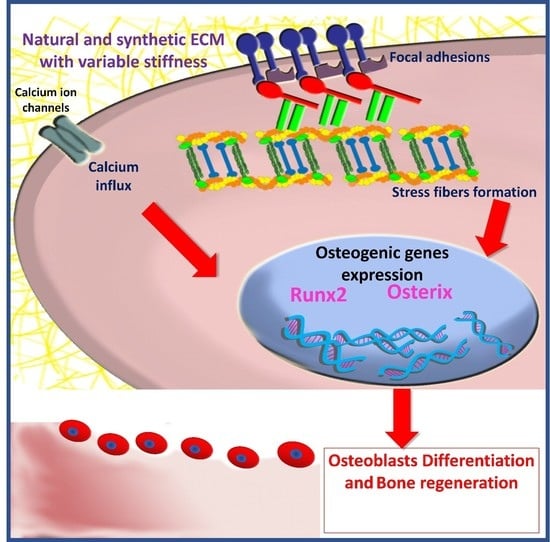
Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review
Abstract
1. Introduction
2. MSCs and Mechanotransduction
2.1. Focal Adhesion and Integrins
2.2. Cytoskeleton Elements
2.3. Mechanosensitive Ion Channels
2.4. MSCs’ Aging and Mechanosensitivity
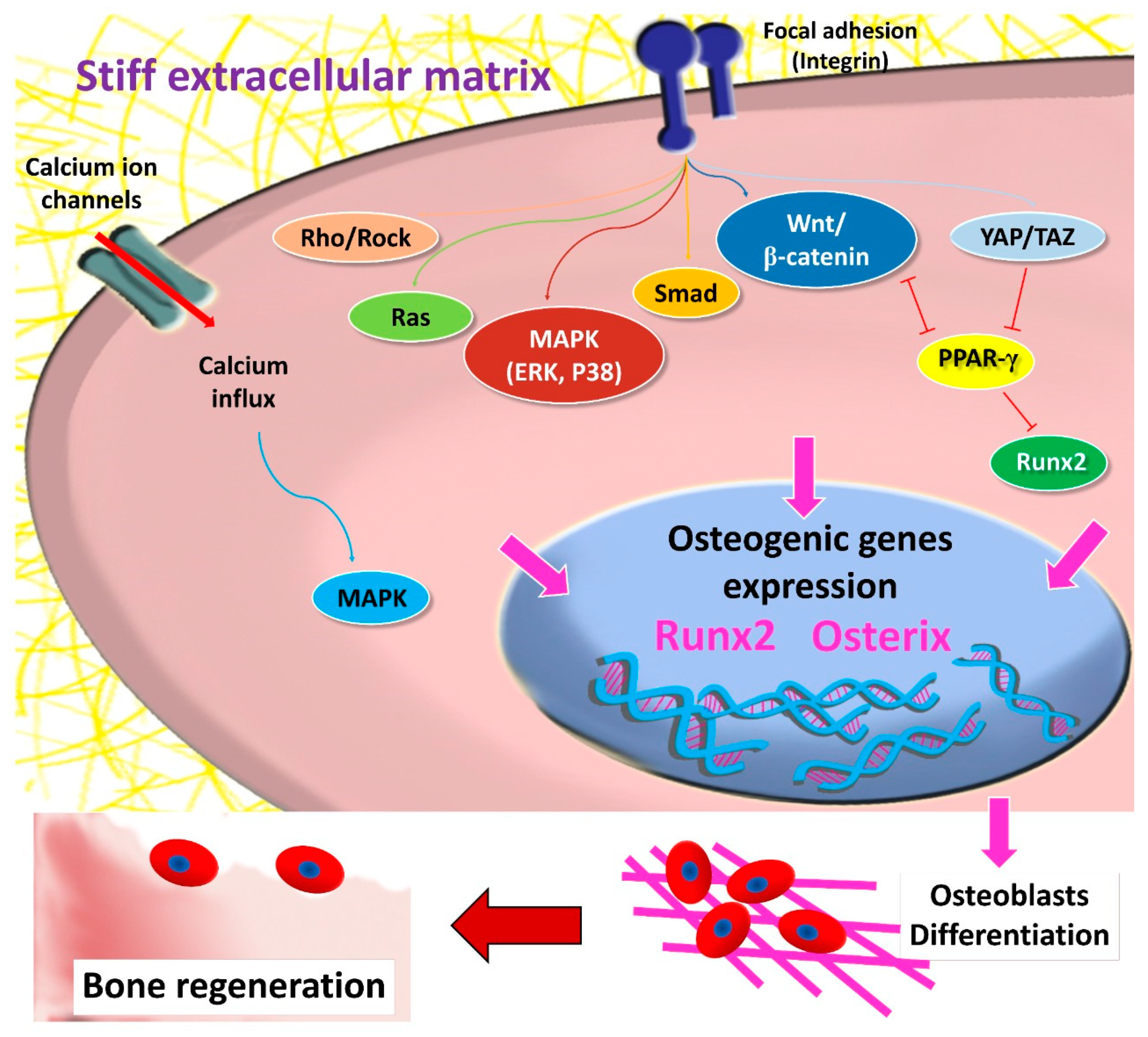
3. The Role of Matrix Stiffness in Triggering MSCs’ Osteogenic Differentiation
4. Matrix-Dependent MSCs’ Osteogenic Differentiation
4.1. Natural Polymers
4.1.1. Alginate
4.1.2. Collagen
4.1.3. Gelatin
4.1.4. Decellularized Matrix and Demineralized Bone
4.1.5. Hyaluronic Acid
4.1.6. Fibrin
4.2. Synthetic Polymers
4.2.1. Polyethylene Glycol
4.2.2. Polydimethylsiloxane
4.2.3. Vinyl Polymers
4.2.4. Polyesters
4.2.5. Polyacrylamide
4.2.6. Self-Assembling Peptides
4.2.7. Other Polymers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ADSCs | Adipose derived stromal cells |
| ALP | Alkaline phosphatase |
| ASCs | adipose derived stromal cells |
| BMMSCs | Bone marrow mesenchymal stem cells |
| BMP-2 | Bone morphogenetic protein 2 |
| dCDMs | Decellularized cell derived matrix |
| DFCs | Dental follicle stem cells |
| ECM | Extracellular matrix |
| EDAC | 1-ethyl-3-(3-dimethylami-nopropyl) carbodiimide |
| GC-TRS | Glycol chitin-based thermo-responsive hydrogel scaffold |
| GelMA | Gelatin with variable degrees of methacrylation |
| HA | Hyaluronic acid |
| HA | Hydroxyapatite |
| hADSCs | Human adipose derived mesenchymal stem cells |
| hBMMSCs | Human Bone marrow mesenchymal stem cells |
| hMSCs | Human mesenchymal stem cells |
| MSCs | Mesenchymal stem cells |
| NHS | N-hydroxysuccinimide |
| OCN | Osteocalcin |
| ON | Osteonectin |
| OPN | osteopontin |
| PCL | poly (ε-caprolactone) |
| PDLSCs | periodontal ligament stem cells |
| PDMS | Polydimethylsiloxane |
| PEEU | Poly (ether-ester-urethane) |
| PEG | Polyethylene glycol |
| PEGDA | Polyethylene glycol diacrylate |
| PEGMC | Polyethylene glycol-maleate-citrate |
| PPDOP | poly (ρ-dioxanone) |
| PV | polyvinyl |
| PVA | polyvinyl alcohol |
| qRT-PCR | Quantitative Reverse transcription real-time polymerase chain reaction |
| RGD | Arginine-glycine-aspartic acid |
| Runx2 | Runt-related transcription factor-2 |
| SCAP | Stem cells of the apical papilla |
| SDF-1α | Stromal derived factor-1alpha |
| SHED | Stem cells isolated from human exfoliated deciduous teeth |
References
- Singh, V.K.; Saini, A.; Kalsan, M.; Kumar, N.; Chandra, R. Describing the Stem Cell Potency: The Various Methods of Functional Assessment and In silico Diagnostics. Front. Cell Dev. Biol. 2016, 4, 134. [Google Scholar] [CrossRef]
- El-Sayed, K.M.; Paris, S.; Graetz, C.; Kassem, N.; Mekhemar, M.; Ungefroren, H.; Fandrich, F.; Dorfer, C. Isolation and characterisation of human gingival margin-derived STRO-1/MACS(+) and MACS(-) cell populations. Int. J. Oral Sci. 2015, 7, 80–88. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Ahmed, G.M.; Abouauf, E.A.; Schwendicke, F. Stem/progenitor cell-mediated pulpal tissue regeneration: A systematic review and meta-analysis. Int. Endod. J. 2019, 52, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dorfer, C.; Fandrich, F.; Gieseler, F.; Moustafa, M.H.; Ungefroren, H. Adult mesenchymal stem cells explored in the dental field. Adv. Biochem. Eng. Biotechnol. 2013, 130, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dorfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem. Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Elahmady, M.; Adawi, Z.; Aboushadi, N.; Elnaggar, A.; Eid, M.; Hamdy, N.; Sanaa, D.; Dorfer, C.E. The periodontal stem/progenitor cell inflammatory-regenerative cross talk: A new perspective. J. Periodontal Res. 2019, 54, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Elsalawy, R.; Ibrahim, N.; Gadalla, M.; Albargasy, H.; Zahra, N.; Mokhtar, S.; El Nahhas, N.; El Kaliouby, Y.; Dorfer, C.E. The Dental Pulp Stem/Progenitor Cells-Mediated Inflammatory-Regenerative Axis. Tissue Eng. Part B Rev. 2019, 25, 445–460. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Jakusz, K.; Jochens, A.; Dorfer, C.; Schwendicke, F. Stem Cell Transplantation for Pulpal Regeneration: A Systematic Review. Tissue Eng. Part B Rev. 2015, 21, 451–460. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Mekhemar, M.K.; Beck-Broichsitter, B.E.; Bahr, T.; Hegab, M.; Receveur, J.; Heneweer, C.; Becker, S.T.; Wiltfang, J.; Dorfer, C.E. Periodontal regeneration employing gingival margin-derived stem/progenitor cells in conjunction with IL-1ra-hydrogel synthetic extracellular matrix. J. Clin. Periodontol. 2015, 42, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Paris, S.; Becker, S.T.; Neuschl, M.; De Buhr, W.; Sälzer, S.; Wulff, A.; Elrefai, M.; Darhous, M.S.; El-Masry, M.; et al. Periodontal regeneration employing gingival margin-derived stem/progenitor cells: An animal study. J. Clin. Periodontol. 2012, 39, 861–870. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar] [CrossRef]
- Zhou, L.L.; Liu, W.; Wu, Y.M.; Sun, W.L.; Dorfer, C.E.; Fawzy El-Sayed, K.M. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem. Cells Int. 2020, 2020, 1327405. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Shah, J.; Srivastava, A.S. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem. Cells Int. 2013, 2013, 496218. [Google Scholar] [CrossRef]
- Via, A.G.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar]
- Lee, O.K.; Kuo, T.K.; Chen, W.-M.; Lee, K.-D.; Hsieh, S.-L.; Chen, T.-H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; Bueno, C.; Insausti, C.L.; Meseguer, L.; Ramírez, M.C.; Blanquer, M.; Marín, N.; Martínez, S.; Moraleda, J.M. Mesenchymal stem cells derived from dental tissues. Int. Endod. J. 2011, 44, 800–806. [Google Scholar] [CrossRef]
- Jorgenson, K.D.; Hart, D.A.; Krawetz, R.; Sen, A. Production of Adult Human Synovial Fluid-Derived Mesenchymal Stem Cells in Stirred-Suspension Culture. Stem. Cells Int. 2018, 2018, 8431053. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Assis-Ribas, T.; Forni, M.F.; Winnischofer, S.M.B.; Sogayar, M.C.; Trombetta-Lima, M. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev. Biol. 2018, 437, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Jhala, D.; Vasita, R. A Review on Extracellular Matrix Mimicking Strategies for an Artificial Stem Cell Niche. Polym. Rev. 2015, 55, 561–595. [Google Scholar] [CrossRef]
