PlascticsEurope. Plasctics-the Facts. 2020. Available online: https://www.plasticseurope.org/application/files/8016/1125/2189/AF_Plastics_the_facts-WEB-2020-ING_FINAL.pdf (accessed on 28 January 2021).
- Zhang, S.; Sun, Y.; Liu, B.; Li, R. Full size microplastics in crab and fish collected from the mangrove wetland of Beibu Gulf: Evidences from Raman Tweezers (1–20 μm) and spectroscopy (20–5000 μm). Sci. Total. Environ. 2021, 759. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Analen Physik 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Heffels, C.M.G.; Verheijen, P.J.T.; Heitzmann, D.; Scarlett, B. Correction of the effect of particle shape on the size distribution measured with a laser diffraction instrument. Part. Part. Syst. Charact. 1996, 13, 271–279. [Google Scholar] [CrossRef]
- Mühlenweg, H.; Hirleman, E.D. Laser Diffraction Spectroscopy: Influence of Particle Shape and a Shape Adaptation Technique. Part. Part. Syst. Charact. 1998, 15, 163–169. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Hebner, T.S.; Maurer-Jones, M.A. Characterizing microplastic size and morphology of photodegraded polymers placed in simulated moving water conditions. Environ. Sci. Process. Impacts 2020, 22, 398–407. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Crocker, B.K.; Gray, A.D. From macroplastic to microplastic: Degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ. Toxicol. Chem. 2016, 35, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- McMullan, D. Scanning electron microscopy 1928–1965. Scanning 1995, 17, 175–185. [Google Scholar] [CrossRef]
- Imhof, H.K.; Ivleva, N.P.; Schmid, J.; Niessner, R.; Laforsch, C. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 2013, 23, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Sujathan, S.; Kniggendorf, A.-K.; Kumar, A.; Roth, B.; Rosenwinkel, K.-H.; Nogueira, R. Heat and Bleach: A Cost-Efficient Method for Extracting Microplastics from Return Activated Sludge. Arch. Environ. Contam. Toxicol. 2017, 73, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of Sample Preparation Methods for Microplastic Analysis in Wastewater Matrices–Reproducibility and Standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Gambardella, C.; Piazza, V.; Albentosa, M.; Bebianno, M.J.; Cardoso, C.; Faimali, M.; Garaventa, F.; Garrido, S.; González, S.; Pérez, S.; et al. Microplastics do not affect standard ecotoxicological endpoints in marine unicellular organisms. Mar. Pollut. Bull. 2019, 143, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Frydkjær, C.K.; Iversen, N.; Roslev, P. Ingestion and Egestion of Microplastics by the Cladoceran Daphnia magna: Effects of Regular and Irregular Shaped Plastic and Sorbed Phenanthrene. Bull. Environ. Contam. Toxicol. 2017, 99, 655–661. [Google Scholar] [CrossRef]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What are the drivers of microplastic toxicity? Comparing the toxicity of plastic chemicals and particles to Daphnia magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar] [CrossRef]
- Gray, A.D.; Weinstein, J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Beiras, R.; Bellas, J.; Cachot, J.; Cormier, B.; Cousin, X.; Engwall, M.; Gambardella, C.; Garaventa, F.; Keiter, S.; Le Bihanic, F.; et al. Ingestion and contact with polyethylene microplastics does not cause acute toxicity on marine zooplankton. J. Hazard. Mater. 2018, 360, 452–460. [Google Scholar] [CrossRef]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic Bag Derived-Microplastics as a Vector for Metal Exposure in Terrestrial Invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, A.; Cao, S.; Sun, F.; Wang, L.; Guo, H.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total. Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Dehaut, A.; Cassone, A.-L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [PubMed]
