Composites of a Polypropylene Random Copolymer and Date Stone Flour: Crystalline Details and Mechanical Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Chemical Modification of Date Stone
2.3. Preparation of Composites and Films
2.4. Scanning Electron Microscopy
2.5. X-ray Diffraction Experiments
2.6. Differential Scanning Calorimetry
2.7. Microhardness
3. Results
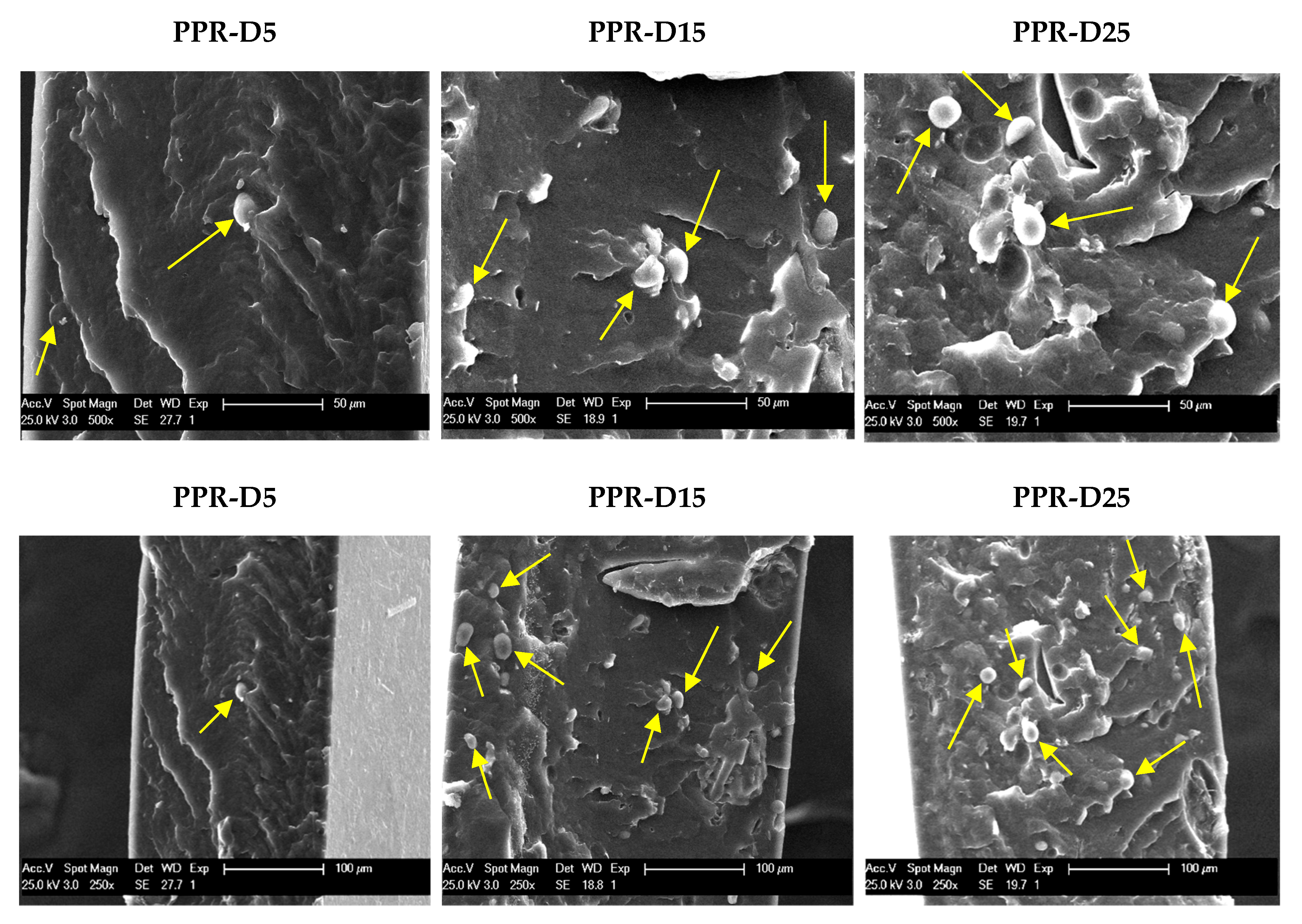