- Even-Ram, S.; Artym, V.; Yamada, K.M. Matrix control of stem cell fate. Cell 2006, 126, 645–647. [Google Scholar] [CrossRef]
- Watt, F.M.; Huck, W.T.S. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2012, 13, 27–38. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, M.; Xiao, G.; Franceschi, R.T. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol. Ther. 2005, 12, 247–253. [Google Scholar] [CrossRef]
- Fujii, M.; Takeda, K.; Imamura, T.; Aoki, H.; Sampath, T.K.; Enomoto, S.; Kawabata, M.; Kato, M.; Ichijo, H.; Miyazono, K. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol. Biol. Cell 1999, 10, 3801–3813. [Google Scholar] [CrossRef]
- Xu, D.-J.; Zhao, Y.-Z.; Wang, J.; He, J.-W.; Weng, Y.-G.; Luo, J.-Y. Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep. 2012, 45, 247–252. [Google Scholar] [CrossRef]
- Javed, A.; Bae, J.-S.; Afzal, F.; Gutierrez, S.; Pratap, J.; Zaidi, S.K.; Lou, Y.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J. Biol. Chem. 2008, 283, 8412–8422. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; De Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Kang, S.; Bennett, C.N.; Gerin, I.; Rapp, L.A.; Hankenson, K.D.; MacDougald, O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2007, 282, 14515–14524. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Claes, V.; Duthoit, G.; Hébert, J.-L. Circadian rhythms, Wnt/beta-catenin pathway and PPAR alpha/gamma profiles in diseases with primary or secondary cardiac dysfunction. Front. Physiol. 2014, 5, 429. [Google Scholar] [CrossRef]
- Day, T.F.; Guo, X.; Garrett-Beal, L.; Yang, Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 2005, 8, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Behrens, J. The Wnt signalling pathway. J. Cell Sci. 2002, 115, 3977–3978. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, D.; Zhou, C.; Yuan, Q.; Ye, L.; Zhou, X. Substrate elasticity regulates adipose-derived stromal cell differentiation towards osteogenesis and adipogenesis through β-catenin transduction. Acta Biomater. 2018, 79, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kshitiz; Afzal, J.; Chang, H.; Goyal, R.; Levchenko, A. Mechanics of Microenvironment as Instructive Cues Guiding Stem Cell Behavior. Curr. Stem. Cell Rep. 2016, 2, 62–72. [Google Scholar] [CrossRef]
- Kshitiz; Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.-H. Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. 2012, 4, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Kim, J.K.; Lee, G.; Kim, D.H. Mechanical Properties of Materials for Stem Cell Differentiation. Adv. Biosyst. 2020, 4, 2000247. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- MacQueen, L.; Sun, Y.; Simmons, C.A. Mesenchymal stem cell mechanobiology and emerging experimental platforms. J. R. Soc. Interface 2013, 10, 20130179. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, M.B.; Shim, J.-H.; Zou, W.; Sitara, D.; Schweitzer, M.; Hu, D.; Lotinun, S.; Sano, Y.; Baron, R.; Park, J.M.; et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Investig. 2010, 120, 2457–2473. [Google Scholar] [CrossRef]
- Thouverey, C.; Caverzasio, J. The p38α MAPK positively regulates osteoblast function and postnatal bone acquisition. Cell Mol. Life Sci. 2012, 69, 3115–3125. [Google Scholar] [CrossRef]
- Rodríguez-Carballo, E.; Gámez, B.; Sedó-Cabezón, L.; Sánchez-Feutrie, M.; Zorzano, A.; Manzanares-Céspedes, C.; Rosa, J.L.; Ventura, F. The p38α MAPK function in osteoprecursors is required for bone formation and bone homeostasis in adult mice. PLoS ONE 2014, 9, e102032. [Google Scholar] [CrossRef]
- Chakladar, A.; Dubeykovskiy, A.; Wojtukiewicz, L.J.; Pratap, J.; Lei, S.; Wang, T.C. Synergistic activation of the murine gastrin promoter by oncogenic Ras and beta-catenin involves SMAD recruitment. Biochem. Biophys. Res. Commun. 2005, 336, 190–196. [Google Scholar] [CrossRef]
- Yamashita, M.; Otsuka, F.; Mukai, T.; Otani, H.; Inagaki, K.; Miyoshi, T.; Goto, J.; Yamamura, M.; Makino, H. Simvastatin antagonizes tumor necrosis factor-alpha inhibition of bone morphogenetic proteins-2-induced osteoblast differentiation by regulating Smad signaling and Ras/Rho-mitogen-activated protein kinase pathway. J. Endocrinol. 2008, 196, 601–613. [Google Scholar] [CrossRef]
- Xue, R.; Li, J.Y.; Yeh, Y.; Yang, L.; Chien, S. Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation. J. Orthop. Res. 2013, 31, 1360–1365. [Google Scholar] [CrossRef]
- Xu, J.; Sun, M.; Tan, Y.; Wang, H.; Wang, H.; Li, P.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Effect of matrix stiffness on the proliferation and differentiation of umbilical cord mesenchymal stem cells. Differentiation 2017, 96, 30–39. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef]
- Ahmed, M.; Ffrench-Constant, C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr. Stem Cell Rep. 2016, 2, 197–206. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Zhang, N.; Kohn, D.H. Using polymeric materials to control stem cell behavior for tissue regeneration. Birth Defects Res. Part. C Embryo Today Rev. 2012, 96, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, H.; Zhang, Z.; Yang, W.; Liu, W.; Li, Y.; Li, L. Biomaterial stiffness determines stem cell fate. Life Sci. 2017, 178, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Lv, H.; Li, L.; Sun, M.; Zhang, Y.; Chen, L.; Rong, Y.; Li, Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015, 6, 103. [Google Scholar] [CrossRef]
- Guo, R.; Lu, S.; Merkel, A.R.; Sterling, J.A.; Guelcher, S.A. Substrate Modulus Regulates Osteogenic Differentiation of Rat Mesenchymal Stem Cells through Integrin β1 and BMP Receptor Type IA. J. Mater. Chem. B 2016, 4, 3584–3593. [Google Scholar] [CrossRef]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef]
- Naqvi, S.M.; McNamara, L.M. Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 597661. [Google Scholar] [CrossRef]
- Shih, Y.R.; Tseng, K.F.; Lai, H.Y.; Lin, C.H.; Lee, O.K. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 2011, 26, 730–738. [Google Scholar] [CrossRef]
- Miller, A.E.; Hu, P.; Barker, T.H. Feeling Things Out: Bidirectional Signaling of the Cell–ECM Interface, Implications in the Mechanobiology of Cell Spreading, Migration, Proliferation, and Differentiation. Adv. Healthc. Mater. 2020, 9, 1901445. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure and Function, 8th ed.; El Sevier Mosby: St Louis, Hong Kong, 2013; pp. 81–83. [Google Scholar]
