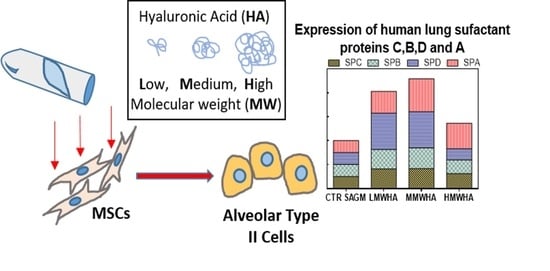
Effect of Hyaluronic Acid on the Differentiation of Mesenchymal Stem Cells into Mature Type II Pneumocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hyaluronic Acid Preparation
2.3. Cell Culture
2.4. Cell Viability Test
2.5. Viscosity Characterization
2.6. ATII Differentiation Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of HA Solution
3.2. Pneumocyetes Differentiation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- White, E.S. Lung extracellular matrix and fibroblast function. Ann. Am. Thorac. Soc. 2015, 12, S30–S33. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Jiang, D.; Noble, P.W. Hyaluronan as a therapeutic target in human diseases. Adv. Drug Deliv. Rev. 2016, 97, 186–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Máiz Carro, L.; Martínez-García, M.A. Use of Hyaluronic Acid (HA) in Chronic Airway Diseases. Cells 2020, 9, 2210. [Google Scholar] [CrossRef] [PubMed]
- Allegra, L.; Patrona, S.D.; Petrigni, G. Hyaluronic Acid. In Heparin—A Century of Progress; Lever, R., Mulloy, B., Page, C.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 385–401. [Google Scholar] [CrossRef]
- Turino, G.M.; Cantor, J.O. Hyaluronan in respiratory injury and repair. Am. J. Respir. Crit. Care Med. 2003, 167, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Umbrello, M.; Formenti, P.; Bolgiaghi, L.; Chiumello, D. Current concepts of ARDS: A narrative review. Int. J. Mol. Sci. 2017, 18, 64. [Google Scholar] [CrossRef] [Green Version]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Weiss, D.J. Cell-based Therapy for Chronic Obstructive Pulmonary Disease. Rebuilding the Lung. Ann. Am. Thorac. Soc. 2018, 15, S253–S259. [Google Scholar] [CrossRef]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary dysplasia: Chronic lung disease of infancy and long-term pulmonary outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Marsilio, E.; Goldstein, R.H.; Yannas, I.V.; Spector, M. Formation of lung alveolar-like structures in collagen–glycosaminoglycan scaffolds in vitro. Tissue Eng. 2005, 11, 1436–1448. [Google Scholar] [CrossRef]
- Crapo, J.D.; Barry, B.E.; Gehr, P.; Bachofen, M.; Weibel, E.R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 1982, 126, 332–337. [Google Scholar] [PubMed]
- Weibel, E.R. On the tricks alveolar epithelial cells play to make a good lung. Am. J. Respir. Crit. Care Med. 2015, 191, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Hogan, B.L.; Barkauskas, C.E.; Chapman, H.A.; Epstein, J.A.; Jain, R.; Hsia, C.C.; Niklason, L.; Calle, E.; Le, A.; Randell, S.H. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 2014, 15, 123–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Gai, H.; Mei, J.; Ding, F.B.; Bao, C.R.; Nguyen, D.M.; Zhong, H. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol. Int. 2011, 35, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Geiger, S.; Hirsch, D.; Hermann, F.G. Cell therapy for lung disease. Eur. Respir. Rev. 2017, 26, 170044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapudom, J.; Ullm, F.; Martin, S.; Kalbitzer, L.; Naab, J.; Möller, S.; Schnabelrauch, M.; Anderegg, U.; Schmidt, S.; Pompe, T. Molecular weight specific impact of soluble and immobilized hyaluronan on CD44 expressing melanoma cells in 3D collagen matrices. Acta Biomater. 2017, 50, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.; Adams, S.; Tigges, B.; Sprague, S.; Wang, X.; Collins, D.; McKenna, D.H. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy 2006, 8, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size matters: Molecular weight specificity of hyaluronan effects in cell biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Chisti, Y. Hydrodynamic damage to animal cells. Crit. Rev. Biotechnol. 2001, 21, 67–110. [Google Scholar] [CrossRef]
- Maltese, A.; Borzacchiello, A.; Mayol, L.; Bucolo, C.; Maugeri, F.; Nicolais, L.; Ambrosio, L. Novel polysaccharides-based viscoelastic formulations for ophthalmic surgery: Rheological characterization. Biomaterials 2006, 27, 5134–5142. [Google Scholar] [CrossRef]
- Johansson, J. Structure and properties of surfactant protein C. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1998, 1408, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Sehlmeyer, K.; Ruwisch, J.; Roldan, N.; Lopez-Rodriguez, E. Alveolar dynamics and beyond–the importance of surfactant protein c and cholesterol in lung homeostasis and fibrosis. Front. Physiol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Victor, I.A.; Andem, A.B.; Archibong, I.A.; Iwok, E.O. Interplay between Cell Proliferation and Cellular Differentiation: A mutually exclusive paradigm. GSJ 2020, 8, 1328–1338. [Google Scholar]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [Green Version]