- von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef]
- Hall, N.M.; Berry, K.L.E.; Rintoul, L.; Hoogenboom, M.O. Microplastic ingestion by scleractinian corals. Mar. Biol. 2015, 162, 725–732. [Google Scholar] [CrossRef]
- Crespy, D.; Landfester, K. Preparation of Nylon 6 Nanoparticles and Nanocapsules by Two Novel Miniemulsion/Solvent Displacement Hybrid Techniques. Macromol. Chem. Phys. 2007, 208, 457–466. [Google Scholar] [CrossRef]
- Ito, F.; Ma, G.; Nagai, M.; Omi, S. Study of particle growth by seeded emulsion polymerization accompanied by electrostatic coagulation. Colloids Surf. A Physicochem. Eng. Asp. 2002, 201, 131–142. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Pinto-Alphandary, H.; Puisieux, F.; Barratt, G. Poly(D,L-Lactide) Nanocapsules Prepared by a Solvent Displacement Process: Influence of the Composition on Physicochemical and Structural Properties. J. Pharm. Sci. 2000, 89, 614–626. [Google Scholar] [CrossRef]
- Horák, D. Uniform polymer beads of micrometer size. Acta Polym. 1996, 47, 20–28. [Google Scholar] [CrossRef]
- Ugelstad, J.; Mork, P.C. Swelling of Oligo-Polymer Particles: New Methods of Preparation of Emulsions and Polymer Dispersions. Adv. Colloid Interface Sci. 1980, 13, 101–140. [Google Scholar] [CrossRef]
- Goodall, A.R.; Wilkinson, M.C.; Hearn, J. Mechanism of emulsion polymerization of styrene in soap-free systems. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 2193–2218. [Google Scholar] [CrossRef]
- Arshady, R. Suspension, emulsion, and dispersion polymerization: A methodological survey. Colloid Polym. Sci. 1992, 270, 717–732. [Google Scholar] [CrossRef]
- Serra, C.A.; Chang, Z. Microfluidic-Assisted Synthesis of Polymer Particles. Chem. Eng. Technol. 2008, 31, 1099–1115. [Google Scholar] [CrossRef]
- Esen, C.; Schweiger, G. Preparation of Monodisperse Polymer Particles by Photopolymerization. J. Colloid Interface Sci. 1996, 179, 276–280. [Google Scholar] [CrossRef]
- Pérez-Moral, N.; Mayes, A. Comparative study of imprinted polymer particles prepared by different polymerisation methods. Anal. Chim. Acta 2004, 504, 15–21. [Google Scholar] [CrossRef]
- Soriano, I.; Delgado, A.; Diaz, R.V.; Evora, C. Use of Surfactants in Polylactic Acid Protein Microspheres. Drug Dev. Ind. Pharm. 1995, 21, 549–558. [Google Scholar] [CrossRef]
- Rancan, F.; Papakostas, D.; Hadam, S.; Hackbarth, S.; Delair, T.; Primard, C.; Verrier, B.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Investigation of Polylactic Acid (PLA) Nanoparticles as Drug Delivery Systems for Local Dermatotherapy. Pharm. Res. 2009, 26, 2027–2036. [Google Scholar] [CrossRef]
- Maurus, P.B.; Kaeding, C.C. Bioabsorbable implant material review. Oper. Tech. Sports Med. 2004, 12, 158–160. [Google Scholar] [CrossRef]
- Giordano, R.A.; Wu, B.M.; Borland, S.W.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J. Biomater. Sci. Polym. Ed. 1997, 8, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef]
- Kedem, M.; Margel, S. Synthesis and characterization of micrometer-sized particles of narrow size distribution with chloromethyl functionality on the basis of single-step swelling of uniform polystyrene template microspheres. J. Polym. Sci. A Polym. Chem. 2002, 40, 1342–1352. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Cospheric LLC. Cospheric White Polyethylene Microspheres: Particle Diameters 10µm–1200µm. Available online: https://www.cospheric.com/polyethylene_PE_microspheres_beads.htm (accessed on 1 April 2020).
