Nanoparticles Addition in PU Foams: The Dramatic Effect of Trapped-Air on Nucleation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
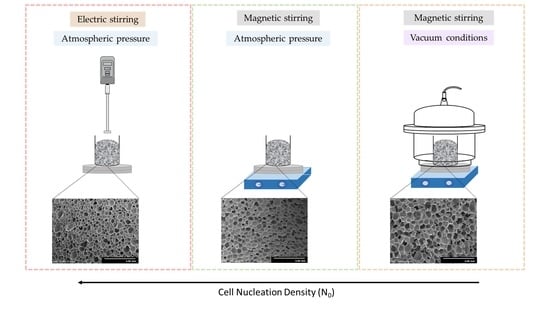
2.2. Fabrication Method
2.3. Characterization
2.3.1. Viscosity
2.3.2. Density
2.3.3. Cell Size
2.3.4. Cell Density and Number of Nucleation Points
3. Results and Discussion
3.1. Viscosity
3.2. Foam Density
3.3. Cellular Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hickel, R. Directive 2010/31/EU of the European Parliament and of the Council on the energy performance of buildings. Presse Med. 1955, 63, 619. [Google Scholar] [PubMed]
- Thoemen, H.; Walther, T.; Wiegmann, A. 3D simulation of macroscopic heat and mass transfer properties from the microstructure of wood fibre networks. Compos. Sci. Technol. 2008, 68, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Lopez Hurtado, P.; Rouilly, A.; Vandenbossche, V.; Raynaud, C. A review on the properties of cellulose fibre insulation. Build. Environ. 2016, 96, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Wells, C.M.; Carrington, C.G.; Hewitt, N.J. Thermal conductivity of wool and wool-hemp insulation. Int. J. Energy Res. 2006, 30, 37–49. [Google Scholar] [CrossRef]
- Van De Walle, W.; Janssen, H. Validation of a 3D pore scale prediction model for the thermal conductivity of porous building materials. Energy Procedia 2017, 132, 225–230. [Google Scholar] [CrossRef]
- Beck, A.; Heinemann, U.; Reidinger, M. Thermal Transport in Straw Insulation. J. Build. Phys. 2004, 27, 227–234. [Google Scholar] [CrossRef]
- Viel, M.; Collet, F.; Lanos, C. Development and characterization of thermal insulation materials from renewable resources. Constr. Build. Mater. 2019, 214, 685–697. [Google Scholar] [CrossRef]
- Nagy, B.; Simon, T.K.; Nemes, R. Effect of built-in mineral wool insulations durability on its thermal and mechanical performance. J. Therm. Anal. Calorim. 2020, 139, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Feldman, D. Polymeric foam materials for insulation in buildings. In Materials for Energy Efficiency and Thermal Comfort in Buildings; Woodhead Publishing: Shaxton, UK, 2010; pp. 257–273. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Azdast, T.; Doniavi, A.; Lee, R.E. Multi-objective optimization of heat transfer mechanisms of microcellular polymeric foams from thermal-insulation point of view. Therm. Sci. Eng. Prog. 2019, 9, 21–29. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, flame-retardant, and bio-based rigid polyurethane/polyisocyanurate foams for thermal insulation application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, H.; Choi, Y.; Kim, J.H. Threshold cell diameter for high thermal insulation of water-blown rigid polyurethane foams. J. Ind. Eng. Chem. 2019, 73, 344–350. [Google Scholar] [CrossRef]
- Ferkl, P.; Toulec, M.; Laurini, E.; Pricl, S.; Fermeglia, M.; Auffarth, S.; Eling, B.; Settels, V.; Kosek, J. Multi-scale modelling of heat transfer in polyurethane foams. Chem. Eng. Sci. 2017, 172, 323–334. [Google Scholar] [CrossRef]
- Sacadura, J.F.; Baillis, D. Experimental characterization of thermal radiation properties of dispersed media. Int. J. Therm. Sci. 2002, 41, 699–707. [Google Scholar] [CrossRef]
