Postharvest Quality of Cherry Tomatoes Coated with Mucilage from Dragon Fruit and Irradiated with UV-C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Plant Mucilage
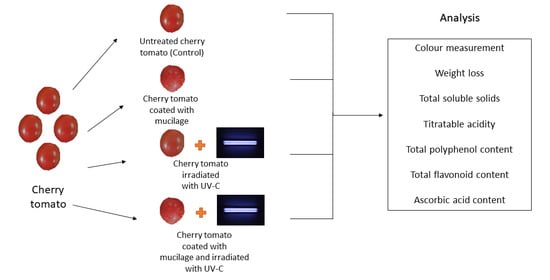
2.2. Treatment of Cherry Tomatoes
2.3. Color Measurement
2.4. Weight Loss
2.5. Antioxidant Analysis
2.5.1. Preparation of Extract
2.5.2. Total Polyphenol Content
2.5.3. Total Flavonoid Content
2.5.4. Ascorbic Acid Content
2.6. Total Soluble Solid
2.7. Titratable Acidity
2.8. Microbial Inactivation Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Color Analysis
3.2. Weight Loss
3.3. Titratable Acidity
3.4. Total Soluble Solids
3.5. Antioxidant Analysis
3.6. Ascorbic Acid Content
3.7. Microbial Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tzortzakis, N.; Borland, A.; Singleton, I.; Barnes, J. Impact of atmospheric ozone-enrichment on quality-related attributes of tomato fruit. Postharvest Biol. Technol. 2007, 45, 317–325. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Raffo, A.; La Malfa, G.; Fogliano, V.; Maiani, G.; Quaglia, G. Seasonal variations in antioxidant components of cherry tomatoes (Lycopersicon esculentum cv. Naomi F1). J. Food Compos. Anal. 2006, 19, 11–19. [Google Scholar] [CrossRef]
- Madureira, J.; Severino, A.; Cojocaru, M.; Garofalide, S.; Santos, P.; Carolino, M.; Margaça, F.; Verde, S.C. E-beam treatment to guarantee the safety and quality of cherry tomatoes. Innov. Food Sci. Emerg. Technol. 2019, 55, 57–65. [Google Scholar] [CrossRef]
- Lin, F.; Xue, Y.; Huang, Z.; Jiang, M.; Lu, F.; Bie, X.; Miao, S.; Lu, Z. Bacillomycin D inhibits growth of Rhizopus stolonifer and induces defense-related mechanism in cherry tomato. Appl. Microbiol. Biotechnol. 2019, 103, 7663–7674. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J. The climacteric respiration rise in attached and detached tomato fruit. Postharvest Biol. Technol. 1995, 6, 287–292. [Google Scholar] [CrossRef]
- Park, M.H.; Sangwanangkul, P.; Choi, J.W. Reduced chilling injury and delayed fruit ripening in tomatoes with modified atmosphere and humidity packaging. Sci. Hortic. 2018, 231, 66–72. [Google Scholar] [CrossRef]
- Lima, G.; Castoria, R.; De Curtis, F.; Raiola, A.; Ritieni, A.; de Cicco, V. Integrated control of blue mould using new fungicides and biocontrol yeasts lowers levels of fungicide residues and patulin contamination in apples. Postharvest Biol. Technol. 2011, 60, 164–172. [Google Scholar] [CrossRef]
- Mencarelli, F.; Saltveit, M.E. Ripening of mature-green tomato fruit slices. J. Am. Soc. Hortic. Sci. 1988, 113, 742–745. [Google Scholar]
- Suzuki, Y.; Nagata, Y. Postharvest ethanol vapor treatment of tomato fruit stimulates gene expression of ethylene biosynthetic enzymes and ripening related transcription factors, although it suppresses ripening. Postharvest Biol. Technol. 2019, 152, 118–126. [Google Scholar] [CrossRef]
- Salas-Méndez, E.D.J.; Vicente, A.; Pinheiro, A.C.; Ballesteros, L.F.; Silva, P.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.D.L.V.; López, M.L.F.; Villarreal-Quintanilla, J.; et al. Application of edible nanolaminate coatings with antimicrobial extract of Flourensia cernua to extend the shelf-life of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2018, 150, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Liplap, P.; Vigneault, C.; Toivonen, P.; Charles, M.T.; Raghavan, G.V. Effect of hyperbaric pressure and temperature on respiration rates and quality attributes of tomato. Postharvest Biol. Technol. 2013, 86, 240–248. [Google Scholar] [CrossRef]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the Shelf Life of Cherry Tomatoes by Pullulan Coating with Ethanol Extract of Propolis During Refrigerated Storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Petersen, K.; Nielsen, P.; Bertelsen, G.; Lawther, M.; Olsen, M.B.; Nilsson, N.H.; Mortensen, G. Potential of biobased materials for food packaging. Trends Food Sci. Technol. 1999, 10, 52–68. [Google Scholar] [CrossRef]