- Ciobanasu, C.; Faivre, B.; Le Clainche, C. Actomyosin-dependent formation of the mechanosensitive talin-vinculin complex reinforces actin anchoring. Nat. Commun. 2014, 5, 3095. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, C.Y. Focal Adhesion Kinase; Springer International Publishing: New York, NY, USA, 2018; pp. 1800–1812. [Google Scholar]
- Bradbury, P.M.; Turner, K.; Mitchell, C.; Griffin, K.R.; Middlemiss, S.; Lau, L.; Dagg, R.; Taran, E.; Cooper-White, J.; Fabry, B.; et al. The focal adhesion targeting domain of p130Cas confers a mechanosensing function. J. Cell Sci. 2017, 130, 1263–1273. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Carman, C.V.; Springer, T.A. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 2003, 301, 1720–1725. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Poleszak, K.; Pasierbinska, M.; Kaminska, B. Integrin Signaling in Glioma Pathogenesis: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 888. [Google Scholar] [CrossRef] [PubMed]
- Petrie, T.A.; Raynor, J.E.; Dumbauld, D.W.; Lee, T.T.; Jagtap, S.; Templeman, K.L.; Collard, D.M.; García, A.J. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci. Transl. Med. 2010, 2, 45ra60. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar] [CrossRef]
- Frith, J.E.; Mills, R.J.; Hudson, J.E.; Cooper-White, J.J. Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem. Cells Dev. 2012, 21, 2442–2456. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Xu, J.; Tan, Y.; Xu, J.; Lv, S.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin α5. Stem. Cell Res. Ther. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Hamidouche, Z.; Fromigué, O.; Ringe, J.; Häupl, T.; Vaudin, P.; Pagès, J.C.; Srouji, S.; Livne, E.; Marie, P.J. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 18587–18591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ni, N.; Wang, Y.; Tang, Z.; Gao, H.; Ju, Y.; Sun, N.; He, X.; Gu, P.; Fan, X. CircRNA-vgll3 promotes osteogenic differentiation of adipose-derived mesenchymal stem cells via modulating miRNA-dependent integrin α5 expression. Cell Death Differ. 2021, 28, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Abdeen, A.A.; Tang, X.; Saif, T.A.; Kilian, K.A. Geometric guidance of integrin mediated traction stress during stem cell differentiation. Biomaterials 2015, 69, 174–183. [Google Scholar] [CrossRef]
- Liu, J.; DeYoung, S.M.; Zhang, M.; Zhang, M.; Cheng, A.; Saltiel, A.R. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2005, 2, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.G.; Wehrle-Haller, B.; Strömblad, S. Cell-matrix adhesion complexes: Master control machinery of cell migration. Semin. Cancer Biol. 2008, 18, 65–76. [Google Scholar] [CrossRef]
- Gavazzo, P.; Viti, F.; Donnelly, H.; Oliva, M.A.G.; Salmeron-Sanchez, M.; Dalby, M.J.; Vassalli, M. Biophysical phenotyping of mesenchymal stem cells along the osteogenic differentiation pathway. Cell Biol. Toxicol. 2021, 2021, 1–19. [Google Scholar] [CrossRef]
- Saidova, A.A.; Vorobjev, I.A. Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells. Tissue Eng. Part B Rev. 2020, 26, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Byron, A.; Frame, M.C. Adhesion protein networks reveal functions proximal and distal to cell-matrix contacts. Curr. Opin. Cell Biol. 2016, 39, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Bielawski, K.S.; Ting, L.H.; Rodriguez, M.L.; Sniadecki, N.J. Decoupling substrate stiffness, spread area, and micropost density: A close spatial relationship between traction forces and focal adhesions. Biophys. J. 2012, 103, 640–648. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Lavenus, S.; Berreur, M.; Trichet, V.; Pilet, P.; Louarn, G.; Layrolle, P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur. Cell Mater. 2011, 22, 84–96. [Google Scholar] [CrossRef]
- Frith, J.E.; Mills, R.J.; Cooper-White, J.J. Lateral spacing of adhesion peptides influences human mesenchymal stem cell behaviour. J. Cell Sci. 2012, 125, 317–327. [Google Scholar] [CrossRef]
- Rosenberg, N. The role of the cytoskeleton in mechanotransduction in human osteoblast-like cells. Hum. Exp. Toxicol. 2003, 22, 271–274. [Google Scholar] [CrossRef]
- Wang, N.; Naruse, K.; Stamenović, D.; Fredberg, J.J.; Mijailovich, S.M.; Tolić-Nørrelykke, I.M.; Polte, T.; Mannix, R.; Ingber, D.E. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA 2001, 98, 7765–7770. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Jreij, P.; Fletcher, D.A. Mechanotransduction by the actin cytoskeleton: Converting mechanical stimuli into biochemical signals. Annu. Rev. Biophys. 2018, 47, 617–631. [Google Scholar] [CrossRef]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Kim, D.H.; Wirtz, D. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials 2015, 48, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Khatau, S.B.; Hale, C.M.; Stewart-Hutchinson, P.J.; Patel, M.S.; Stewart, C.L.; Searson, P.C.; Hodzic, D.; Wirtz, D. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA 2009, 106, 19017–19022. [Google Scholar] [CrossRef]
- Kim, D.H.; Khatau, S.B.; Feng, Y.; Walcott, S.; Sun, S.X.; Longmore, G.D.; Wirtz, D. Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Sci. Rep. 2012, 2, 555. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers—Assembly, dynamics and biological roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chen, M.H.; Young, T.H.; Jeng, J.H.; Chen, Y.J. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J. Cell Biochem. 2009, 108, 1263–1273. [Google Scholar] [CrossRef]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, H.; Qiu, W.; Shi, K.; Kassem, M. Actin depolymerization enhances adipogenic differentiation in human stromal stem cells. Stem Cell Res. 2018, 29, 76–83. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; González, M.; Ríos, S.; Cambiazo, V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J. Cell Biochem. 2004, 93, 721–731. [Google Scholar] [CrossRef]
- Sonowal, H.; Kumar, A.; Bhattacharyya, J.; Gogoi, P.K.; Jaganathan, B.G. Inhibition of actin polymerization decreases osteogeneic differentiation of mesenchymal stem cells through p38 MAPK pathway. J. Biomed. Sci. 2013, 20, 71. [Google Scholar] [CrossRef]
- Sen, B.; Xie, Z.; Case, N.; Thompson, W.R.; Uzer, G.; Styner, M.; Rubin, J. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J. Bone Miner. Res. 2014, 29, 78–89. [Google Scholar] [CrossRef]