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901. [Google Scholar] [CrossRef]
- Almog, Y.; Reich, S.; Levy, M. Monodisperse polymeric spheres in the micron size range by a single step process. Brit. Poly. J. 1982, 14, 131–136. [Google Scholar] [CrossRef]
- Bamnolker, H.; Margel, S. Dispersion polymerization of styrene in polar solvents: Effect of reaction parameters on microsphere surface composition and surface properties, size and size distribution, and molecular weight. J. Polym. Sci. A Polym. Chem. 1996, 34, 1857–1871. [Google Scholar] [CrossRef]
- ter Halle, A.; Ladirat, L.; Martignac, M.; Mingotaud, A.F.; Boyron, O.; Perez, E. To what extent are microplastics from the open ocean weathered? Environ. Pollut. 2017, 227, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H. Kollektive Zerkleinerung von Polyethylen im Tieftemperaturbereich. Chem. Ing. Tech. 1982, 54, 252–254. [Google Scholar] [CrossRef]
- Woldt, D. Zerkleinerung nicht-spröder Stoffe in Rotorscheren und -reißern. Chem. Ing. Tech. 2004, 75, 1860–1863. [Google Scholar] [CrossRef]
- Oprea, C.V.; Neguleanu, C.; Simionescu, C. On the mechano-chemical destruction of polyethylene terephthalate by vibratory milling. Eur. Polym. J. 1970, 6, 181–198. [Google Scholar] [CrossRef]
- Molina-Boisseau, S.; Le Bolay, N. Fine grinding of polymers in a vibrated bead mill. Powder Technol. 1999, 105, 321–327. [Google Scholar] [CrossRef]
- Bai, C.; Spontak, R.J.; Koch, C.C.; Saw, C.K.; Balik, C.M. Structural changes in poly (ethylene terephthalate) induced by mechanical milling. Polymer 2000, 41, 7147–7157. [Google Scholar] [CrossRef]