- Wu, J.W.; Sung, W.F.; Chu, H. Sen Thermal conductivity of polyurethane foams. Int. J. Heat Mass Transf. 1999, 42, 2211–2217. [Google Scholar] [CrossRef]
- Nield, D.A.; Bejan, A. Convection in porous media. In Convection in Porous Media; Springer: Cham, Switzerland, 2013; pp. 1–778. [Google Scholar] [CrossRef]
- Solórzano, E.; Rodriguez-Perez, M.A.; Lazaro, J.; De Saja, J.A. Influence of solid phase conductivity and cellular structure on the heat transfer mechanisms of cellular materials: Diverse case studies. Adv. Eng. Mater. 2009, 11, 818–824. [Google Scholar] [CrossRef]
- Siaueira, G.; Bras, J.; Dufresne, A. Cellulose whiskers versus microfibrils: Influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 2009, 10, 425–432. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Annamalai, P.K.; Martin, D.J. The use of cellulose nanocrystals to enhance the thermal insulation properties and sustainability of rigid polyurethane foam. Ind. Crops Prod. 2017, 107, 114–121. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. J. Non. Cryst. Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Thi, N.H.; Pham, D.L.; Hanh, N.T.; Oanh, H.T.; Yen Duong, T.H.; Nguyen, T.N.; Tuyen, N.D.; Phan, D.L.; Trinh, H.T.; Nguyen, H.T.; et al. Influence of Organoclay on the Flame Retardancy and Thermal Insulation Property of Expandable Graphite/Polyurethane Foam. J. Chem. 2019, 2019, 19–21. [Google Scholar] [CrossRef]
- Verdejo, R.; Saiz-Arroyo, C.; Carretero-Gonzalez, J.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; Lopez-Manchado, M.A. Physical properties of silicone foams filled with carbon nanotubes and functionalized graphene sheets. Eur. Polym. J. 2008, 44, 2790–2797. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.H.; Ahn, J.H.; Kim, J.D.; Park, S.; Park, K.H.; Lee, J.M. Synthesis of nanoparticle-enhanced polyurethane foams and evaluation of mechanical characteristics. Compos. Part B Eng. 2018, 136, 28–38. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C. Polymer Nanocomposites: Electrical and Thermal Properties; Springer: Berlin, Germany, 2016; ISBN 978-3-319-28238-1. [Google Scholar]
- Marhoon, I.I. Effect of Silica-Fume Microparticles on Rigid Polyurethane Foam Properties. Int. J. Sci. Technol. Res. 2015, 4, 96–100. [Google Scholar]
- Chen, H.; Lu, H.; Zhou, Y.; Zheng, M.; Ke, C.; Zeng, D. Study on thermal properties of polyurethane nanocomposites based on organo- sepiolite. Polym. Degrad. Stab. 2012, 97, 242–247. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, H.C.; Jeong, H.M.; Kim, B.K. Glass fiber reinforced rigid polyurethane foams. J. Mater. Sci. 2010, 45, 2675–2680. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym. Plast. Technol. Eng. 2018, 57, 346–369. [Google Scholar] [CrossRef]
- Widya, T.; Macosko, C.W. Nanoclay-modified rigid polyurethane foam. J. Macromol. Sci.-Phys. 2005, 44B, 897–908. [Google Scholar] [CrossRef]
- Saha, M.C.; Kabir, M.E.; Jeelani, S. Enhancement in thermal and mechanical properties of polyurethane foam infused with nanoparticles. Mater. Sci. Eng. A 2008, 479, 213–222. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, M.C.; Kim, H.D.; Park, H.C.; Jeong, H.M.; Yoon, K.S.; Nanoclay, B.K.K. Reinforced Rigid Polyurethane Foams. J. Appl. Polym. Sci. 2010, 117, 1992–1997. [Google Scholar] [CrossRef]