- Chen, L.; Shi, K.; Frary, C.E.; Ditzel, N.; Hu, H.; Qiu, W.; Kassem, M. Inhibiting actin depolymerization enhances osteoblast differentiation and bone formation in human stromal stem cells. Stem Cell Res. 2015, 15, 281–289. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Qu, R.; Fan, T.; Ouyang, J.; Dai, J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 283. [Google Scholar] [CrossRef]
- Kobayashi, T.; Sokabe, M. Sensing substrate rigidity by mechanosensitive ion channels with stress fibers and focal adhesions. Curr. Opin. Cell Biol. 2010, 22, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wong, K.; Glogauer, M.; Ellen, R.P.; McCulloch, C.A.G. Regulation of Stretch-Activated Intracellular Calcium Transients by Actin Filaments. Biochem. Biophys. Res. Commun. 1999, 261, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Zaidi, M. Molecular regulation of mechanotransduction. Biochem. Biophys. Res. Commun. 2005, 328, 751–755. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Jin, Y.-Q.; Liu, W.; Zhang, W.J.; Hong, T.-H.; Zhou, G.; Baggett, L.S.; Mikos, A.G.; Cao, Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008, 9, 1–13. [Google Scholar] [CrossRef]
- Stenderup, K.; Justesen, J.; Clausen, C.; Kassem, M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone Miner. 2003, 33, 919–926. [Google Scholar] [CrossRef]
- Asumda, F.Z.; Chase, P.B. Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol. 2011, 12, 1–11. [Google Scholar] [CrossRef]
- Shen, J.; Tsai, Y.-T.; DiMarco, N.M.; Long, M.A.; Sun, X.; Tang, L. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci. Rep. Cetacean Res. 2011, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.h.; Gibon, E.; Loi, F.; Pajarinen, J.; Córdova, L.A.; Nabeshima, A.; Lu, L.; Yao, Z.; Goodman, S.B. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-κB activity. J. Orth Res. 2017, 35, 281–288. [Google Scholar] [CrossRef]
- Pelissier, F.A.; Garbe, J.C.; Ananthanarayanan, B.; Miyano, M.; Lin, C.; Jokela, T.; Kumar, S.; Stampfer, M.R.; Lorens, J.B.; LaBarge, M.A. Age-Related Dysfunction in Mechanotransduction Impairs Differentiation of Human Mammary Epithelial Progenitors. Cell Rep. 2014, 7, 1926–1939. [Google Scholar] [CrossRef]
- Barreto, S.; Gonzalez-Vazquez, A.; Cameron, A.R.; Cavanagh, B.; Murray, D.J.; O′Brien, F.J. Identification of the mechanisms by which age alters the mechanosensitivity of mesenchymal stromal cells on substrates of differing stiffness: Implications for osteogenesis and angiogenesis. Acta Biomater. 2017, 53, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zouani, O.F.; Kalisky, J.; Ibarboure, E.; Durrieu, M.-C. Effect of BMP-2 from matrices of different stiffnesses for the modulation of stem cell fate. Biomaterials 2013, 34, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.-L. The Hippo pathway: Biology and pathophysiology. Annu Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Taouk, G.M. A Potential role of YAP/TAZ in the interplay between metastasis and metabolic alterations. Front. Oncol. 2020, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Dobrokhotov, O.; Samsonov, M.; Sokabe, M.; Hirata, H. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. J. Transl. Med. 2018, 7, 1–14. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Wang, L.; Wang, C.-Y.; Yu, J.; Guan, K.-L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012, 26, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 2017, 171, 1397–1410.e1314. [Google Scholar] [CrossRef] [PubMed]
- Lorthongpanich, C.; Thumanu, K.; Tangkiettrakul, K.; Jiamvoraphong, N.; Laowtammathron, C.; Damkham, N.; U-pratya, Y.; Issaragrisil, S. YAP as a key regulator of adipo-osteogenic differentiation in human MSCs. Stem Cell Res. Ther. 2019, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Hwang, E.S.; McManus, M.T.; Amsterdam, A.; Tian, Y.; Kalmukova, R.; Mueller, E.; Benjamin, T.; Spiegelman, B.M.; Sharp, P.A.; et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 2005, 309, 1074–1078. [Google Scholar] [CrossRef]
- Du, J.; Chen, X.; Liang, X.; Zhang, G.; Xu, J.; He, L.; Zhan, Q.; Feng, X.Q.; Chien, S.; Yang, C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 9466–9471. [Google Scholar] [CrossRef]
- Chrzanowska-Wodnicka, M.; Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996, 133, 1403–1415. [Google Scholar] [CrossRef]
- Arnsdorf, E.J.; Tummala, P.; Kwon, R.Y.; Jacobs, C.R. Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. J. Cell Sci. 2009, 122, 546–553. [Google Scholar] [CrossRef]
- Hyväri, L.; Ojansivu, M.; Juntunen, M.; Kartasalo, K.; Miettinen, S.; Vanhatupa, S. Focal Adhesion Kinase and ROCK Signaling Are Switch-Like Regulators of Human Adipose Stem Cell Differentiation towards Osteogenic and Adipogenic Lineages. Stem Cells Int. 2018, 2018, 2190657. [Google Scholar] [CrossRef]
- Rowlands, A.S.; George, P.A.; Cooper-White, J.J. Directing osteogenic and myogenic differentiation of MSCs: Interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 2008, 295, C1037–C1044. [Google Scholar] [CrossRef]
- Wan, W.; Cheng, B.; Zhang, C.; Ma, Y.; Li, A.; Xu, F.; Lin, M. Synergistic Effect of Matrix Stiffness and Inflammatory Factors on Osteogenic Differentiation of MSC. Biophys. J. 2019, 117, 129–142. [Google Scholar] [CrossRef]
- He, X.T.; Wu, R.X.; Xu, X.Y.; Wang, J.; Yin, Y.; Chen, F.M. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018, 71, 132–147. [Google Scholar] [CrossRef]
- Tang, X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Chapter 21—Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 351–371. [Google Scholar]
- Ramkumar, R.; Sundaram, M.M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Yang, J.M.; Olanrele, O.S.; Zhang, X.; Hsu, C.C. Fabrication of Hydrogel Materials for Biomedical Applications. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Springer Singapore: Singapore, 2018; pp. 197–224. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Lee, H.-p.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017, 16, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Wickramaarachchi, K.; Sundaram, M.M.; Henry, D.J.; Gao, X. Alginate Biopolymer Effect on the Electrodeposition of Manganese Dioxide on Electrodes for Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 7040–7051. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Adamek, P.; Paul, G.R.; Qin, X.-H.; Rubert, M.; Müller, R. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020, 114, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Kelly, D.J. Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC fate within bioprinted tissues. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Maia, F.R.; Fonseca, K.B.; Rodrigues, G.; Granja, P.L.; Barrias, C.C. Matrix-driven formation of mesenchymal stem cell–Extracellular matrix microtissues on soft alginate hydrogels. Acta Biomater. 2014, 10, 3197–3208. [Google Scholar] [CrossRef]
