Capsule Independent Antimicrobial Activity Induced by Nanochitosan against Streptococcus pneumoniae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNPs
2.3. Characterization of Formulated NPs
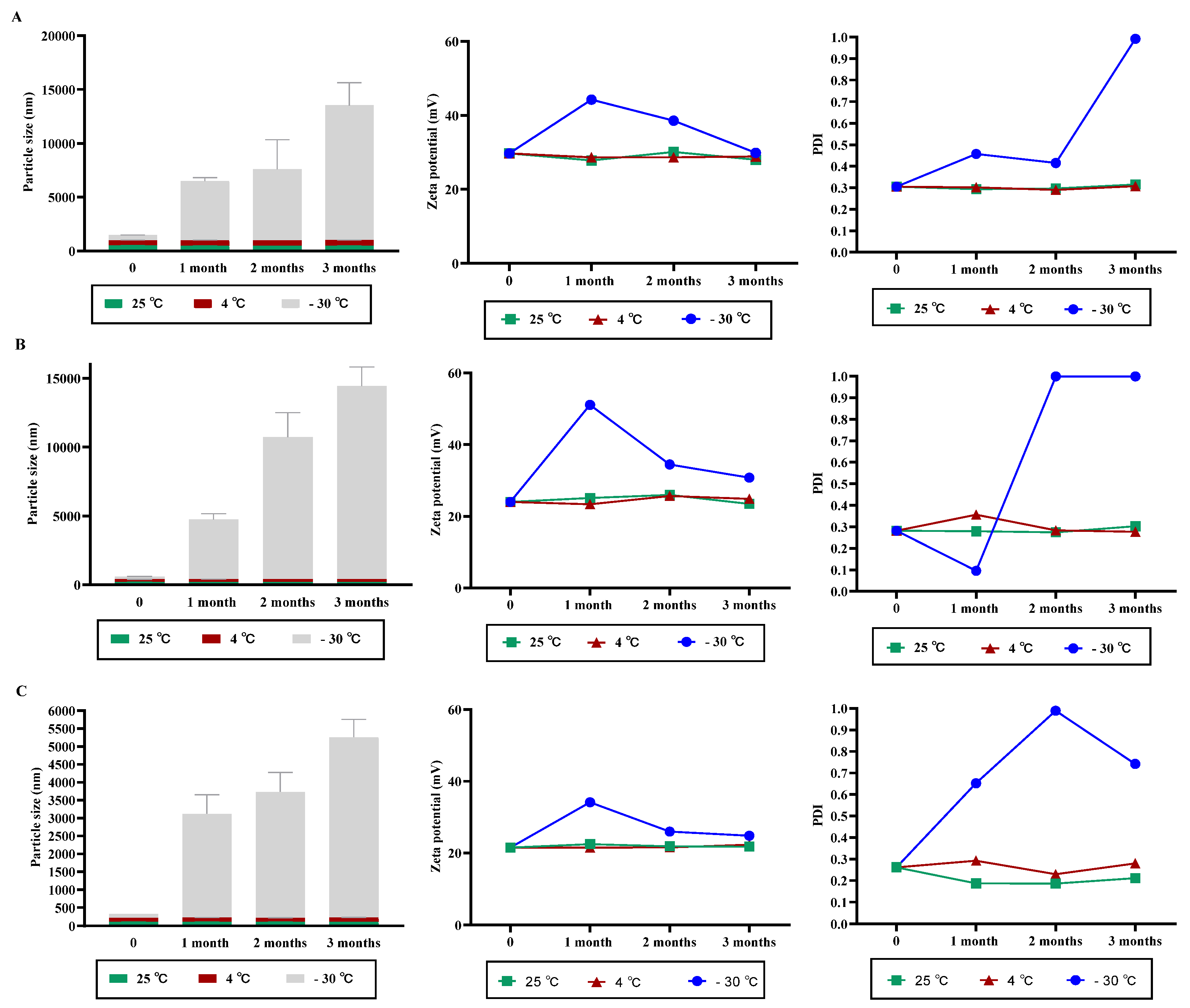
2.4. Stability Studies
2.5. Bacterial Strains and Growth Conditions