- Cao, X.; James Lee, L.; Widya, T.; Macosko, C. Polyurethane/clay nanocomposites foams: Processing, structure and properties. Polymer 2005, 46, 775–783. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of a nanoclay on the mechanical, thermal and flame retardant properties of rigid polyurethane foam. J. Macromol. Sci. Part A Pure Appl. Chem. 2009, 46, 704–712. [Google Scholar] [CrossRef]
- Pardo-Alonso, S.; Solórzano, E.; Estravís, S.; Rodríguez-Perez, M.A.; De Saja, J.A. In situ evidence of the nanoparticle nucleating effect in polyurethane-nanoclay foamed systems. Soft Matter 2012, 8, 11262–11270. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, J.M.; Kim, M.S.; Kim, Y.H.; Kim, W.N.; Jang, W.; Shin, D.S. Effects of nucleating agents on the morphological, mechanical and thermal insulating properties of rigid polyurethane foams. Macromol. Res. 2009, 17, 856–862. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Ruiz-Herrero, J.L.; Rodríguez-Pérez, M.Á.; Villafañe, F. The effects of functional nanofillers on the reaction kinetics, microstructure, thermal and mechanical properties of water blown rigid polyurethane foams. Polymer 2018, 150, 138–149. [Google Scholar] [CrossRef]
- Estravís, S.; Tirado-Mediavilla, J.; Santiago-Calvo, M.; Ruiz-Herrero, J.L.; Villafañe, F.; Rodríguez-Pérez, M.Á. Rigid polyurethane foams with infused nanoclays: Relationship between cellular structure and thermal conductivity. Eur. Polym. J. 2016, 80, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Kanti, A.; Karan, T.; Gandhi, S.; Rahaman, A.; Rehaan, M. Mechanical property enhancement of flexible polyurethane foam using alumina particles. Mater. Today Proc. 2021, 45, 4040–4044. [Google Scholar] [CrossRef]
- Kanner, B.; Decker, T.G. Urethane Foam Formation & Mdash; Role of the Silicone Surfactant. J. Cell. Plast. 1969, 5, 32–39. [Google Scholar] [CrossRef]
- Pérez-tamarit, S.; Solórzano, E.; Mokso, R.; Rodríguez-pérez, M.A. In-situ understanding of pore nucleation and growth in polyurethane foams by using real-time synchrotron X-ray tomography. Polymer 2019, 166, 50–54. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Foaming and Blowing Agents, 1st ed.; ChemTec Publishing: Toronto, ON, Canada, 2017; Volume 1, ISBN 9781895198997. [Google Scholar]
- Brondi, C.; Di, E.; Bertucelli, L.; Parenti, V.; Mosciatti, T. Competing bubble formation mechanisms in rigid polyurethane foaming. Polymer 2021, 228, 123877. [Google Scholar] [CrossRef]
- ASTM D4878-15. Standard Test Methods for Polyurethane Raw Materials: Determination of Viscosity of Polyols; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM D1622-08. Standard Test Method for Apparent Density of Rigid Cellular Plastics; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Pinto, J.; Solórzano, E.; Rodriguez-Perez, M.A.; de Saja, J.A. Characterization of the cellular structure based on user-interactive image analysis procedures. J. Cell. Plast. 2013, 49, 555–575. [Google Scholar] [CrossRef]
- Kumar, V.; Suh, N.P. A process for making microcellular thermoplastic parts. Polym. Eng. Sci. 1990, 30, 1323–1329. [Google Scholar] [CrossRef]



| Polyol Properties | |
|---|---|
| Polyol/isocyanate ratio | 100/183 |
| OH index (mg KOH g−1) | 455 |
| Polyol viscosity (25 °C) (mPa·s) | 5250 |
| Polyol molecular weight (g mol−1) | 555 |