- Schmidt, J.; Plata, M.; Tröger, S.; Peukert, W. Production of polymer particles below 5μm by wet grinding. Powder Technol. 2012, 228, 84–90. [Google Scholar] [CrossRef]
- Wolff, M.; Antonyuk, S.; Heinrich, S.; Schneider, G.A. Attritor-milling of poly(amide imide) suspensions. Particuology 2014, 17, 92–96. [Google Scholar] [CrossRef][Green Version]
- Papaspyrides, C.D.; Poulakis, J.G.; Varelides, P.C. A model recycling process for low density polyethylene. Resour. Conserv. Recycl. 1994, 12, 177–184. [Google Scholar] [CrossRef]
- Hadi, A.J.; Faisal, G. Reconditioning Process of Waste Low Density Polyethylene Using New. J. Purity Util. Recation Environ. 2012, 1, 373–383. [Google Scholar]
- Hirsjärvi, S. Preparation and Characterization of Poly (Lactic Acid) Nanoparticles for Pharmaceutical Use. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2008. [Google Scholar]
- Miguel, F.; Martín, A.; Mattea, F.; Cocero, M.J. Precipitation of lutein and co-precipitation of lutein and poly-lactic acid with the supercritical anti-solvent process. Chem. Eng. Process. Process. Intensif. 2008, 47, 1594–1602. [Google Scholar] [CrossRef]
- Smith, A.P.; Shay, J.S.; Spontak, R.J.; Balik, C.M.; Ade, H.; Smith, S.D.; Koch, C.C. High-energy mechanical milling of poly(methyl methacrylate), polyisoprene and poly(ethylene-alt-propylene). Polymer 2000, 41, 6271–6283. [Google Scholar] [CrossRef]
- Pan, J.; Shaw, W.J. Properties of a mechanically processed polymeric material. J. Appl. Polym. Sci. 1994, 52, 507–514. [Google Scholar] [CrossRef]