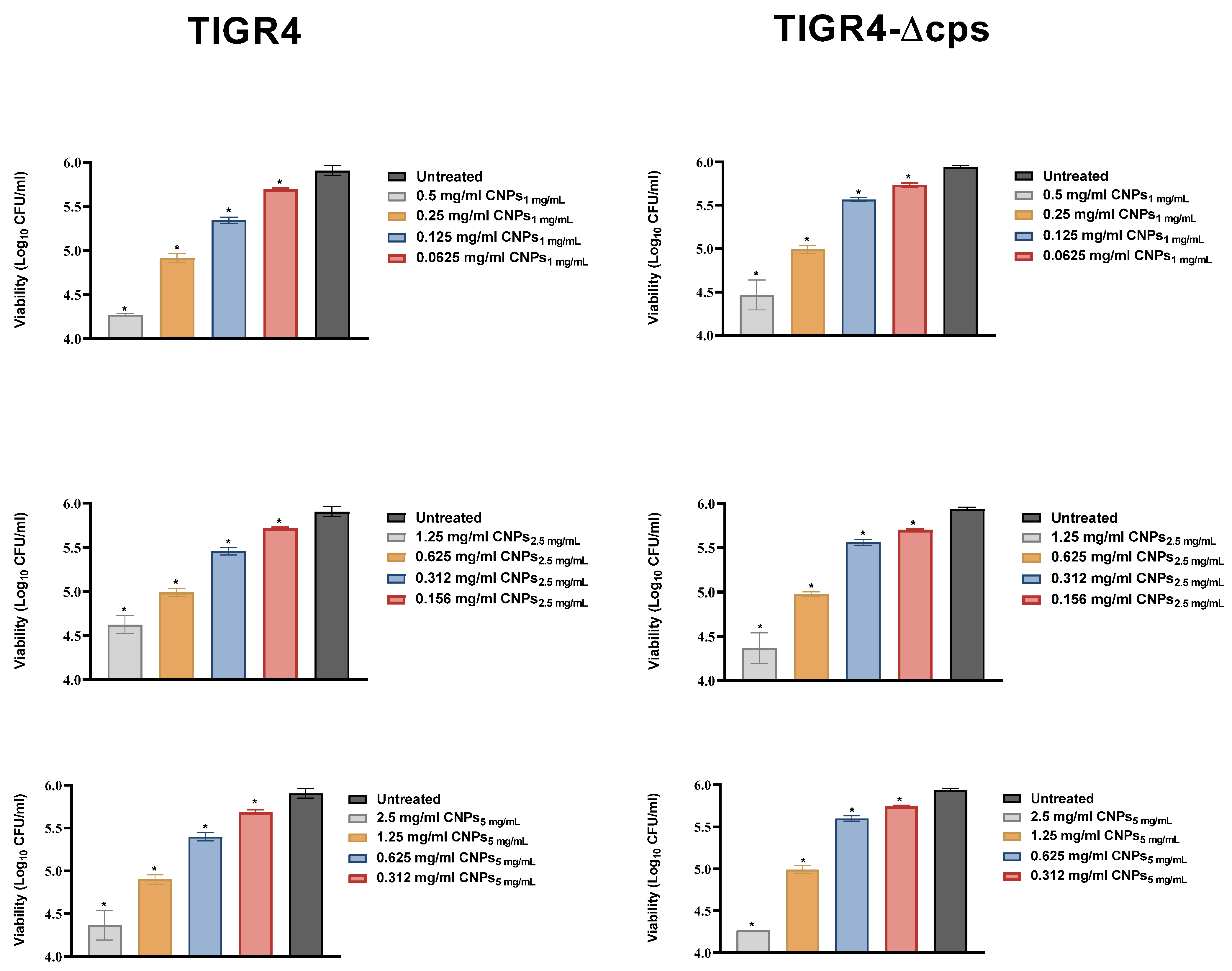
2.6. Antimicrobial Assay
2.7. Hemolytic Activity Assay
2.8. Cytotoxicity Assay
2.9. Statistical Analysis
3. Results
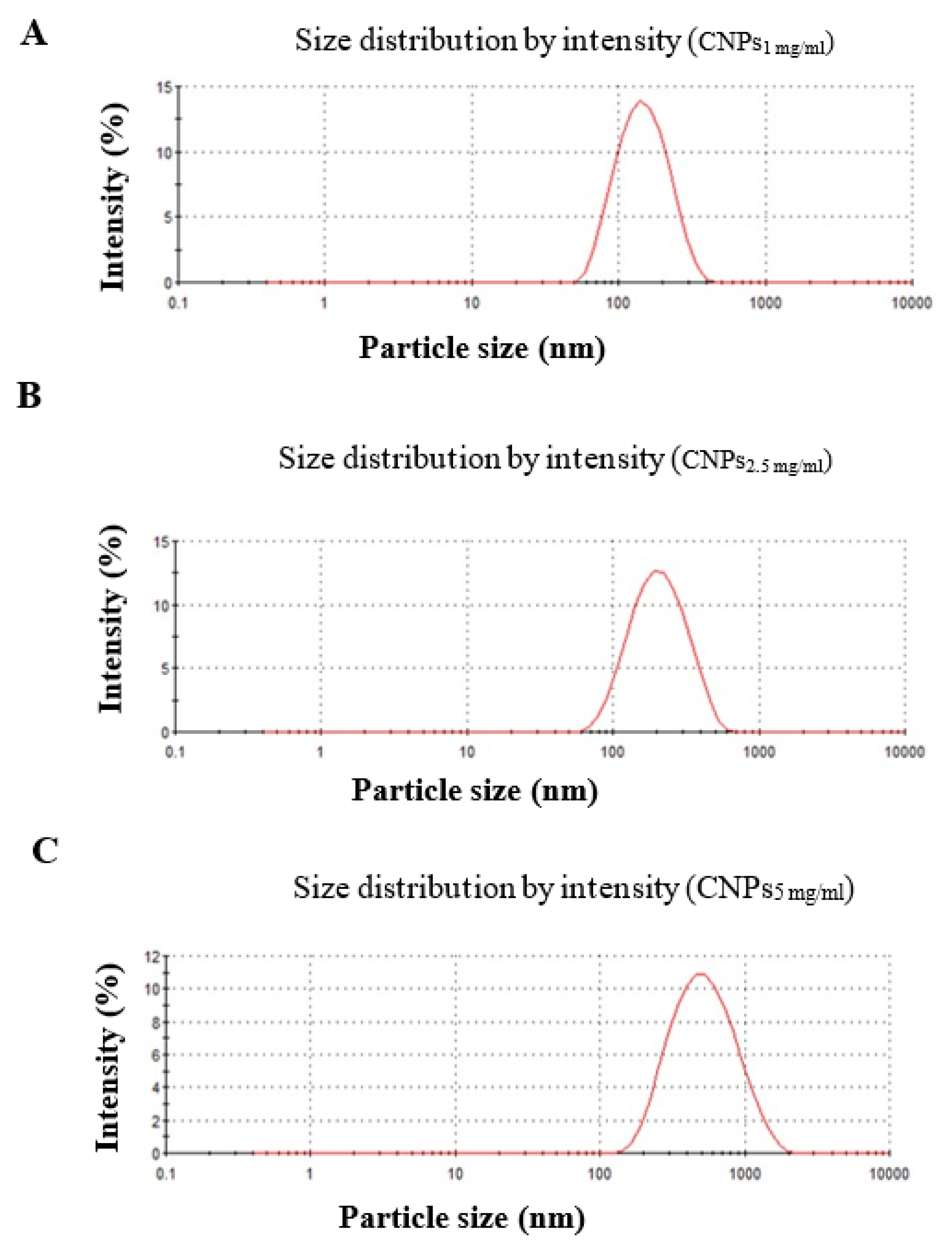
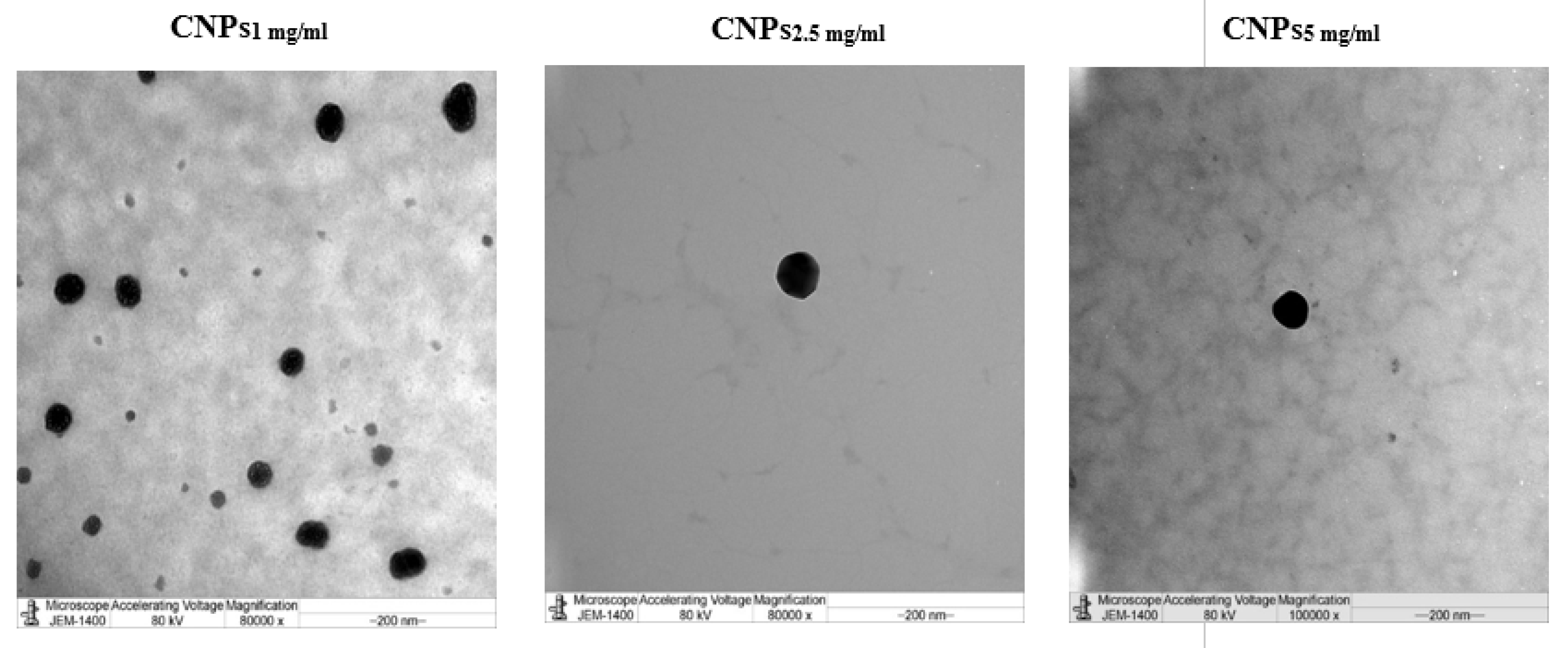
3.1. Characterization of Nanochitosan
3.2. NPs Stability under Variable Storage Condition