- Yener, B.; Acar, E.; Aguis, P.; Bennett, K.; Vandenberg, S.L.; Plopper, G.E. Multiway modeling and analysis in stem cell systems biology. BMC Syst. Biol. 2008, 2, 1–17. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B.; Owen, T.A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990, 4, 3111–3123. [Google Scholar] [CrossRef]
- Xie, J.; Bao, M.; Bruekers, S.p.M.; Huck, W.T. Collagen gels with different fibrillar microarchitectures elicit different cellular responses. ACS Appl. Mater. Interfaces 2017, 9, 19630–19637. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.M.; Mozdzen, L.C.; Harley, B.A.; Bailey, R.C. The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells. Biomaterials 2014, 35, 8951–8959. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Han, U.; Yang, M.; Choi, Y.; Choi, J.; Lee, J.-M.; Jung, H.-S.; Hong, J.; Hong, J.-H. Artificial cellular nano-environment composed of collagen-based nanofilm promotes osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2019, 86, 247–256. [Google Scholar] [CrossRef]
- Zhou, Q.; Lyu, S.; Bertrand, A.A.; Hu, A.C.; Chan, C.H.; Ren, X.; Dewey, M.J.; Tiffany, A.S.; Harley, B.A.; Lee, J.C. Stiffness of Nanoparticulate Mineralized Collagen Scaffolds Triggers Osteogenesis via Mechanotransduction and Canonical Wnt Signaling. Macromol. Biosci. 2021, 21, 2000370. [Google Scholar] [CrossRef]
- Tsimbouri, P.M.; Childs, P.G.; Pemberton, G.D.; Yang, J.; Jayawarna, V.; Orapiriyakul, W.; Burgess, K.; Gonzalez-Garcia, C.; Blackburn, G.; Thomas, D. Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor. Nat. Biomed. Eng. 2017, 1, 758–770. [Google Scholar] [CrossRef]
- Murphy, C.M.; Matsiko, A.; Haugh, M.G.; Gleeson, J.P.; O’Brien, F.J. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen–glycosaminoglycan scaffolds. J. Mech. Behav. Biomed. Mater. 2012, 11, 53–62. [Google Scholar] [CrossRef]
- Chen, G.; Dong, C.; Yang, L.; Lv, Y. Interfaces 3D scaffolds with different stiffness but the same microstructure for bone tissue engineering. ACS Appl. Mater. 2015, 7, 15790–15802. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Krishnan, U.M.; Sethuraman, S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol. Adv. 2014, 32, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.J.; Ren, J.R.; Huang, N.; Wang, J.; Chen, J.Y.; Leng, Y.X.; Liu, H.Q. Surface engineering of Ti–O films by photochemical immobilization of gelatin. Mater. Sci Eng. C 2008, 28, 1495–1500. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; He, T.; Shen, J. Endothelium regeneration on luminal surface of polyurethane vascular scaffold modified with diamine and covalently grafted with gelatin. Biomaterials 2004, 25, 423–430. [Google Scholar] [CrossRef]
- Salamon, A.; van Vlierberghe, S.; van Nieuwenhove, I.; Baudisch, F.; Graulus, G.-J.; Benecke, V.; Alberti, K.; Neumann, H.-G.; Rychly, J.; Martins, J.C.; et al. Gelatin-Based Hydrogels Promote Chondrogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells In Vitro. Materials 2014, 7, 1342–1359. [Google Scholar] [CrossRef]
- Zhou, D.; Ito, Y. Inorganic material surfaces made bioactive by immobilizing growth factors for hard tissue engineering. RSC Adv. 2013, 3, 11095–11106. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Salamon, A.; Adam, S.; Dubruel, P.; Van Vlierberghe, S.; Peters, K. Gelatin- and starch-based hydrogels. Part B: In vitro mesenchymal stem cell behavior on the hydrogels. Carbohydr. Polym. 2017, 161, 295–305. [Google Scholar] [CrossRef]
- Jiang, P.; Mao, Z.; Gao, C. Combinational effect of matrix elasticity and alendronate density on differentiation of rat mesenchymal stem cells. Acta Biomater. 2015, 19, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, F.; Hu, Q.; Krebsbach, P.H. Controlling stem cell-mediated bone regeneration through tailored mechanical properties of collagen scaffolds. Biomaterials 2014, 35, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Ventre, M.; Coppola, V.; Natale, C.F.; Netti, P.A. Aligned fibrous decellularized cell derived matrices for mesenchymal stem cell amplification. J. Biomed. Mater. Res. A 2019, 107, 2536–2546. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, M.; Chen, G.; Xu, Z.; Lv, Y. Demineralized Bone Scaffolds with Tunable Matrix Stiffness for Efficient Bone Integration. ACS Appl. Mater. Interfaces 2018, 10, 27669–27680. [Google Scholar] [CrossRef]
- Lee, J.Y.; Spicer, A.P. Hyaluronan: A multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000, 12, 581–586. [Google Scholar] [CrossRef]
- Garg, H.G.; Hales, C.A. Chemistry and Biology of Hyaluronan; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Fawzy El-Sayed, K.M.; Dahaba, M.A.; Aboul-Ela, S.; Darhous, M.S. Local application of hyaluronan gel in conjunction with periodontal surgery: A randomized controlled trial. Clin. Oral Investig. 2012, 16, 1229–1236. [Google Scholar] [CrossRef]
- Xing, F.; Li, L.; Zhou, C.; Long, C.; Wu, L.; Lei, H.; Kong, Q.; Fan, Y.; Xiang, Z.; Zhang, X. Regulation and directing stem cell fate by tissue engineering functional microenvironments: Scaffold physical and chemical cues. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Itano, N. Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J. Biochem. 2008, 144, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Anseth, K.S.; Burdick, J.A. New directions in photopolymerizable biomaterials. MRS Bull. 2002, 27, 130–136. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Liu, X.; Zhang, N.; Wen, X. Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2014, 40, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, B.D.; Mui, K.L.; Driscoll, T.P.; Caliari, S.R.; Mehta, K.D.; Assoian, R.K.; Burdick, J.A.; Mauck, R.L. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 2016, 15, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Dorcemus, D.L.; George, E.O.; Dealy, C.N.; Nukavarapu, S.P. Harnessing External Cues: Development and Evaluation of an In Vitro Culture System for Osteochondral Tissue Engineering. Tissue Eng. Part A 2017, 23, 719–737. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Song, J.; Ravikrishnan, A.; Dicker, K.T.; Fowler, E.W.; Zerdoum, A.B.; Li, Y.; Zhang, H.; Rajasekaran, A.K.; Fox, J.M.; et al. Rapid Bioorthogonal Chemistry Enables in Situ Modulation of the Stem Cell Behavior in 3D without External Triggers. ACS Appl Mater. Interfaces 2018, 10, 26016–26027. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Koh, W.-G.J.P. Composite Hydrogel of Methacrylated Hyaluronic Acid and Fragmented Polycaprolactone Nanofiber for Osteogenic Differentiation of Adipose-Derived. Stem Cells 2020, 12, 902. [Google Scholar] [CrossRef]