- von der Esch, E.; Lanzinger, M.; Kohles, A.J.; Schwaferts, C.; Weisser, J.; Hofmann, T.; Glas, K.; Elsner, M.; Ivleva, N.P. Simple Generation of Suspensible Secondary Microplastic Reference Particles via Ultrasound Treatment. Front. Chem. 2020, 8, 169. [Google Scholar] [CrossRef] [PubMed]
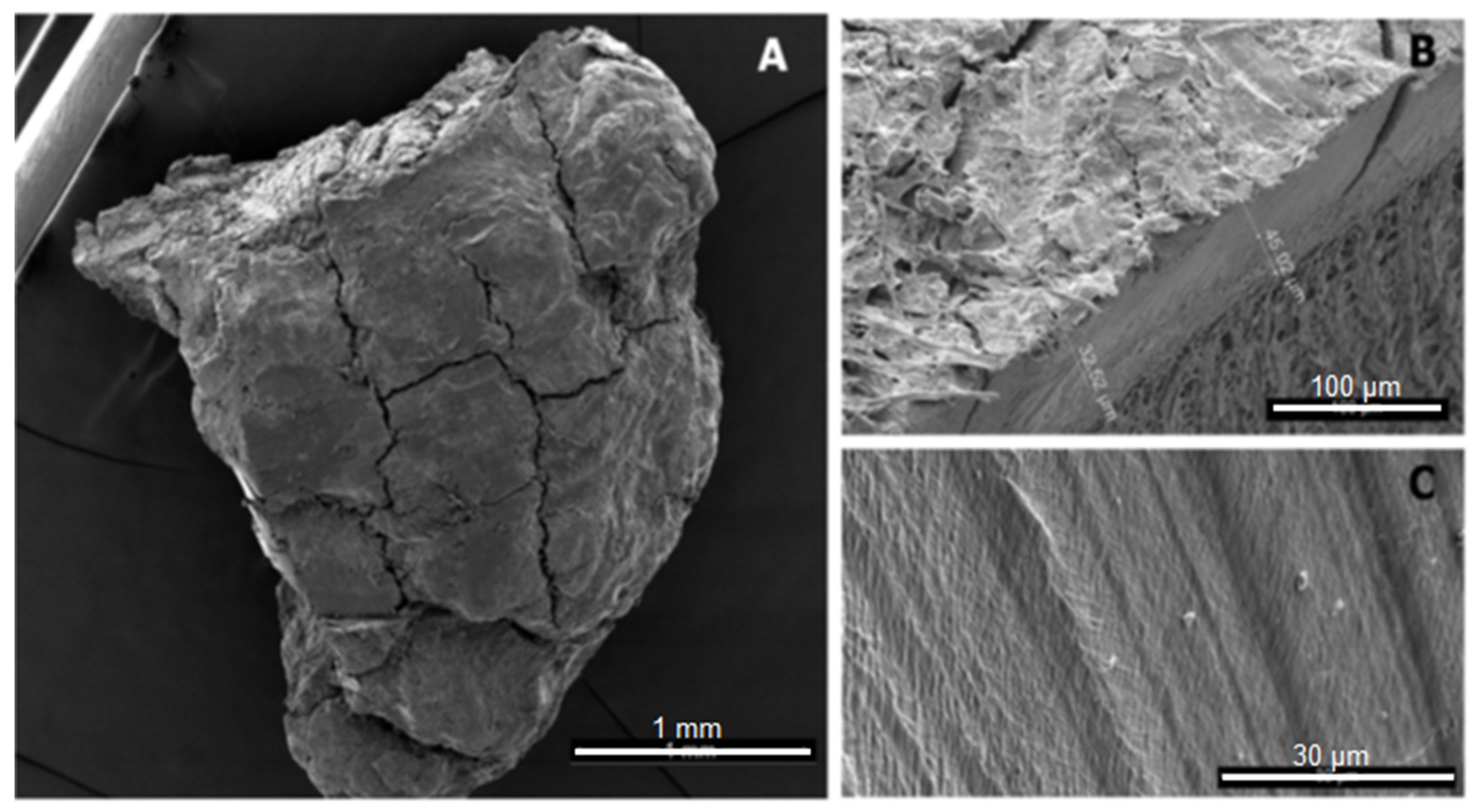
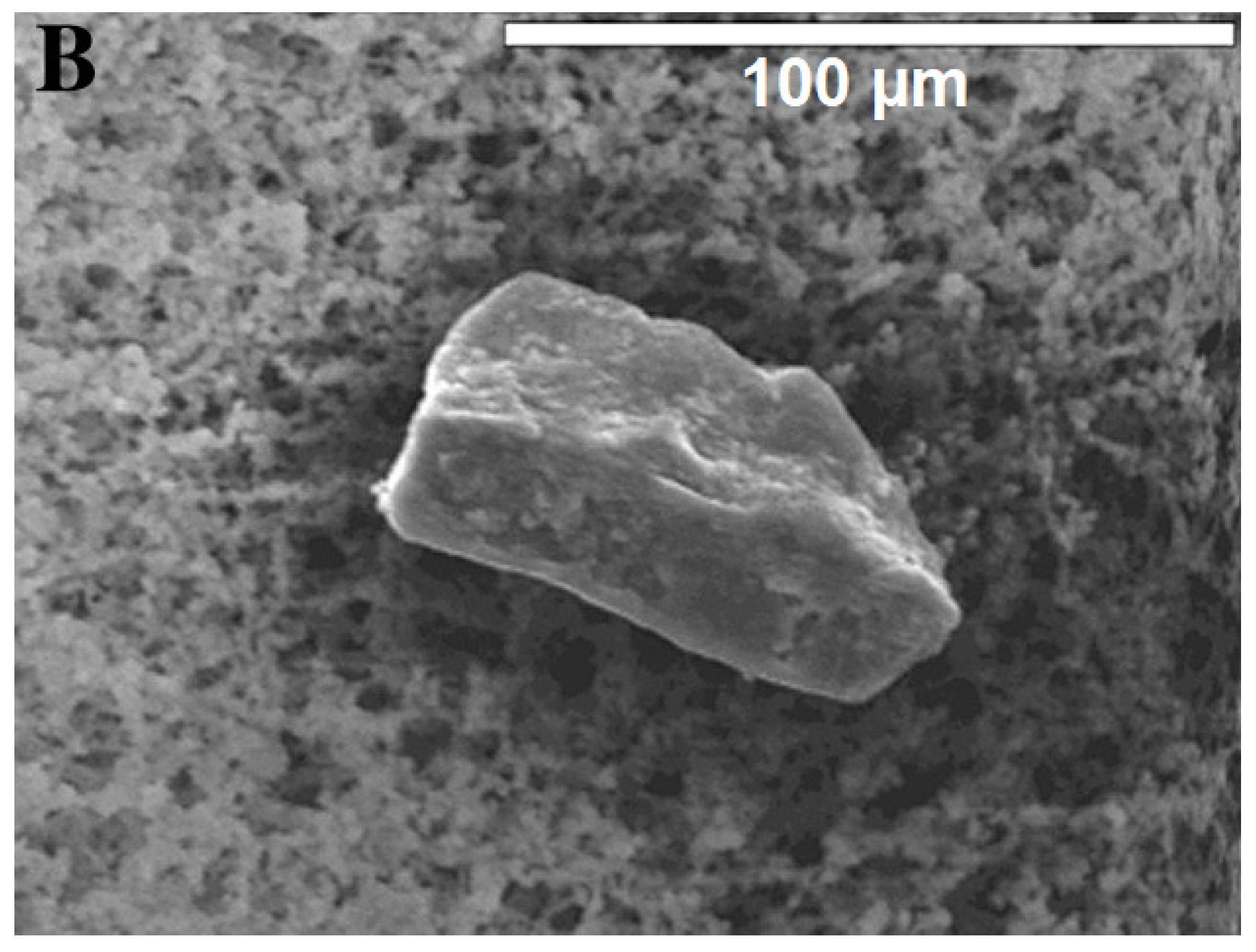
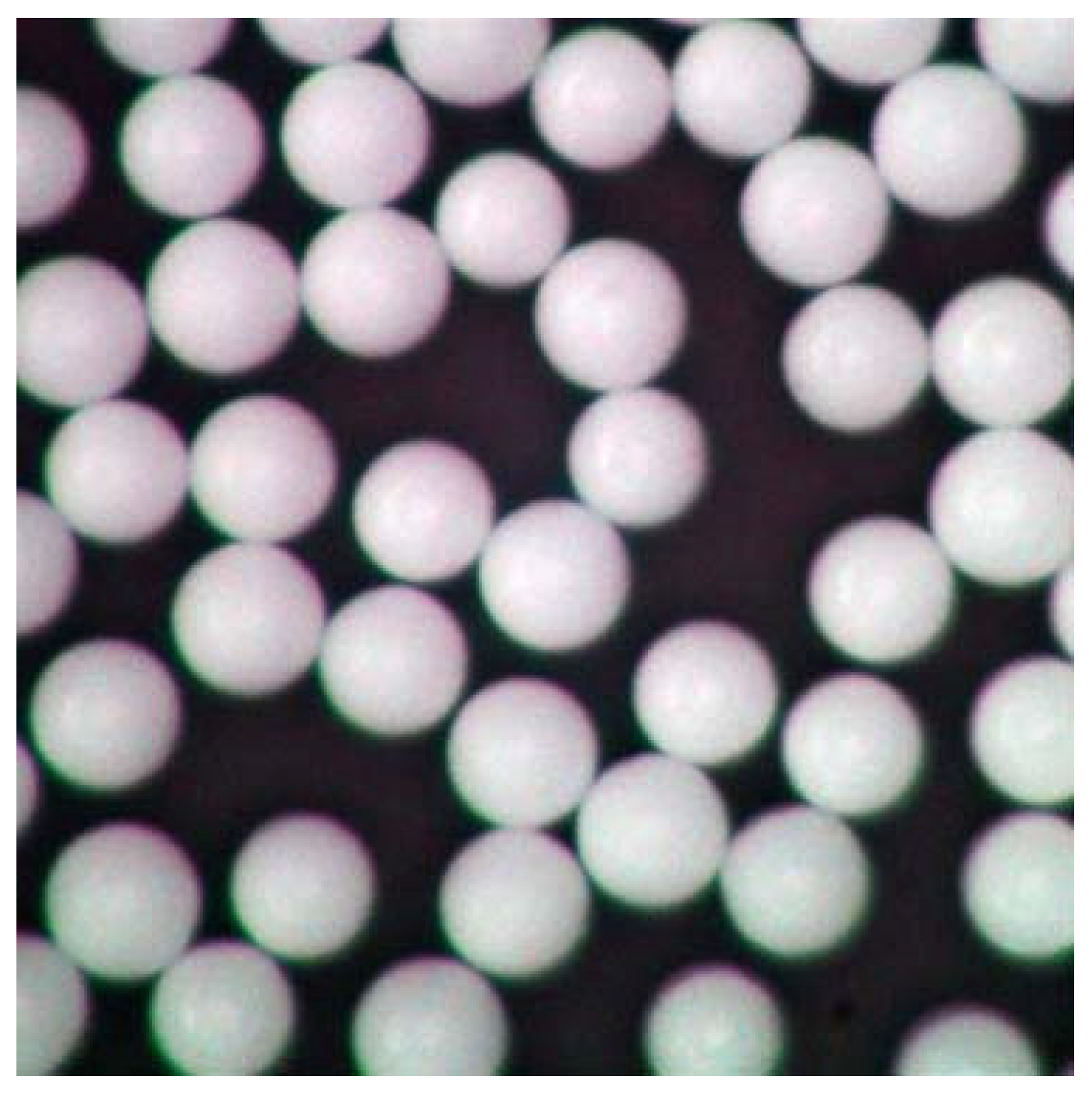

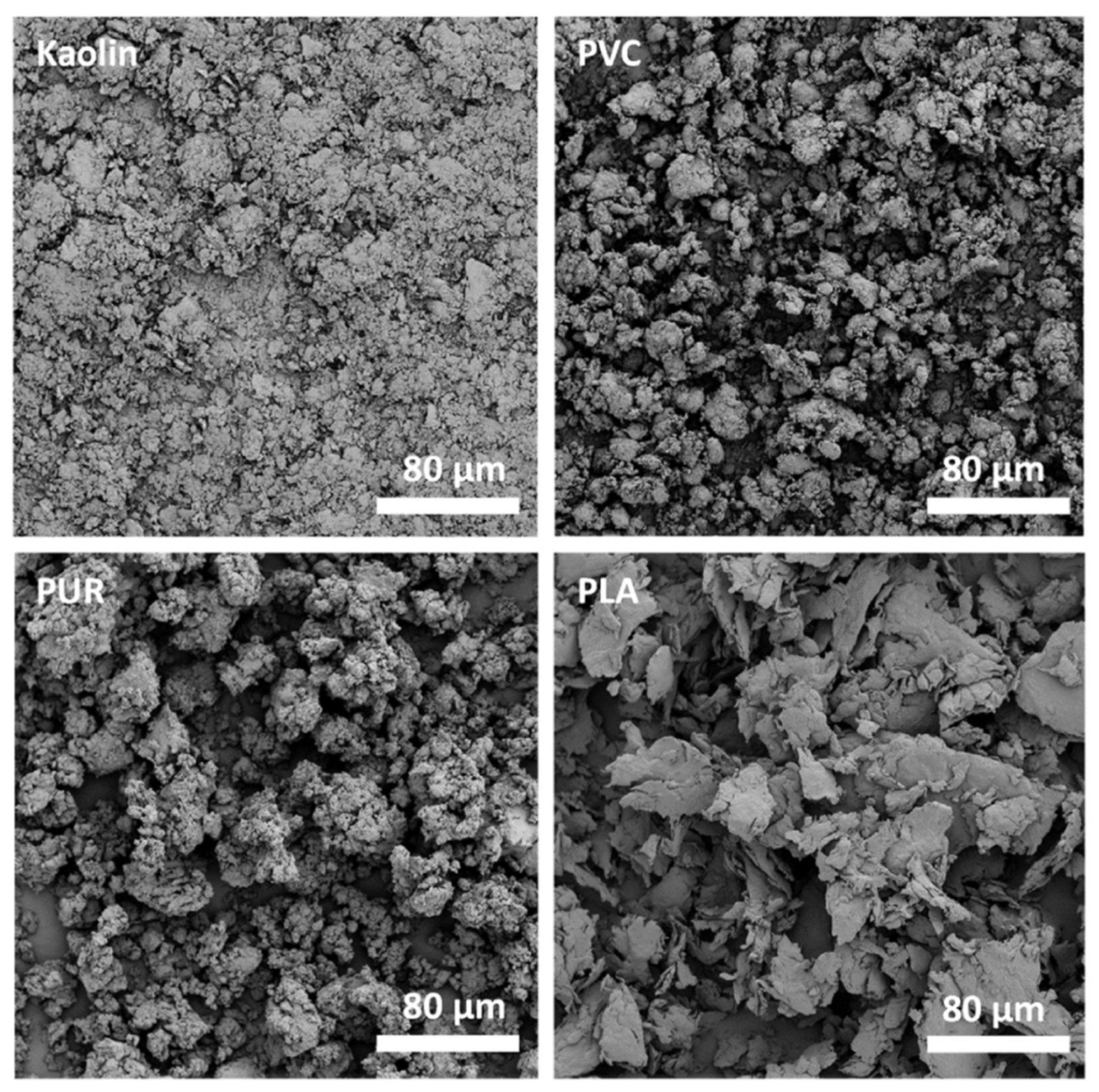
| Sampling Site | Sampling Method | Reference | |
|---|---|---|---|
| Soil | Core sampling, shovel, | [51,60,61] | |
| Sand (land-based) | Trowel, spatula, tube, spoon, shovel | [50,62,63,64,65,66] | |
| Lake | Open Water | Grab, pump, trawl net, bottles, bucket | [56,62,67,68,69,70] |
| Coastal Water | Bottles, bucket, trawl net | [55,56,67,70,71] | |
| Sediment | Grab sampler, gravity corer | [54,57,67,72] | |
| River | Open Water | Pump, trawl net, bucket | [62,63,68,73,74,75,76,77] |
| Coastal Water | Bucket, bottles, | [51,78,79] | |
| Sediment | Grab, shovel, dredge sampler, gravity corer | [51,62,63,73,74,76,79,80] | |
| Sea | Open Water | Pump, trawl net | [7,10,53,81,82] |
| Coastal Water | Jar, bucket, pump, trawl net, bottles | [7,52,75,78,83,84,85,86] | |
| Sediment | Trowel, spatula, box corer, grab, dredge sampler, gravity corer, tube, shovel | [11,50,65,80,81,86,87,88,89,90,91,92] | |
| Wastewater treatment plant | Grit and grease removal | Bottles | [26] |
| First effluent | Containers, bottles | [26,93] | |
| Second effluent | Containers, bottles, pump | [26,93,94] | |
| Activated sludge bioreactor | Containers, bottles | [26,93] | |