| Polyol density (25 °C) (g cm−3) | 1.08 |
| Polyol water content (%) | 0.1 |
| Component | pphp |
|---|---|
| TEGOSTAB® B 8522 | 1.0 |
| PMDETA | 0.3 |
| TEGOAMIN® DMCHA | 1.0 |
| Water | 5.0 |
| Properties | Cloisite® Na+ | Cloisite® 30B |
|---|---|---|
| Treatment | None | Methyl tallow-bis-2-hydroxyethyl quaternary ammonium |
| Density (g cm−3) | 2.86 | 1.98 |
| Loose bulk density (g cm−3) | 0.20 | 0.23 |
| Packed bulk density (g cm−3) | 0.34 | 0.36 |
| X-ray diffraction d-spacing (nm) | 1.17 | 1.85 |
| Sample | Mixing Conditions | Stirrer Used | Clay 30B | Clay Na+ |
|---|---|---|---|---|
| E-A-Pure | Air | Electric | - | - |
| M-A-Pure | Air | Magnetic | - | - |
| M-V-Pure | Vacuum | Magnetic | - | - |
| E-A-0.5% 30B | Air | Electric | 0.5 wt% | - |
| M-A-0.5% 30B | Air | Magnetic | 0.5 wt% | - |
| M-V-0.5% 30B | Vacuum | Magnetic | 0.5 wt% | - |
| E-A-1% 30B | Air | Electric | 1 wt% | - |
| M-A-1% 30B | Air | Magnetic | 1 wt% | - |
| M-V-1% 30B | Vacuum | Magnetic | 1 wt% | - |
| E-A-0.5% Na | Air | Electric | - | 0.5 wt% |
| M-A-0.5% Na | Air | Magnetic | - | 0.5 wt% |
| M-V-0.5% Na | Vacuum | Magnetic | - | 0.5 wt% |
| E-A-1% Na | Air | Electric | - | 1 wt% |
| M-A-1% Na | Air | Magnetic | - | 1 wt% |
| M-V-1% Na | Vacuum | Magnetic | - | 1 wt% |
| Sample | Density (kg m−3) | Relative Density | Cell Size (µm) | NSD | N0 |
|---|---|---|---|---|---|
| E-A-Pure | 44.18 ± 1.92 | 0.038 | 519.6 | 0.24 | 3.4 × 105 |
| M-A-Pure | 60.43 ± 5.47 | 0.052 | 570.2 | 0.16 | 1.9 × 105 |
| M-V-Pure | 62.11 ± 4.61 | 0.054 | 799.9 | 0.18 | 6.6 × 104 |
| E-A-0.5% 30B | 43.08 ± 0.11 | 0.037 | 471.9 | 0.17 | 4.7 × 105 |
| M-A-0.5% 30B | 67.57 ± 4.88 | 0.058 | 527.4 | 0.16 | 2.1 × 105 |
| M-V-0.5% 30B | 61.26 ± 2.96 | 0.053 | 991.7 | 0.13 | 3.5 × 104 |
| E-A-1% 30B | 41.49 ± 2.05 | 0.036 | 465.8 | 0.26 | 5.1 × 105 |
| M-A-1% 30B | 72.22 ± 2.67 | 0.062 | 507.9 | 0.20 | 2.2 × 105 |
| M-V-1% 30B | 73.27 ± 3.58 | 0.063 | 680.5 | 0.14 | 9.0 × 104 |
| E-A-0.5% Na | 44.90 ± 0.76 | 0.039 | 496.6 | 0.25 | 3.9 × 105 |
| M-A-0.5% Na | 71.51 ± 6.41 | 0.062 | 499.5 | 0.16 | 2.3 × 105 |
| M-V-0.5% Na | 73.73 ± 1.05 | 0.064 | 688.2 | 0.16 | 8.6 × 104 |
| E-A-1% Na | 43.83 ± 0.88 | 0.038 | 402.1 | 0.23 | 7.5 × 105 |
| M-A-1% Na | 80.27 ± 3.61 | 0.069 | 479.6 | 0.18 | 2.3 × 105 |
| M-V-1% Na | 77.62 ± 1.54 | 0.067 | 706.5 | 0.14 | 7.6 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merillas, B.; Villafañe, F.; Rodríguez-Pérez, M.Á. Nanoparticles Addition in PU Foams: The Dramatic Effect of Trapped-Air on Nucleation. Polymers 2021, 13, 2952. https://doi.org/10.3390/polym13172952
Merillas B, Villafañe F, Rodríguez-Pérez MÁ. Nanoparticles Addition in PU Foams: The Dramatic Effect of Trapped-Air on Nucleation. Polymers. 2021; 13(17):2952. https://doi.org/10.3390/polym13172952
Chicago/Turabian StyleMerillas, Beatriz, Fernando Villafañe, and Miguel Ángel Rodríguez-Pérez. 2021. "Nanoparticles Addition in PU Foams: The Dramatic Effect of Trapped-Air on Nucleation" Polymers 13, no. 17: 2952. https://doi.org/10.3390/polym13172952
APA StyleMerillas, B., Villafañe, F., & Rodríguez-Pérez, M. Á. (2021). Nanoparticles Addition in PU Foams: The Dramatic Effect of Trapped-Air on Nucleation. Polymers, 13(17), 2952. https://doi.org/10.3390/polym13172952