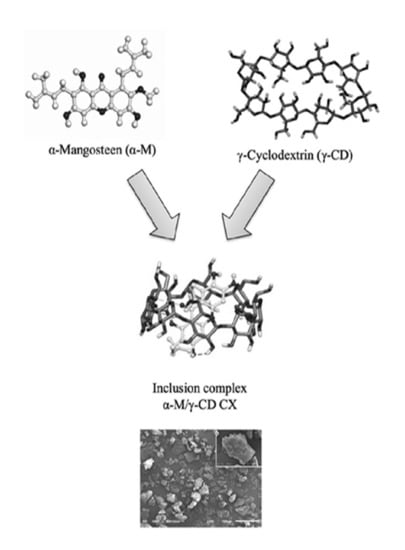
α-Mangostin/γ-Cyclodextrin Inclusion Complex: Formation and Thermodynamic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of α-Mangostin and γ-Cyclodextrin Physical Mixture (α-M/γ-CD PM)
2.2.2. Stoichiometry Determination of α-M/γ-CD CX Formation
2.2.3. Phase Solubility Studies
2.2.4. Preparation of α-Mangostin and γ-Cyclodextrin Complex (α-M/γ-CD CX)
2.2.5. Characterization of α-Mangostin (α-M), γ-Cyclodextrin (γ-CD), Physical Mixture (α-M/γ-CD PM), and Inclusion Complex (α-M/γ-CD CX)
FTIR Spectrometry
X-ray Diffractometry (XRD)
Scanning Electron Microscopy (SEM)
NMR Spectrometry
2.2.6. Thermodynamic Study of α-M/γ-CD CX Formation
Determination of Enthalpy (∆H) and Gibbs Energy (∆G)
2.2.7. Data Analysis
3. Results and Discussion
3.1. Phase Solubility Studies
3.2. Stoichiometry Determination of α-M/γ-CD CX Complex Formation
3.3. Characterization of α-M, γ-CD, the Physical Mixture (α-M/γ-CD PM), and the Inclusion Complex (α-M/γ-CD CX)
3.3.1. FTIR Spectrometry
3.3.2. X-ray Diffractometry (XRD)
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. NMR Spectrometry
3.4. Thermodynamic Study of α-M/γ-CD CX Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dermawan, D.; Wathoni, N.; Muchtaridi, M. Host-guest interactions of α−mangostin with (A,β,γ)−cyclodextrins: Semi-empirical quantum mechanical methods of PM6 and PM7. J. Young Pharm. 2018, 11, 31–35. [Google Scholar] [CrossRef]
- Herrera-Aco, D.R.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Sciutto-Conde, E.; Rosas-Salgado, G.; Fragoso-González, G. Alpha-mangostin: Anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem. Toxicol. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Lee, H.N.; Jang, H.Y.; Kim, H.J.; Shin, S.A.; Choo, G.S.; Park, Y.S.; Kim, S.K.; Jung, J.Y. Antitumor and apoptosis-inducing effects of α-mangostin extracted from the pericarp of the mangosteen fruit (Garcinia mangostana L.) in YD-15 tongue mucoepidermoid carcinoma cells. Int. J. Mol. Med. 2016, 37, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef]
- Pothitirat, W.; Chomnawang, M.; Gritsanapan, W. Anti-acne-inducing bacterial activity of mangosteen fruit rind extracts. Med Princ. Pr. 2010, 19, 281–286. [Google Scholar] [CrossRef]
- Tadtong, S.; Viriyaroj, A.; Vorarat, S.; Nimkulrat, S.; Suksamrarn, S. Antityrosinase and antibacterial activities of mangosteen pericarp extract. Health 2009, 23, 99–102. [Google Scholar]
- Aisha, A.F.A.; Ismail, Z.; Abu-Salah, K.M.; Majid, A.M.S.A. Solid dispersions of α-mangostin improve its aqueous solubility through self-assembly of nanomicelles. J. Pharm. Sci. 2012, 101, 815–825. [Google Scholar] [CrossRef]
- Hotarat, W.; Phunpee, S.; Rungnim, C.; Wolschann, P.; Kungwan, N.; Ruktanonchai, U.; Rungrotmongkol, T.; Hannongbua, S. Encapsulation of alpha-mangostin and hydrophilic beta-cyclodextrins revealed by all-atom molecular dynamics simulations. J. Mol. Liq. 2019, 288, 110965. [Google Scholar] [CrossRef]
- Rungnim, C.; Phunpee, S.; Kunaseth, M.; Namuangruk, S.; Rungsardthong, K.; Rungrotmongkol, T.; Ruktanonchai, U. Co-solvation effect on the binding mode of the α-mangostin/β-cyclodextrin inclusion complex. Beilstein J. Org. Chem. 2015, 11, 2306–2317. [Google Scholar] [CrossRef] [Green Version]
- Hotarat, W.; Nutho, B.; Wolschann, P.; Rungrotmongkol, T.; Hannongbua, S. Delivery of alpha-mangostin using cyclodextrins through a biological membrane: Molecular dynamics simulation. Molecules 2020, 25, 2532. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef]