- Ducret, M.; Montembault, A.; Josse, J.; Pasdeloup, M.; Celle, A.; Benchrih, R.; Mallein-Gerin, F.; Alliot-Licht, B.; David, L.; Farges, J.C. Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. Dent. Mater. 2019, 35, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, H.; Allahverdi, A.; Ghorbani, M.; Soleymani, H.; Kocsis, Á.; Fischer, M.B.; Ertl, P.; Naderi-Manesh, H. Gold Nanowires/Fibrin Nanostructure as Microfluidics Platforms for Enhancing Stem Cell Differentiation: Bio-AFM Study. Micromachines 2020, 11, 50. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Pek, Y.S.; Wan, A.C.; Ying, J.Y. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 2010, 31, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Wang, X.; Cao, L.; Li, S.; Li, Z.; Yu, L.; Ding, J. Matrix stiffness and nanoscale spatial organization of cell-adhesive ligands direct stem cell fate. Nano Lett. 2015, 15, 4720–4729. [Google Scholar] [CrossRef]
- Steinmetz, N.J.; Aisenbrey, E.A.; Westbrook, K.K.; Qi, H.J.; Bryant, S.J. Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering. Acta Biomater. 2015, 21, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, Y.; Qu, R.; Li, Q.; Rong, D.; Fan, T.; Yang, Y.; Sun, B.; Bi, Z.; Khan, A.U. Synthesis of aligned porous polyethylene glycol/silk fibroin/hydroxyapatite scaffolds for osteoinduction in bone tissue engineering. Stem. Cell Res. Ther. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Yang, C.; DelRio, F.W.; Ma, H.; Killaars, A.R.; Basta, L.P.; Kyburz, K.A.; Anseth, K.S. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci. USA 2016, 113, E4439–E4445. [Google Scholar] [CrossRef]
- Gandavarapu, N.R.; Alge, D.L.; Anseth, K.S. Osteogenic differentiation of human mesenchymal stem cells on α5 integrin binding peptide hydrogels is dependent on substrate elasticity. Biomater. Sci. 2014, 2, 352–361. [Google Scholar] [CrossRef]
- Chou, S.-Y.; Cheng, C.-M.; LeDuc, P.R. Composite polymer systems with control of local substrate elasticity and their effect on cytoskeletal and morphological characteristics of adherent cells. Biomaterials 2009, 30, 3136–3142. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Völlner, F.; Möhl, C.; Küpper, K.; Brockhoff, G.; Reichert, T.E.; Schmalz, G.; Morsczeck, C. Soft matrix supports osteogenic differentiation of human dental follicle cells. Biochem. Biophys. Res. Commun. 2011, 410, 587–592. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Gosau, M.; Küpper, K.; Möhl, C.; Brockhoff, G.; Reichert, T.E.; Schmalz, G.; Ettl, T.; Morsczeck, C. Rigid matrix supports osteogenic differentiation of stem cells from human exfoliated deciduous teeth (SHED). Differentiation 2012, 84, 366–370. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Tsai, W.-B.; Voelcker, N.H. Screening of rat mesenchymal stem cell behaviour on polydimethylsiloxane stiffness gradients. Acta Biomater. 2012, 8, 519–530. [Google Scholar] [CrossRef]
- Khoramgah, M.S.; Ranjbari, J.; Abbaszadeh, H.-A.; Mirakabad, F.S.T.; Hatami, S.; Hosseinzadeh, S.; Ghanbarian, H. Freeze-dried multiscale porous nanofibrous three dimensional scaffolds for bone regenerations. BioImpacts BI 2020, 10, 73. [Google Scholar] [CrossRef]
- Oh, S.H.; An, D.B.; Kim, T.H.; Lee, J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016, 35, 23–31. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Byun, M.R.; Kim, A.R.; Kim, K.M.; Cho, H.J.; Lee, Y.H.; Kim, J.; Jeong, M.G.; Hwang, E.S.; Hong, J.-H. Extracellular matrix stiffness regulates osteogenic differentiation through MAPK activation. PLoS ONE 2015, 10, e0135519. [Google Scholar] [CrossRef]
- Sun, X.; Tung, W.; Wang, W.; Xu, X.; Zou, J.; Gould, O.E.; Kratz, K.; Ma, N.; Lendlein, A. The effect of stiffness variation of electrospun fiber meshes of multiblock copolymers on the osteogenic differentiation of human mesenchymal stem cells. Clin. Hemorheol. Microcirc. 2019, 73, 219–228. [Google Scholar] [CrossRef]
- Shams, M.; Karimi, M.; Heydari, M.; Salimi, A. Nanocomposite scaffolds composed of Apacite (apatite-calcite) nanostructures, poly (ε-caprolactone) and poly (2-hydroxyethylmethacrylate): The effect of nanostructures on physico-mechanical properties and osteogenic differentiation of human bone marrow mesenchymal stem cells in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111271. [Google Scholar] [CrossRef]
- Tang, X.; Ali, M.Y.; Saif, M.T.A. A Novel Technique for Micro-patterning Proteins and Cells on Polyacrylamide Gels. Soft Matter 2012, 8, 7197–7206. [Google Scholar] [CrossRef] [PubMed]
- Daliri, K.; Pfannkuche, K.; Garipcan, B. Effects of physicochemical properties of polyacrylamide (PAA) and (polydimethylsiloxane) PDMS on cardiac cell behavior. Soft Matter 2021, 17, 1156–1172. [Google Scholar] [CrossRef]
- Winer, J.P.; Janmey, P.A.; McCormick, M.E.; Funaki, M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng. Part A 2009, 15, 147–154. [Google Scholar] [CrossRef]
- Lee, J.; Abdeen, A.A.; Huang, T.H.; Kilian, K.A. Controlling cell geometry on substrates of variable stiffness can tune the degree of osteogenesis in human mesenchymal stem cells. J. Mech. Behav. Biomed. Mater. 2014, 38, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.E.; Tong, X.; Yang, F. Extracellular matrix type modulates mechanotransduction of stem cells. Acta Biomater. 2019, 96, 310–320. [Google Scholar] [CrossRef]
- Gungordu, H.I.; Bao, M.; van Helvert, S.; Jansen, J.A.; Leeuwenburgh, S.C.G.; Walboomers, X.F. Effect of mechanical loading and substrate elasticity on the osteogenic and adipogenic differentiation of mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2019, 13, 2279–2290. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Gooch, K.J. Direct influence of culture dimensionality on human mesenchymal stem cell differentiation at various matrix stiffnesses using a fibrous self-assembling peptide hydrogel. J. Biomed. Mater. Res. A 2016, 104, 2356–2368. [Google Scholar] [CrossRef]