3.3. Antimicrobial Activity of CNPs
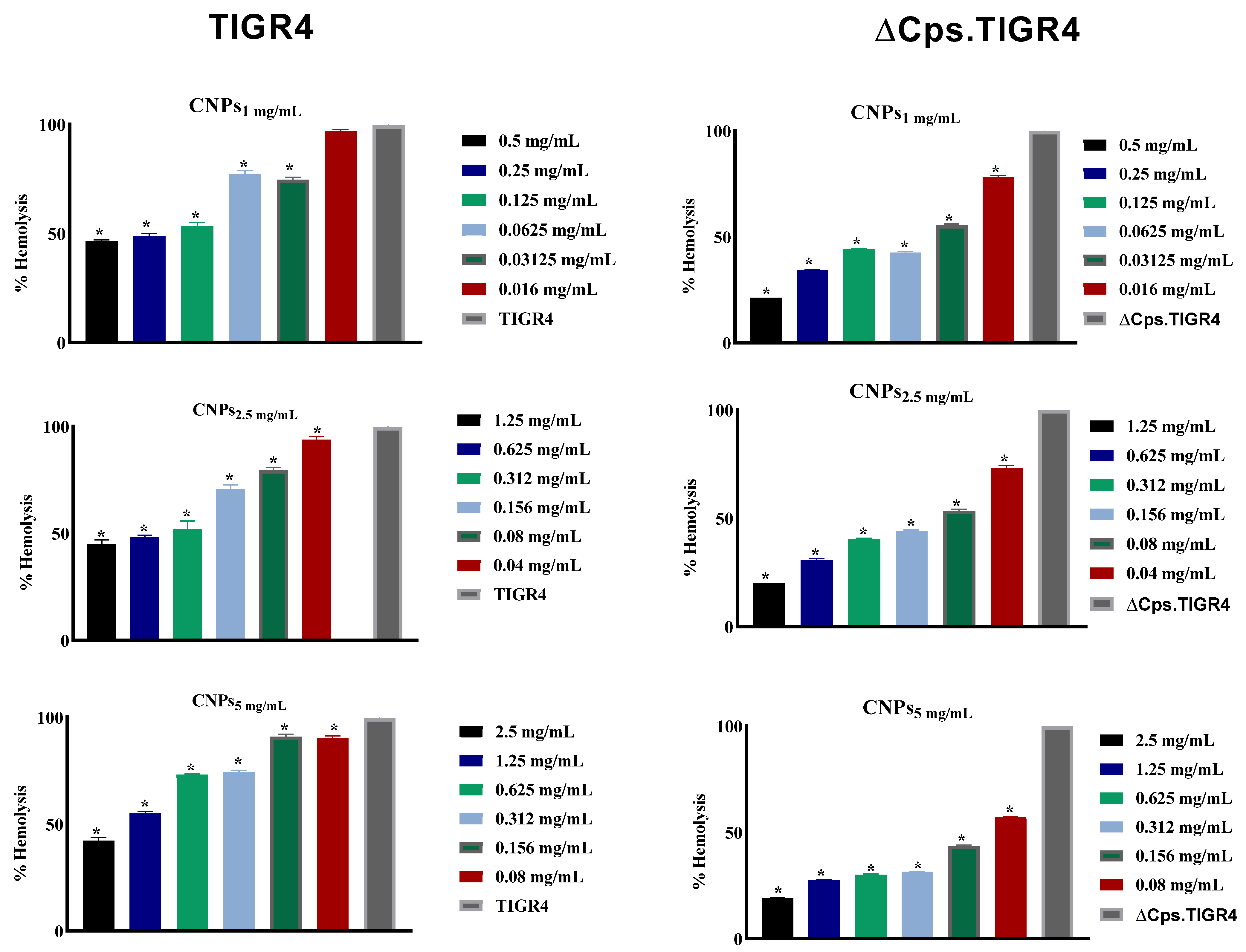
3.4. The CNPs Reduced Pneumococcal Hemolysis Activity
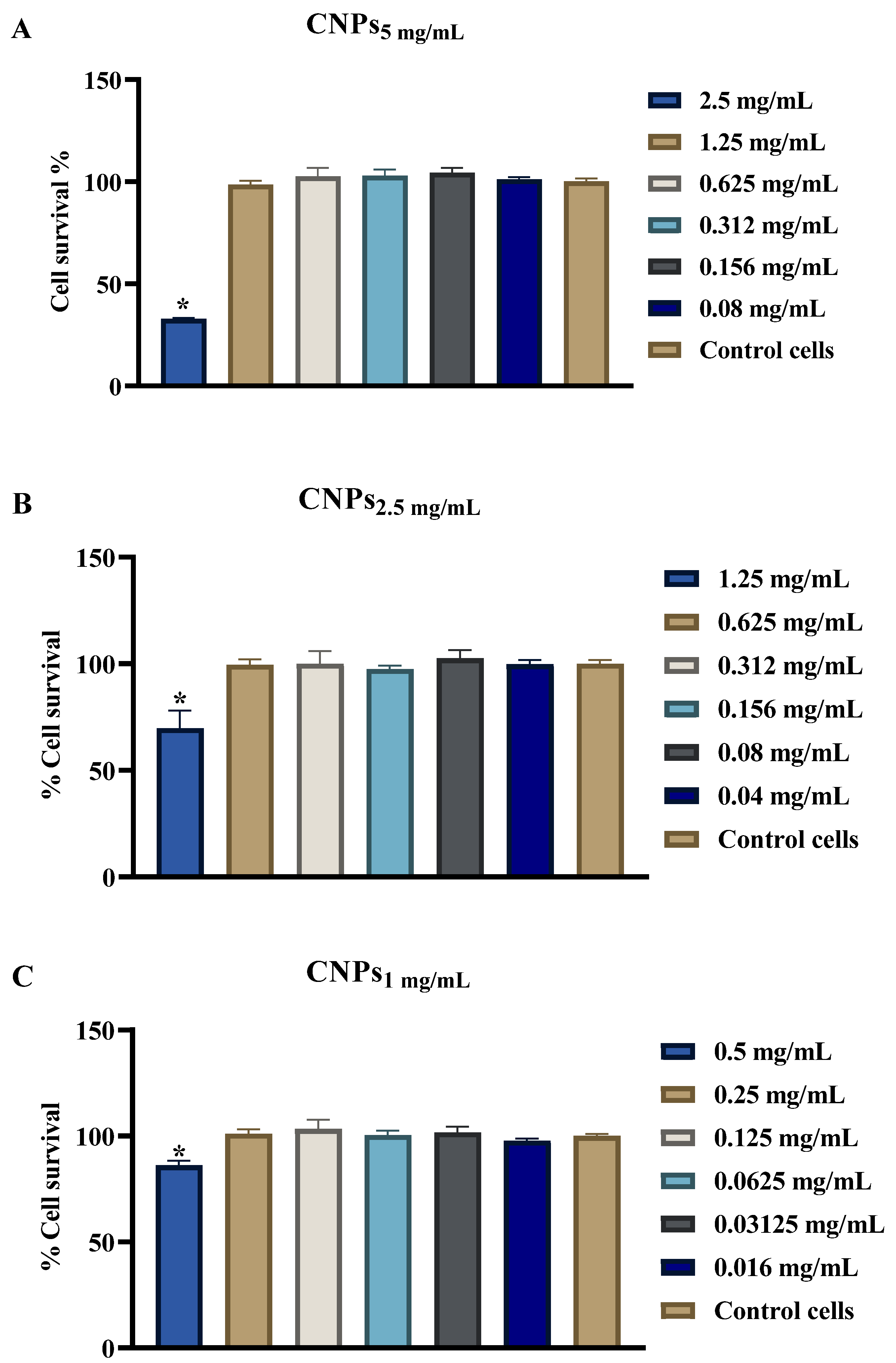
3.5. Evaluation of CNPs Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bogaert, D.; De Groot, R.; Hermans, P.W. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Appelbaum, P.C. Resistance among Streptococcus pneumoniae: Implications for drug selection. Clin. Infect. Dis. 2002, 34, 1613–1620. [Google Scholar] [CrossRef] [Green Version]
- WHO. Antimicrobial Resistance: Global Report on Surveillance 2014; Antimicrobial Resistance Global Surveillance Report; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panja, S.; Bharti, R.; Dey, G.; Lynd, N.A.; Chattopadhyay, S. Coordination-assisted self-assembled polypeptide nanogels to selectively combat bacterial infection. ACS Appl. Mater. Interfaces 2019, 11, 33599–33611. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial activity of chitosan nanoparticles: A review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.B.; Lopez-Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Shrimp-Derived Chitosan GRAS Notification. 2012. Available online: www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000443pdf (accessed on 10 August 2021).