- Salama, H.E.; Aziz, M.S.A. Optimised alginate and Aloe vera gel edible coating reinforced with nTiO2 for the shelf-life extension of tomatoes. Int. J. Biol. Macromol. 2020, 165, 2693–2701. [Google Scholar] [CrossRef]
- Abdipour, M.; Malekhossini, P.S.; Hosseinifarahi, M.; Radi, M. Integration of UV irradiation and chitosan coating: A powerful treatment for maintaining the postharvest quality of sweet cherry fruit. Sci. Hortic. 2020, 264, 109197. [Google Scholar] [CrossRef]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa, Duch.). Postharvest Biol. Technol. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- Fritzemeier, K.-H.; Kindl, H. Coordinate induction by UV light of stilbene synthase, phenylalanine ammonia-lyase and cinnamate 4-hydroxylase in leaves of vitaceae. Planta 1981, 151, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.; Pineda, C.; Lemoine, M.L.; Civello, P.M.; Martinez, G.A.; Chaves, A.R. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005, 35, 69–78. [Google Scholar] [CrossRef]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Charles, M.T.; Tano, K.; Asselin, A.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. V. Constitutive defence enzymes and inducible pathogenesis-related proteins. Postharvest Biol. Technol. 2009, 51, 414–424. [Google Scholar] [CrossRef]
- Pombo, M.A.; Dotto, M.; Martínez, G.A.; Civello, P.M. UV-C irradiation delays strawberry fruit softening and modifies the expression of genes involved in cell wall degradation. Postharvest Biol. Technol. 2009, 51, 141–148. [Google Scholar] [CrossRef]
- Ippolito, A.; El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol. Technol. 2000, 19, 265–272. [Google Scholar] [CrossRef]
- Erkan, M.; Wang, S.Y.; Wang, C.Y. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol. Technol. 2008, 48, 163–171. [Google Scholar] [CrossRef]
- Maharaj, R.; Arul, J.; Nadeau, P. UV-C irradiation effects on levels of enzymic and non-enzymic phytochemicals in tomato. Innov. Food Sci. Emerg. Technol. 2014, 21, 99–106. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Muñoz, L.; Cobos, A.; Diaz, O.; Aguilera, J. Chia seeds: Microstructure, mucilage extraction and hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products; Tata McGraw-Hill Education: New York, NY, USA, 1986. [Google Scholar]
- Redd, J.B.; Hendrix, C.M.; Hendrix, D.L. Quality control manual for citrus processing plants. Santhirasegaram, V.; Razali, Z.; Somasundram, C. Effects of thermal treatment and sonication on quality attributes of Chokanan mango (Mangifera indica L.) juice. Ultrason. Sonochem. 2013, 20, 1276–1282. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Caminiti, I.M.; Noci, F.; Morgan, D.J.; Cronin, D.A.; Lyng, J. The effect of pulsed electric fields, ultraviolet light or high intensity light pulses in combination with manothermosonication on selected physico-chemical and sensory attributes of an orange and carrot juice blend. Food Bioprod. Process. 2012, 90, 442–448. [Google Scholar] [CrossRef]
- Lim, R.; Stathopoulos, C.E.; Golding, J.B. Effect of edible coatings on some quality characteristics of sweet cherries. Int. Food Res. J. 2011, 18, 1237–1241. [Google Scholar]
- Li, X.-Y.; Du, X.-L.; Liu, Y.; Tong, L.-J.; Wang, Q.; Li, J.-L. Rhubarb extract incorporated into an alginate-based edible coating for peach preservation. Sci. Hortic. 2019, 257, 108685. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Rahman, M.A.; Arfin, M.S.; Islam, M.N.; Ullah, M.A. Effect of novel coconut oil and beeswax edible coating on postharvest quality of lemon at ambient storage. J. Agric. Food Res. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Zgórska, K.; Grudzinska, M. Zmiany wybranych cech jakości bulw ziemniaka w czasie przechowywania. Acta Agrophys. 2012, 19, 203–214. Available online: https://www.semanticscholar.org/paper/Zmiany-wybranych-cech-jako%C5%9Bci-bulw-ziemniaka-w-Zg%C3%B3rska-Grudzi%C5%84ska/96444dc8683e8b89c43e7a0ab10ef6286dcb7cc9 (accessed on 23 July 2021).