- Wu, L.; Magaz, A.; Wang, T.; Liu, C.; Darbyshire, A.; Loizidou, M.; Emberton, M.; Birchall, M.; Song, W. Stiffness memory of indirectly 3D-printed elastomer nanohybrid regulates chondrogenesis and osteogenesis of human mesenchymal stem cells. Biomaterials 2018, 186, 64–79. [Google Scholar] [CrossRef]
- Chen, G.; Xu, R.; Zhang, C.; Lv, Y. Responses of MSCs to 3D scaffold matrix mechanical properties under oscillatory perfusion culture. ACS Appl. Mater. 2017, 9, 1207–1218. [Google Scholar] [CrossRef]


| Study | Cell Source | Polymer | Modification | Modulus of Elasticity | Results |
|---|---|---|---|---|---|
| Alginate | |||||
| Zhang et al., 2020 [141] | hMSCs | Alginate–gelatin scaffold | 3D bioprinted porous scaffolds different alginate concentration (0.8%alg and 1.8%alg) and different initial cell seeding density (1.67, 5, and 15 M cells/mL) | Soft scaffold 0.66 ± 0.08 kPa Stiff scaffold 5.4 ± 1.2 kPa | UpregulatedALP-activity-related, 3D-bone-like-tissue-related, osteoblast-related, and early osteocyte-related gene expression |
| Freeman and Kelly, 2017 [142] | MSCs | Alginate hydrogel | 3D bioprinting matrix with varying alginate molecular weight and cross linker ratio | Osteogenic differentiation with increased ALP staining | |
| Maia et al., 2014 [143] | hMSCs | Alginate hydrogel | 3D matrix with bimodal molecular weight distribution at different polymer concentrations (1 and 2 wt.%) and RGD densities (0, 100 or 200 μM | 2 wt.% hydrogels (tan ∂ ᵙ 0.2), 1 wt.% hydrogels (tan ∂ ᵙ 0.4–0.6). | 1 wt.% alginate hydrogel matrices upregulated hMSCs osteogenic differentiation and expressed high levels of ALP and OCN |
| Collagen | |||||
| Xie et al., 2017 [146] | hMSCs | Collagen gel | Varying polymerization temperature 4, 21, and 37 °C. | Fiber stiffness: 1.1 to 9.3 kPa Bulk stiffness: 16.4 to 151.5 Pa | Collagen gel polymerized at 37 °C resulted in 34.1% ALP positive staining |
| Banks et al., 2014 [147] | ADSCs | Collagen–glycosaminoglycan (CG) | Chemical Crosslinking with EDAC and NHS Covalent immobilization of PDGF-BB and BMP-2 by benzophenone photolithography | 2.85 to 5 MPa | Upregulated expression of collagen 1, ALP, and OCN with increased stiffness |
| Hwang et al., 2019 [148] | hMSCs | Three bilayers of collagen/alginate nano film | 24 and 53 MPa | Increase in alkaline phosphatase activity | |
| Zhou et al., 2021 [149] | hMSCs | Nano-particulate mineralized collagen glycosaminoglycan | Chemical crosslinking with EDAC and NHS | 3.90 −/+ 0.36 kPa | Increase in expression of ALP, collagen 1, and Runx2 |
| Tsimbouri et al., 2017 [150] | MSCs | Collagen gel | 3D collagen gel culture on the vibrational bioreactor | ~108 Pa | Increased expression of Runx2, collagen I, ALP, OPN, OCN, and BMP2. |
| Murphy et al., 2012 [151] | MSCs | Collagen/glycosaminoglycan | DHT and EDAC crosslinking | 0.5, 1, and 1.5 kPa | Osteogenic differentiation with Runx2 expression |
| Chen et al., 2015 [152] | Rat MSCs | 3D scaffold collagen and hydroxyapatite | Coated on decellularized cancellous bone | 13.00 ± 5.55 kPa, 13.87 ± 1.51 kPa, and 37.7 ± 19.6 kPa | Highest scaffold stiffness promoted higher expressions of OPN and OC |
| Chen et al., 2017 [205] | Rat MSCs | Collagen and hydroxyapatite, coated on decellularized cancellous bone | 3D oscillatory perfusion bioreactor system | 6.74 ± 1.16 kPa- 8.82 ± 2.12 kPa- 23.61 ± 8.06 kPa | Osteogenic differentiation of MSCs |
| Gelatin | |||||
| Wan et al., 2019 [133] | PDLSCs | Gelatin | Crosslinked with variable concentrations of methacryloyl | GelMA concentrations of 10, 12, and 14 wt% stiffness 25.75 ± 1.21, 59.71 ± 8.87, and 117.82 ± 9.83 kPa, respectively | Increasing matrix stiffness increased osteogenic differentiation of PDLSCs, with upregulated expression of OCN and Runx2 |
| He et al., 2018 [134] | BMMSCs | Gelatin 3%, 6%, and 9%. | Crosslinked with transglutaminase | 9% gelatin gave rise to the highest stiffness (60.54 ± 10.45 kPa), while 3% gelatin resulted in the lowest stiffness (1.58 ± 0.42 kPa) | BMMSCs encapsulated in hydrogel with highest stiffness demonstrated the highest osteogenic differentiation |
| Van Nieuwenhove et al., 2017 [162] | ADSCs | Gelatin with variable degrees of methacrylation (GelMA 31%, GelMA 72%, and GelMA 95%) | Covalently bound to variable ratios of pentenoates modified starch (10 v% starch and 20 v% starch) | Increase in matrix stiffness promoted osteogenic differentiation of ADSCs | |
| Jiang et al., 2015 [163] | BMMSCs | GelMA encapsulating alendronate | Crosslinked by PEG diacrylate | stiffness increased from 4 to 40 kPa | Increased osteogenic differentiation of BMMSCs on stiffer hydrogel with higher alendronate concentration with upregulated ALP, collagen I, OCN, and calcium deposition |
| Sun et al., 2014 [164] | BMMSCs | Three-dimensional porous gelatin scaffolds | Crosslinked using EDC | Crosslinked scaffold demonstrated an increase in the elastic modulus from w 0.6 to ≈ 2.5 kP without any change in the scaffold internal structure | Increased stiffness increased osteogenic differentiation evidenced by increased Runx2 and OCN in vitro and increased bone formation in vivo |
| Decellularized matrix and Demineralized Bone | |||||
| Ventre et al., 2019 [165] | Murine MSCs | Decellularized MC3T3-E1-cell-derived matrix on replica from PDMS | Genipin crosslinking | Young’s modulus increased from (0.01–0.1 kPa) to (0.1–1.5 kPa). | MSCs on stiff dCDMs, revealed significant adipogenic and osteogenic differentiation potentials |
| Hu et al., 2018 [166] | BMMSC | Demineralized bone matrices | Controlling the decalcification duration (1 h, 12 h, and 5 d, respectively) | High: 66.06 ± 27.83 MPa, Medium: 26.90 ± 13.16 MPa Low: 0.67 ± 0.14 MPa | Low stiffness scaffolds promoted osteogenesis in vitro. Subcutaneous implantation in a rat model and in a femoral condylar defect rabbit model revealed positive OCN and OPN expression |
| Hyaluronic acid (HA) | |||||
| Zhao et al., 2014 [174] | hBMMSCs | Thiol functionalized hyaluronic acid (HA) and thiol functionalized recombinant human gelatin | Crosslinked by poly (ethylene glycol) tetra-acrylate | 0.15, 1.5, and 4 kPa | Change in cell morphologies with different stiffness. Cells cultured on the 4 kPa hydrogel revealed an enhanced expression of late osteogenic genes |