- Simoes, S.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef]
- Di Donato, C.; Lavorgna, M.; Fattorusso, R.; Isernia, C.; Isidori, M.; Malgieri, G.; Piscitelli, C.; Russo, C.; Russo, L.; Iacovino, R. Alpha- and beta-cyclodextrin inclusion complexes with 5-fluorouracil: Characterization and cytotoxic activity evaluation. Molecules 2016, 21, 1644. [Google Scholar] [CrossRef]
- Heydari, S.; Kakhki, R.M. Thermodynamic study of complex formation of β-cyclodextrin with ibuprofen by conductometric method and determination of ibuprofen in pharmaceutical drugs. Arab. J. Chem. 2017, 10, S1223–S1226. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Electrospun polymer-free nanofibers incorporating hydroxypropyl-β-cyclodextrin/Difenoconazole via supramolecular assembly for antifungal activity. J. Agric. Food Chem. 2021, 69, 5871–5881. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Thiram/hydroxypropyl-β-cyclodextrin inclusion complex electrospun nanofibers for a fast dissolving water-based drug delivery system. Colloids Surf. B Biointerfaces 2021, 201, 111625. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Jiang, J.; Zhao, L.; Fu, Y.; Ye, F. Fabrication and characterization of thiophanate methyl/hydroxypropyl-β-cyclodextrin inclusion complex nanofibers by electrospinning. J. Mol. Liq. 2021, 335, 116228. [Google Scholar] [CrossRef]
- Inoue, Y.; Hirano, A.; Murata, I.; Kobata, K.; Kanamoto, I. Assessment of the physical properties of inclusion complexes of forchlorfenuron and γ-cyclodextrin derivatives and their promotion of plant growth. ACS Omega 2018, 3, 13160–13169. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F.; et al. Cyclodextrins in drug delivery systems and their effects on biological barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Inoue, Y.; Limmatvapirat, S.; Kanamoto, I.; Murata, I. Molecular interactions of the inclusion complexes of hinokitiol and various cyclodextrins. AAPS PharmSciTech 2017, 18, 2717–2726. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Suryani, D.; Qosim, W.A.; Saptarini, N.M. Quantitative analysis of A-mangostin in mangosteen (Garcinia mangostana L.) pericarp extract from four district of West Java by HPLC method. Int. J. Pharm. Pharm. Sci. 2016, 8, 232–236. [Google Scholar]
- Mader, W.J.; Higuchi, T. Phase solubility analysis. CRC Crit. Rev. Anal. Chem. 1970, 1, 193–215. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef] [PubMed]
- Caso, J.V.; Russo, L.; Palmieri, M.; Malgieri, G.; Galdiero, S.; Falanga, A.; Isernia, C.; Iacovino, R. Investigating the inclusion properties of aromatic amino acids complexing beta-cyclodextrins in model peptides. Amino Acids 2015, 47, 2215–2227. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.J.; Arias-Blanco, M.J.; Moyano, J.R.; Muñoz, P.; Gines, J.M.; Justo, A.; Giordano, F. Study of omeprazole-γ-cyclodextrin complexation in the solid state. Drug Dev. Ind. Pharm. 2000, 26, 253–259. [Google Scholar] [CrossRef]
- Jug, M.; Jablan, J.; Kövér, K.; Weitner, T.; Gabričević, M. Thermodynamic study of inclusion complexes of zaleplon with natural and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2013, 79, 391–400. [Google Scholar] [CrossRef]
- Del Valle, E. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Ikeda, N.; Inoue, Y.; Ogata, Y.; Murata, I.; Meiyan, X.; Takayama, J.; Sakamoto, T.; Okazaki, M.; Kanamoto, I. Improvement of the solubility and evaluation of the physical properties of an inclusion complex formed by a new ferulic acid derivative and γ-cyclodextrin. ACS Omega 2020, 5, 12073–12080. [Google Scholar] [CrossRef]