- Eshetu, A.; Ibrahim, A.M.; Forsido, S.F.; Kuyu, C.G. Effect of beeswax and chitosan treatments on quality and shelf life of selected mango (Mangifera indica L.) cultivars. Heliyon 2019, 5, e01116. [Google Scholar] [CrossRef] [Green Version]
- Sanganamoni, M.; Patil, H.B.; Ajjappalavara, P.S. Correlation and path coefficient studies on growth earliness and yield parameters in okra, Abelmoschus esculentus (L) Moench. Int. J. Farm Sci. 2017, 7, 81–86. [Google Scholar]
- Bassetto, E.; Jacomino, A.P.; Pinheiro, A.L. Conservation of ‘Pedro Sato’ guavas under treatment with 1-methylcyclopropene. Pesqui. Agropecuária Bras. 2005, 40, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Charles, M.T.; Arul, J.; Charlebois, D.; Yaganza, E.-S.; Rolland, D.; Roussel, D.; Merisier, M.J. Postharvest UV-C treatment of tomato fruits: Changes in simple sugars and organic acids contents during storage. LWT 2016, 65, 557–564. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.; Opara, U.L. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Pataro, G.; Donsi, G.; Ferrari, G. Post-harvest UV-C and PL irradiation of fruits and vegetables. Chem. Eng. Trans. 2015, 44, 31–36. [Google Scholar]
- Swarup, K.R.L.A.; Sattar, M.A.; Abdullah, N.A.; Abdulla, M.H.; Salman, I.M.; Rathore, H.; Johns, E. Effect of dragon fruit extract on oxidative stress and aortic stiffness in streptozotocin-induced diabetes in rats. Pharmacogn. Res. 2010, 2, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aguilar, G.A.; Villa-Rodriguez, J.A.; Zavala, J.F.A.; Yahia, E. Improvement of the antioxidant status of tropical fruits as a secondary response to some postharvest treatments. Trends Food Sci. Technol. 2010, 21, 475–482. [Google Scholar] [CrossRef]
- Romanazzi, G.; Gabler, F.M.; Margosan, D.; Mackey, B.E.; Smilanick, J.L. Effect of Chitosan Dissolved in Different Acids on Its Ability to Control Postharvest Gray Mold of Table Grape. Phytopathology 2009, 99, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Esua, O.J.; Chin, N.L.; Yusof, Y.A.; Sukor, R. Effects of simultaneous UV-C radiation and ultrasonic energy postharvest treatment on bioactive compounds and antioxidant activity of tomatoes during storage. Food Chem. 2019, 270, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-R.; Cho, H.-S.; Cho, Y.-S.; Kim, D.-O. Changes in phenolics, soluble solids, vitamin C, and antioxidant capacity of various cultivars of hardy kiwifruits during cold storage. Food Sci. Biotechnol. 2020, 29, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, S.L.; Charles, M.T.; Gariepy, Y.; Goyette, B.; Raghavan, G.S.V.; Vigneault, C. Influence of Postharvest UV-C Hormesis on the Bioactive Components of Tomato during Post-treatment Handling. Food Bioprocess Technol. 2009, 4, 1463–1472. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Changes in major antioxidant components of tomatoes during post-harvest storage. Food Chem. 2006, 99, 724–727. [Google Scholar] [CrossRef]
- Kalt, W. Effects of Production and Processing Factors on Major Fruit and Vegetable Antioxidants. J. Food Sci. 2005, 70, R11–R19. [Google Scholar] [CrossRef]
- Eissa, H.A.; Hussein, A.S.; Mostafa, B.E. Rheological properties and quality evaluation on Egyptian balady bread and biscuits supplemented with flours of ungerminated and germinated legume seeds or mushroom. Pol. J. Food Nutr. Sci. 2007, 57, 487–496. [Google Scholar]
- Covas, M.-I. Bioactive effects of olive oil phenolic compounds in humans: Reduction of heart disease factors and oxidative damage. Inflammopharmacology 2008, 16, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.T.; Goulet, A.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit: IV. Biochemical modification of structural barriers. Postharvest Biol. Technol. 2008, 47, 41–53. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible Films from Carrageenan/Orange Essential Oil/Trehalose—Structure, Optical Properties, and Antimicrobial Activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef]
- Abad, J.; Valencia-Chamorro, S.; Castro, A.; Vasco, C. Studying the effect of combining two nonconventional treatments, gamma irradiation and the application of an edible coating, on the postharvest quality of tamarillo (Solanum betaceum Cav.) fruits. Food Control 2017, 72, 319–323. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razali, Z.; Somasundram, C.; Nurulain, S.Z.; Kunasekaran, W.; Alias, M.R. Postharvest Quality of Cherry Tomatoes Coated with Mucilage from Dragon Fruit and Irradiated with UV-C. Polymers 2021, 13, 2919. https://doi.org/10.3390/polym13172919
Razali Z, Somasundram C, Nurulain SZ, Kunasekaran W, Alias MR. Postharvest Quality of Cherry Tomatoes Coated with Mucilage from Dragon Fruit and Irradiated with UV-C. Polymers. 2021; 13(17):2919. https://doi.org/10.3390/polym13172919
Chicago/Turabian StyleRazali, Zuliana, Chandran Somasundram, Siti Zalifah Nurulain, Wijenthiran Kunasekaran, and Matthew Raj Alias. 2021. "Postharvest Quality of Cherry Tomatoes Coated with Mucilage from Dragon Fruit and Irradiated with UV-C" Polymers 13, no. 17: 2919. https://doi.org/10.3390/polym13172919