| Final effluent | Automated liquid samplers | [55] | |
| Procedures Used | Reference |
|---|---|
| Sample pre-classification and rough separation | |
| Sieving | [97] ***, [117] **, [118] ***, [119] *, [63] *, [53,55,64,65,82,84,86,90,93,107,110] |
| Separation of particles from biogenic and inorganic matter | |
| Enzymatic digestion | [120] |
| Flotation/elutriation | [95] *, [60,61,111,113,121] |
| Density separation in water | [83] * |
| Density separation using aqueous solutions of NaCl, CaCl, or ZnCl2 | [117] *, [60] *, [119] ***, [50] **, [94] *, [105] ***, [64] ***, [9,51,53,63,65,66,78,79,82,84,90,91,107] |
| Density separation using aqueous solutions of NaI | [60,61,89,109,110,122] |
| Digestion of biogenic matter using H2O2, HCl or NaOH, HNO3 or Fenton’s reagent | [121] *, [27] ***, [123] ***, [5,83,93,121,124,125,126] |
| Bare eye and tweezers | [78,83] |
| Optical microscope | [5,50,79,83,91,109,125,127] |
| Fluorescence microscope | [9,40,66,91,106,128] |
| Stereo microscope | [117] ***, [26,50,51,52,61,70,78,81,88,93,129,130] |
| Identification and Characterisation | |
| Polymer type | |
| µ-Raman spectroscopy | [53,55,82,84,89,94,118,120,123,126,128,131] |
| Coherent anti-stokes Raman scattering (CARS) | [13] |
| TED-Pyr-GC/MS | [24,95,132] |
| µ-FT-IR | [5,50,52,68,70,78,79,86,87,105,106,107,109,119,120,124] |
| ATR-FT-IR | [51,55,60,61,63,73,83,92,93,117,121,122,126] |
| Energy dispersive x-ray spectroscopy (EDS) | [9,89,106,118,122,128] |
| Particle shape, size and dimensions | |
| Scanning electron microscopy (SEM) | [53,60,61,68,79,89,106,110,118,122] |