- Goy, R.C.; Britto, D.D.; Assis, O.B. A Review of the Antimicrobial Activity of Chitosan. Ciênc. Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- de Campos, A.M.; Diebold, Y.; Carvalho, E.L.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles as new ocular drug delivery systems: In vitro stability, in vivo fate, and cellular toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Wardani, G.; Sudjarwo, S.A. In vitro Antibacterial Activity of Chitosan Nanoparticles against Mycobacterium tuberculosis. Pharmacogn. J. 2018, 10, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Aleanizy, F.S.; Alqahtani, F.Y.; Shazly, G.; Alfaraj, R.; Alsarra, I.; Alshamsan, A.; Abdulhady, H.G. Measurement and evaluation of the effects of pH gradients on the antimicrobial and antivirulence activities of chitosan nanoparticles in Pseudomonas aeruginosa. Saudi Pharm. J. 2018, 26, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Chavez de Paz, L.E.; Resin, A.; Howard, K.A.; Sutherland, D.S.; Wejse, P.L. Antimicrobial effect of chitosan nanoparticles on streptococcus mutans biofilms. Appl. Environ. Microbiol. 2011, 77, 3892–3895. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, F.; Aleanizy, F.; El Tahir, E.; Alhabib, H.; Alsaif, R.; Shazly, G.; AlQahtani, H.; Alsarra, I.; Mahdavi, J. Antibacterial Activity of Chitosan Nanoparticles Against Pathogenic N. gonorrhoea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef]
- Tettelin, H.; Nelson, K.E.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Peterson, S.; Heidelberg, J.; DeBoy, R.T.; Haft, D.H.; Dodson, R.J.; et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 2001, 293, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Thamadilok, S.; Roche-Håkansson, H.; Håkansson, A.P.; Ruhl, S. Absence of capsule reveals glycan-mediated binding and recognition of salivary mucin MUC7 by Streptococcus pneumoniae. Mol. Oral Microbiol. 2016, 31, 175–188. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Supplement M100; Weinstein, M.P., Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Yang, Y.; Lin, J.; Harrington, A.; Cornilescu, G.; Lau, G.W.; Tal-Gan, Y. Designing cyclic competence-stimulating peptide (CSP) analogs with pan-group quorum-sensing inhibition activity in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 2020, 117, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- AbuMousa, R.A.; Baig, U.; Gondal, M.A.; AlSalhi, M.S.; Alqahtani, F.Y.; Akhtar, S.; Aleanizy, F.S.; Dastageer, M.A. Photo-catalytic killing of HeLa cancer cells using facile synthesized pure and Ag loaded WO3 nanoparticles. Sci. Rep. 2018, 8, 15224. [Google Scholar] [CrossRef] [Green Version]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef]
- Jeon, S.J.; Oh, M.; Yeo, W.S.; Galvao, K.N.; Jeong, K.C. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS ONE 2014, 9, e92723. [Google Scholar] [CrossRef] [PubMed]