- Wong, J.W.; Yuen, K.H. Inclusion complexation of artemisinin with α-, β-, and γ-cyclodextrins. Drug Dev. Ind. Pharm. 2003, 29, 1035–1044. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Z.; Xu, X. Gamma-cyclodextrin on enhancement of water solubility and store stability of nystatin. J. Incl. Phenom. Macrocycl. Chem. 2012, 78, 145–150. [Google Scholar] [CrossRef]
- Olson, E.J.; Buhlmann, P. Getting more out of a job plot: Determination of reactant to product stoichiometry in cases of displacement reactions andn: Ncomplex formation. J. Org. Chem. 2011, 76, 8406–8412. [Google Scholar] [CrossRef]
- Tablet, C.; Matei, I.; Hillebr, M. The determination of the stoichiometry of cyclodextrin inclusion complexes by spectral methods: Possibilities and limitations. Stoichiom. Res. 2012. [Google Scholar] [CrossRef] [Green Version]
- Tejamukti, E.P.; Setyaningsih, W.; Irnawati; Yasir, B.; Alam, G.; Rohman, A. Application of FTIR spectroscopy and HPLC combined with multivariate calibration for analysis of xanthones in mangosteen extracts. Sci. Pharm. 2020, 88, 35. [Google Scholar] [CrossRef]
- Maksimowski, P.; Rumianowski, T. Properties of the gamma-cyclodextrin/CL-20 system. Cent. Eur. J. Energetic Mater. 2016, 13, 217–229. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Vieira, T.; Veiga, F.J.B. Physicochemical characterization and in vitro dissolution behavior of nicardipine–cyclodextrins inclusion compounds. Eur. J. Pharm. Sci. 2002, 15, 79–88. [Google Scholar] [CrossRef]
- Higashi, K.; Ideura, S.; Waraya, H.; Moribe, K.; Yamamoto, K. Incorporation of salicylic acid molecules into the intermolecular spaces of γ-cyclodextrin-polypseudorotaxane. Cryst. Growth Des. 2009, 9, 4243–4246. [Google Scholar] [CrossRef]
- Şoica, C.; Dehelean, C.; Danciu, C.; Wang, H.M.; Wenz, G.; Ambrus, R.; Bojin, F.; Anghel, M. Betulin complex in γ-cyclodextrin derivatives: Properties and antineoplasic activities in in vitro and in vivo tumor models. Int. J. Mol. Sci. 2012, 13, 14992–15011. [Google Scholar] [CrossRef]
- Şoica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupkó, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic acid in complex with a gamma-cyclodextrin derivative decreases proliferation and in vivo tumor development of non-metastatic and metastatic B164A5 cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suharyani, I.; Muchtaridi, M.; Mohammed, A.F.A.; Elamin, K.M.; Wathoni, N.; Abdassah, M. α-Mangostin/γ-Cyclodextrin Inclusion Complex: Formation and Thermodynamic Study. Polymers 2021, 13, 2890. https://doi.org/10.3390/polym13172890
Suharyani I, Muchtaridi M, Mohammed AFA, Elamin KM, Wathoni N, Abdassah M. α-Mangostin/γ-Cyclodextrin Inclusion Complex: Formation and Thermodynamic Study. Polymers. 2021; 13(17):2890. https://doi.org/10.3390/polym13172890
Chicago/Turabian StyleSuharyani, Ine, Muchtaridi Muchtaridi, Ahmed Fouad Abdelwahab Mohammed, Khaled M. Elamin, Nasrul Wathoni, and Marline Abdassah. 2021. "α-Mangostin/γ-Cyclodextrin Inclusion Complex: Formation and Thermodynamic Study" Polymers 13, no. 17: 2890. https://doi.org/10.3390/polym13172890
APA StyleSuharyani, I., Muchtaridi, M., Mohammed, A. F. A., Elamin, K. M., Wathoni, N., & Abdassah, M. (2021). α-Mangostin/γ-Cyclodextrin Inclusion Complex: Formation and Thermodynamic Study. Polymers, 13(17), 2890. https://doi.org/10.3390/polym13172890