| Microscopy and Image processing | [83] ***, [10,90,109,128] |
| Sieving | [55,63,64,65,84,86,90,93,107,110,119] |
| Material | Particle Origin | Sample | Extracting Solvents | Result | Reference |
|---|---|---|---|---|---|
| PE | Primary particles extracted from cosmetics | Return activated sludge | 30% H2O2 at 70 °C, NaNO3/Na2S2O3 | Recovery rate: 78% | [159] |
| PE, PP | Ground commercial particles | Field-cleaned sand | NaI, NaCl | Recovery rate: 97% | [60] |
| PA, PE, PET | Comminuted fibres | Sediment, sand | NaCl, 30% H2O2 at 50 °C | Recovery rate: 77.5% | [63] |
| PP, PA, PE-LD, PE-HD, PS, PET, PC, PMMA | Purchased pellets | No environmental samples | Fenton’s reagent, 30% H2O2 at 30 °C and 70 °C, 1 M and 10 M NaOH at 60 °C, KOH at 60 °C | 1 M NaOH damages PET and PC, 10 M degrades them, no significant changes in other treatments | [160] |
| PE-LD, PET, PS, PP, PLA, PVC, PA | Lab-made | Return activated sludge | Fenton’s reagent, 10% KOH at 60 °C, 30% H2O2 at 60 °C | Fenton’s reagent most efficient, H2O2 reaction is slow, KOH destroys polyesters | [161] |
| Material | Starting Aize | Medium | Milling Device | End Size | Reference | Further Results |
|---|---|---|---|---|---|---|
| PET | Unspecified powder | Various gaseous and liquid media | Vibratory mill | Dependent on parameters, only specified in MW decrease | [202] | Milling at low temperatures, wet or oxygen-rich media most efficient |
| PE-HD | Not mentioned | air | Pin mill | 300 µm | [200] | Pre-cooling irrelevant; particle size will increase if milling temperature is above Tg |