- Magee, A.D.; Yother, J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 2001, 69, 3755–3761. [Google Scholar] [CrossRef] [Green Version]
- Sanders, M.E.; Norcross, E.W.; Robertson, Z.M.; Moore, Q.C.; Fratkin, J.; Marquart, M.E. The Streptococcus pneumoniae capsule is required for full virulence in pneumococcal endophthalmitis. Invest. Ophthalmol. Vis. Sci. 2011, 52, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyams, C.; Camberlein, E.; Cohen, J.M.; Bax, K.; Brown, J.S. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 2010, 78, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Hathaway, L.J.; Brugger, S.D.; Morand, B.; Bangert, M.; Rotzetter, J.U.; Hauser, C.; Graber, W.A.; Gore, S.; Kadioglu, A.; Mühlemann, K. Capsule type of Streptococcus pneumoniae determines growth phenotype. PLoS Pathog. 2012, 8, e1002574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyres, K.L.; Lambertsen, L.M.; Croucher, N.J.; McGee, L.; Von Gottberg, A.; Liñares, J.; Jacobs, M.R.; Kristinsson, K.G.; Beall, B.W.; Klugman, K.P.; et al. Pneumococcal capsular switching: A historical perspective. J. Infect. Dis. 2013, 207, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| S. pneumonia Strains | Description | References |
|---|---|---|
| TIGR4 | S. pneumoniae serotype 4 clinical isolate | [20] |
| TIGR4-∆cps | Capsule-free TIGR4, ErmR | [21] |
| Formulation | Size (nm ± SD) | PDI ± SD | ζ-Potential (mV ± SD) |
|---|---|---|---|
| CNPs1 mg/ml | 133.3 ± 0.57 | 0.159 ± 0.006 | 19 ± 0.115 |
| CNPs2.5 mg/ml | 177 ± 2.85 | 0.235 ± 0.011 | 24.7 ± 1.06 |
| CNPs5 mg/ml | 423 ± 12.93 | 0.281 ± 0.014 | 27 ± 0.819 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alowais, H.; Binkelaib, A.; Alwathlan, B.; Al-Bdrawy, A.; Håkansson, A.P.; Alsarra, I. Capsule Independent Antimicrobial Activity Induced by Nanochitosan against Streptococcus pneumoniae. Polymers 2021, 13, 2924. https://doi.org/10.3390/polym13172924
Alqahtani FY, Aleanizy FS, El Tahir E, Alowais H, Binkelaib A, Alwathlan B, Al-Bdrawy A, Håkansson AP, Alsarra I. Capsule Independent Antimicrobial Activity Induced by Nanochitosan against Streptococcus pneumoniae. Polymers. 2021; 13(17):2924. https://doi.org/10.3390/polym13172924
Chicago/Turabian StyleAlqahtani, Fulwah Y., Fadilah S. Aleanizy, Eram El Tahir, Hessa Alowais, Assalh Binkelaib, Bdour Alwathlan, Asmaa Al-Bdrawy, Anders P. Håkansson, and Ibrahim Alsarra. 2021. "Capsule Independent Antimicrobial Activity Induced by Nanochitosan against Streptococcus pneumoniae" Polymers 13, no. 17: 2924. https://doi.org/10.3390/polym13172924
APA StyleAlqahtani, F. Y., Aleanizy, F. S., El Tahir, E., Alowais, H., Binkelaib, A., Alwathlan, B., Al-Bdrawy, A., Håkansson, A. P., & Alsarra, I. (2021). Capsule Independent Antimicrobial Activity Induced by Nanochitosan against Streptococcus pneumoniae. Polymers, 13(17), 2924. https://doi.org/10.3390/polym13172924