- Agassandian, M.; Mallampalli, R.K. Surfactant phospholipid metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 612–625. [Google Scholar] [CrossRef] [Green Version]
- Beers, M.F.; Moodley, Y. When is an alveolar type 2 cell an alveolar type 2 cell? A conundrum for lung stem cell biology and regenerative medicine. Am. J. Respir. Cell Mol. Biol. 2017, 57, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.; Phelps, C.; Sheehan, J. The conformation of the mucopolysaccharides. Hyaluronates. Biochem. J. 1972, 128, 1255–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Rodriguez, E.; Pérez-Gil, J. Structure-function relationships in pulmonary surfactant membranes: From biophysics to therapy. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1568–1585. [Google Scholar] [CrossRef] [Green Version]
- Borzacchiello, A.; Mayol, L.; Ramires, P.A.; Pastorello, A.; Di Bartolo, C.; Ambrosio, L.; Milella, E. Structural and rheological characterization of hyaluronic acid-based scaffolds for adipose tissue engineering. Biomaterials 2007, 28, 4399–4408. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Bray, B.A. The role of hyaluronan in the pulmonary alveolus. J. Theor. Biol. 2001, 210, 121–130. [Google Scholar] [CrossRef]
- Della Sala, F.; Biondi, M.; Guarnieri, D.; Borzacchiello, A.; Ambrosio, L.; Mayol, L. Mechanical behavior of bioactive poly (ethylene glycol) diacrylate matrices for biomedical application. J. Mech. Behav. Biomed. Mater. 2020, 110, 103885. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, L.G. Isolation and culture of alveolar type II cells. Am. J. Physiol.—Lung Cell. Mol. Physiol. 1990, 258, L134–L147. [Google Scholar] [CrossRef]
- Schmidt, J.; Pilbauerova, N.; Soukup, T.; Suchankova-Kleplova, T.; Suchanek, J. Low Molecular Weight Hyaluronic Acid Effect on Dental Pulp Stem Cells In Vitro. Biomolecules 2020, 11, 22. [Google Scholar] [CrossRef]
- Agarwal, S.; Duffy, B.; Curtin, J.; Jaiswal, S. Effect of high-and low-molecular-weight hyaluronic-acid-functionalized-AZ31 Mg and Ti alloys on proliferation and differentiation of osteoblast cells. ACS Biomater. Sci. Eng. 2018, 4, 3874–3884. [Google Scholar] [CrossRef]
- Sato, E.; Ando, T.; Ichikawa, J.; Okita, G.; Sato, N.; Wako, M.; Ohba, T.; Ochiai, S.; Hagino, T.; Jacobson, R.; et al. High molecular weight hyaluronic acid increases the differentiation potential of the murine chondrocytic ATDC5 cell line. J. Orthop. Res. 2014, 32, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R.; Camprubí-Rimblas, M.; Puig, F.; Herrero, R.; Tantinyà, N.; Serrano-Mollar, A.; Artigas, A. Alveolar Type II Cells or Mesenchymal Stem Cells: Comparison of Two Different Cell Therapies for the Treatment of Acute Lung Injury in Rats. Cells 2020, 9, 1816. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.T.; Medina, C.; Kasper, M.; Ehrhardt, C. Interplay between RAGE, CD44, and focal adhesion molecules in epithelial-mesenchymal transition of alveolar epithelial cells. Am. J. Physiol.–Lung Cell. Mol. Physiol. 2011, 300, L548–L559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louderbough, J.M.; Schroeder, J.A. Understanding the dual nature of CD44 in breast cancer progression. Mol. Cancer Res. 2011, 9, 1573–1586. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Sala, F.; di Gennaro, M.; Lista, G.; Messina, F.; Ambrosio, L.; Borzacchiello, A. Effect of Hyaluronic Acid on the Differentiation of Mesenchymal Stem Cells into Mature Type II Pneumocytes. Polymers 2021, 13, 2928. https://doi.org/10.3390/polym13172928
Della Sala F, di Gennaro M, Lista G, Messina F, Ambrosio L, Borzacchiello A. Effect of Hyaluronic Acid on the Differentiation of Mesenchymal Stem Cells into Mature Type II Pneumocytes. Polymers. 2021; 13(17):2928. https://doi.org/10.3390/polym13172928
Chicago/Turabian StyleDella Sala, Francesca, Mario di Gennaro, Gianluca Lista, Francesco Messina, Luigi Ambrosio, and Assunta Borzacchiello. 2021. "Effect of Hyaluronic Acid on the Differentiation of Mesenchymal Stem Cells into Mature Type II Pneumocytes" Polymers 13, no. 17: 2928. https://doi.org/10.3390/polym13172928
APA StyleDella Sala, F., di Gennaro, M., Lista, G., Messina, F., Ambrosio, L., & Borzacchiello, A. (2021). Effect of Hyaluronic Acid on the Differentiation of Mesenchymal Stem Cells into Mature Type II Pneumocytes. Polymers, 13(17), 2928. https://doi.org/10.3390/polym13172928