| PA 6.6 | 200 µm | air | Ball mill | 3 µm | Pan and Shaw 1994 | Milling at temp below Tg is needed; MW decreased with increasing milling time and temp. |
| PE PS PVA | 100–200 µm 80–100 µm 80–100 µm | air | Vibrated bead mill | Dependent on milling time and bead load; 5–100 µm | [203] | |
| PET | Pellet | Argon | Ball mill | 20 µm | [204] | |
| PS PEEK | 250–500 µm d50,3 = 21.5 µm | Denaturated ethanol, n-hexane | Stirred media mill | <5 µm | [205] | Milling in wet media and organic solvent at low temp. produces small particles |
| PAI | d50,3 = 22 µm | Water | Attritor mill | 3 µm | [206] | |
| PUR, PVC, PLA | 0.5 cm | Air | Ball mill | </ = 59 µm | [165] | Milling after liquid N2 application |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kefer, S.; Miesbauer, O.; Langowski, H.-C. Environmental Microplastic Particles vs. Engineered Plastic Microparticles—A Comparative Review. Polymers 2021, 13, 2881. https://doi.org/10.3390/polym13172881
Kefer S, Miesbauer O, Langowski H-C. Environmental Microplastic Particles vs. Engineered Plastic Microparticles—A Comparative Review. Polymers. 2021; 13(17):2881. https://doi.org/10.3390/polym13172881
Chicago/Turabian StyleKefer, Simone, Oliver Miesbauer, and Horst-Christian Langowski. 2021. "Environmental Microplastic Particles vs. Engineered Plastic Microparticles—A Comparative Review" Polymers 13, no. 17: 2881. https://doi.org/10.3390/polym13172881
APA StyleKefer, S., Miesbauer, O., & Langowski, H.-C. (2021). Environmental Microplastic Particles vs. Engineered Plastic Microparticles—A Comparative Review. Polymers, 13(17), 2881. https://doi.org/10.3390/polym13172881