| Cosgrove et al., 2016 [175] | Juvenile bovine MSCs | Methacrylated HA hydrogel | Ligation of the HAVDI adhesive peptide sequence from N-cadherin domain 1 and RGD from fibronectin | 5, 10, and 15 kPa | Lack of myosin IIA incorporated into focal adhesions hindered their maturation with increasing substrate stiffness and decreased osteogenesis |
| Dorcemus et al., 2017 [176] | hMSCs-bone-marrow-derived | Thiol-modified hyaluronan gel | Crosslinked by PEG at ratios ranging from 1:1 to 7:1 | Storage moduli from 10 to 45 Pa | Differences between the top (cartilage-forming) and bottom (bone-forming) regions of the scaffold proved its capability for osteochondral engineering |
| Hao et al., 2018 [177] | hMSCs-bone-marrow-derived | HA carrying sulfhydryl groups and a hydrophilic polymer bearing both acrylate and tetrazine groups | Matrix metalloprotease -degradable peptidic crosslinker and adding HA conjugated with multiple copies of trans-cyclooctene (TCO) | (G’) = 180 ± 42 Pa increased to G′ = 520 ± 80 Pa | The 3D matrix tagged with a TCO- motif promoted the cells to undergo change from a rounded to spindle phenotype |
| Fibrin | |||||
| Hashemzadeh et al., 2019 [180] | hADSCs | Fibrin hydrogels embedding gold nanowires | Altering fibrinogen and thrombin concentration and incorporation of gold nanowires | With high fibrinogen and thrombin concentration, gold nanowires, promoted osteogenic differentiation | |
| Polyethylene glycol (PEG) | |||||
| Pek et al., 2010 [182] | MSCs | Thixotropic polyethylene glycol–silica (PEG–silica) nano composite gel | 3D cell culture Cell-adhesion peptide RGD (Arg–Gly–Asp) sequence immobilization | ≥75 Pa | Higher expression of the osteogenic transcription factor |
| Ye et al., 2015 [183] | Rat BMMSCs | PEG | PEG hydrogels with RGD nano-spacings of 49 and 135 nm and incubated in mixed osteogenic and adipogenic medium | Soft hydrogels (130 kPa) and stiff hydrogels (3170 kPa) | Stiff hydrogels promoted osteogenesis. Large RGD nano-spacing promoted osteogenesis |
| Steinmetz et al., 2015 [184] | hMSCs | Multilayer PEG-based hydrogel | Simple sequential photopolymerization- high RGD concentrations- dynamic mechanical stimulation | 345 kPa | Collagen I generation with mineral deposits were evident |
| Yang et al., 2020 [185] | Rat BMMSCs | PEG/silk fibroin/HA scaffold | Varying HA concentration | 80.98 to 190.51 kPa | Expression of all the osteogenesis-related markers in vitro and superior calvarial defect repair in vivo |
| Yang et al., 2016 [186] | hMSCs | PEG hydrogel | Regularly and randomly patterned photodegradable hydrogel | ∼10–12 kPa | Osteogenic differentiation of MSCs cultured on random patterns |
| Gandavarapu et al., 2014 [187] | hMSCs | PEG hydrogels | functionalized with c(RRETAWA) hydrogels through α5 integrins | ∼25 kPa | Osteogenic differentiation of hMSCs |
| Polydimethylsiloxane (PDMS) | |||||
| Xie et al., 2018 [38] | ASCs | PDMS | 1.014 ± 0.15 MPa | Osteogenic differentiation by ALP stain and upregulation of Runx2 and Osx transcriptional factors | |
| Viale-Bouroncle et al., 2014 [189] | DFCs | PDMS | Coating PDMS with fibronectin and cultured in osteogenic differentiation medium | 11 kPa | High ALP activity and accumulation of calcium on the soft substrate |
| Viale-Bouroncle et al., 2012 [190] | SHED | PDMS | Adding osteogenic differentiation medium | 93 kPa | High osteogenic differentiation |
| Wang et al., 2012 [191] | Rat MSCs | PDMS | Osteogenic medium with temperature gradient curing | 0.19 to 3.10 MPa | Calcein Blue–positive bone-nodule-like colonies |
| Vinyl polymers | |||||
| Khoramgah et al., 2020 [192] | hADSCs | Poly tetra fluoro ethylene (PTFE) and PVA with and without graphene oxide nanoparticles | 3D porous scaffolds- chemical crosslinking with small amounts of boric acids–controlled freeze-drying method | 620 and 130 kPa | Elevation in ALP activity, calcium deposition, and osteogenic-related genes expression |
| Oh et al., 2016 [193] | hBMMSCs | Cylindrical PVA/HA hydrogel | Liquid nitrogen—contacting gradual freezing–thawing method | ~20 kPa and ~200 kPa | Stiffness of ~190 kPa led to osteoblast differentiation |
| Polyesters | |||||
| Sun et al., 2019 [195] | hADSCs | Poly(ether-ester-urethane) (PEEU) containing PPDO and PCL segments | Electrospun into fiber meshes with varying PPDO to PCL weight ratios | 2.6 ± 0.8 MPa (PEEU40), 3.2 ± 0.9 MPa (PEEU50), 4.0 ± 0.9 MPa (PEEU60) 4.5 ± 0.8 MPa (PEEU70) | Enhanced osteogenic differentiation of hADSCs with higher levels of OCN, ALP, and hydroxyapatite detected on the stiffer fiber meshes |
| Self-assembling peptides | |||||
| Hogrebe and Gooch, 2016 [203] | hMSCs | Biomimetic self-assembling peptide hydrogel containing 1 mg/mL RGD-functionalized peptide (KFE–RGD) | hMSCs were encapsulated within 3D culture and grown on top of 2D culture Adding 1:1 mixed adipogenic/osteogenic induction medium | (G′) 10 kPa | Osteogenesis induction and alizarin red-stained calcium deposits |
| Other Polymers | |||||
| Olivares-Navarrete et al., 2017 [76] | MSCs | Methyl acrylate/methyl methacrylate polymer | Altering monomer concentration. | 0.1 MPa to 310 MPa | Chondrogenic and osteogenic differentiation when grown on substrates with less than 10 MPa stiffness |
| Wu et al., 2018 [204] | hBMMSCs | Poly(urea-urethane) (PUU)/POSS elastomeric nano-hybrid scaffolds | Thermoresponsive scaffolds indirectly 3D printed by inverse self-assembling | >10 kPa | Osteogenic differentiation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Rashidy, A.A.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review. Polymers 2021, 13, 2950. https://doi.org/10.3390/polym13172950
El-Rashidy AA, El Moshy S, Radwan IA, Rady D, Abbass MMS, Dörfer CE, Fawzy El-Sayed KM. Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review. Polymers. 2021; 13(17):2950. https://doi.org/10.3390/polym13172950
Chicago/Turabian StyleEl-Rashidy, Aiah A., Sara El Moshy, Israa Ahmed Radwan, Dina Rady, Marwa M. S. Abbass, Christof E. Dörfer, and Karim M. Fawzy El-Sayed. 2021. "Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review" Polymers 13, no. 17: 2950. https://doi.org/10.3390/polym13172950
APA StyleEl-Rashidy, A. A., El Moshy, S., Radwan, I. A., Rady, D., Abbass, M. M. S., Dörfer, C. E., & Fawzy El-Sayed, K. M. (2021). Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review. Polymers, 13(17), 2950. https://doi.org/10.3390/polym13172950