3.1. Scanning Electron Microscopy
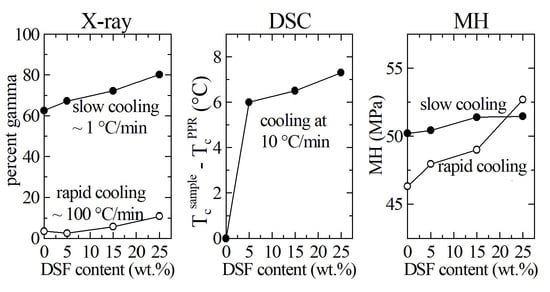
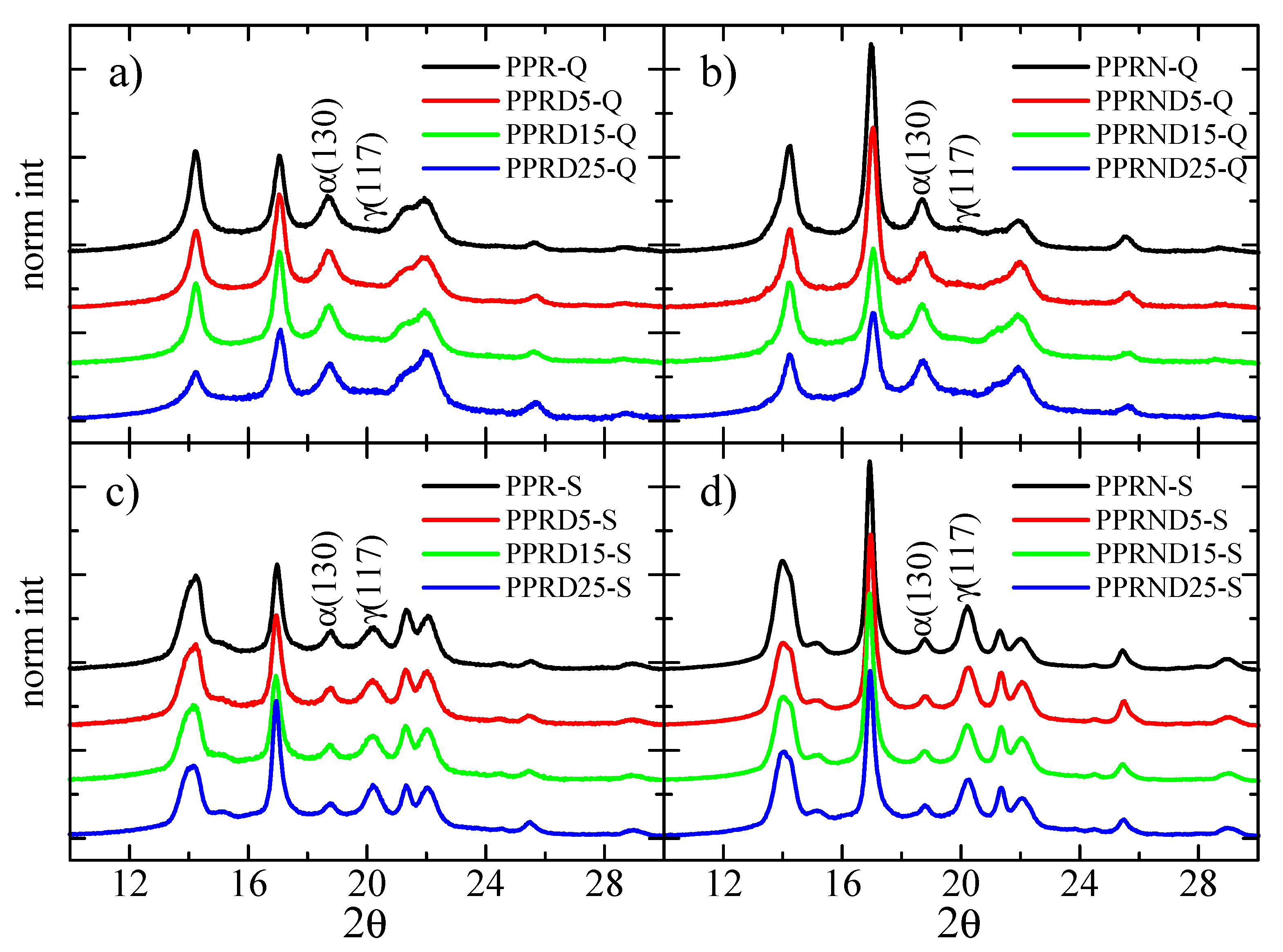
3.2. X-ray Diffraction
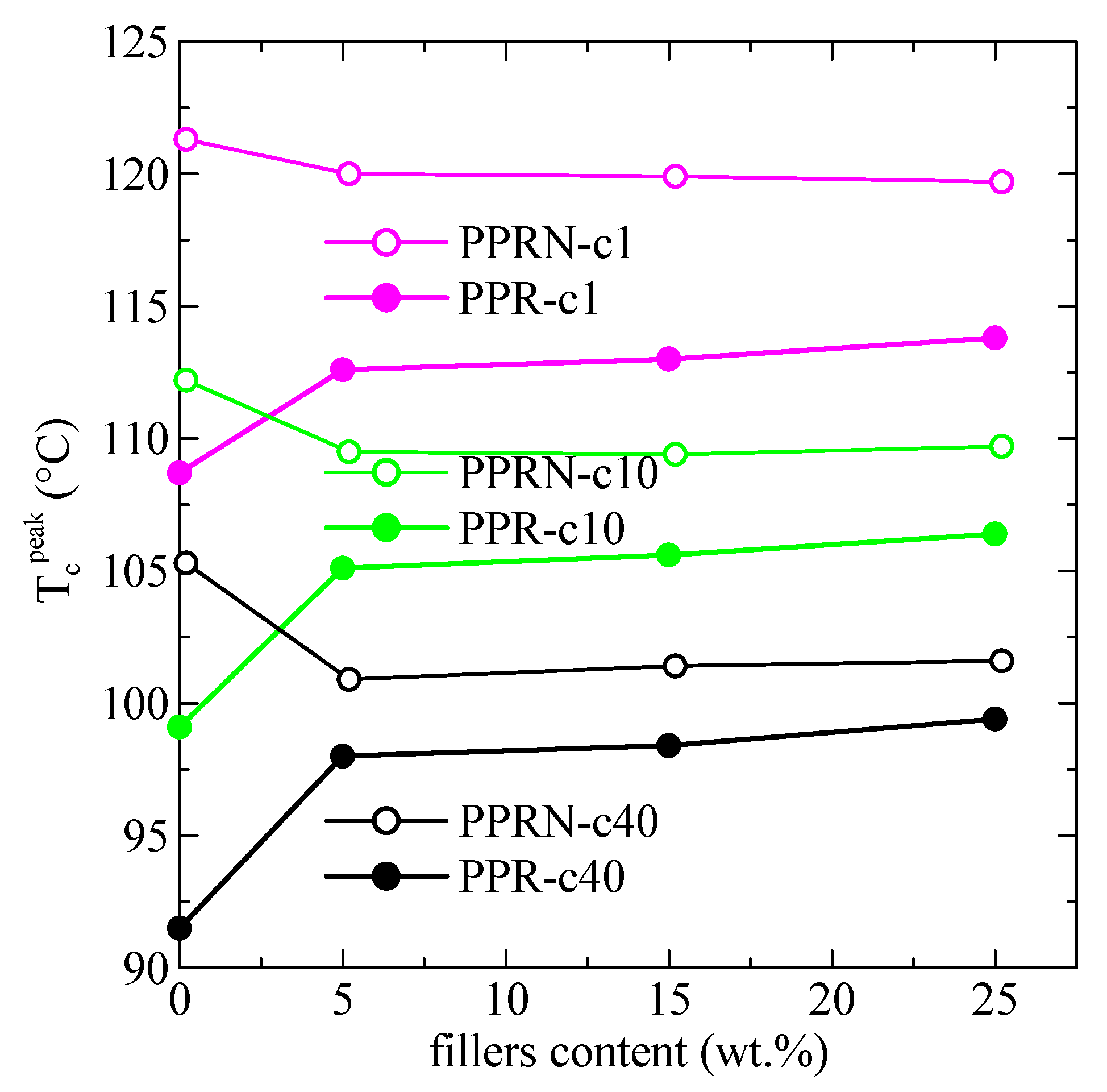
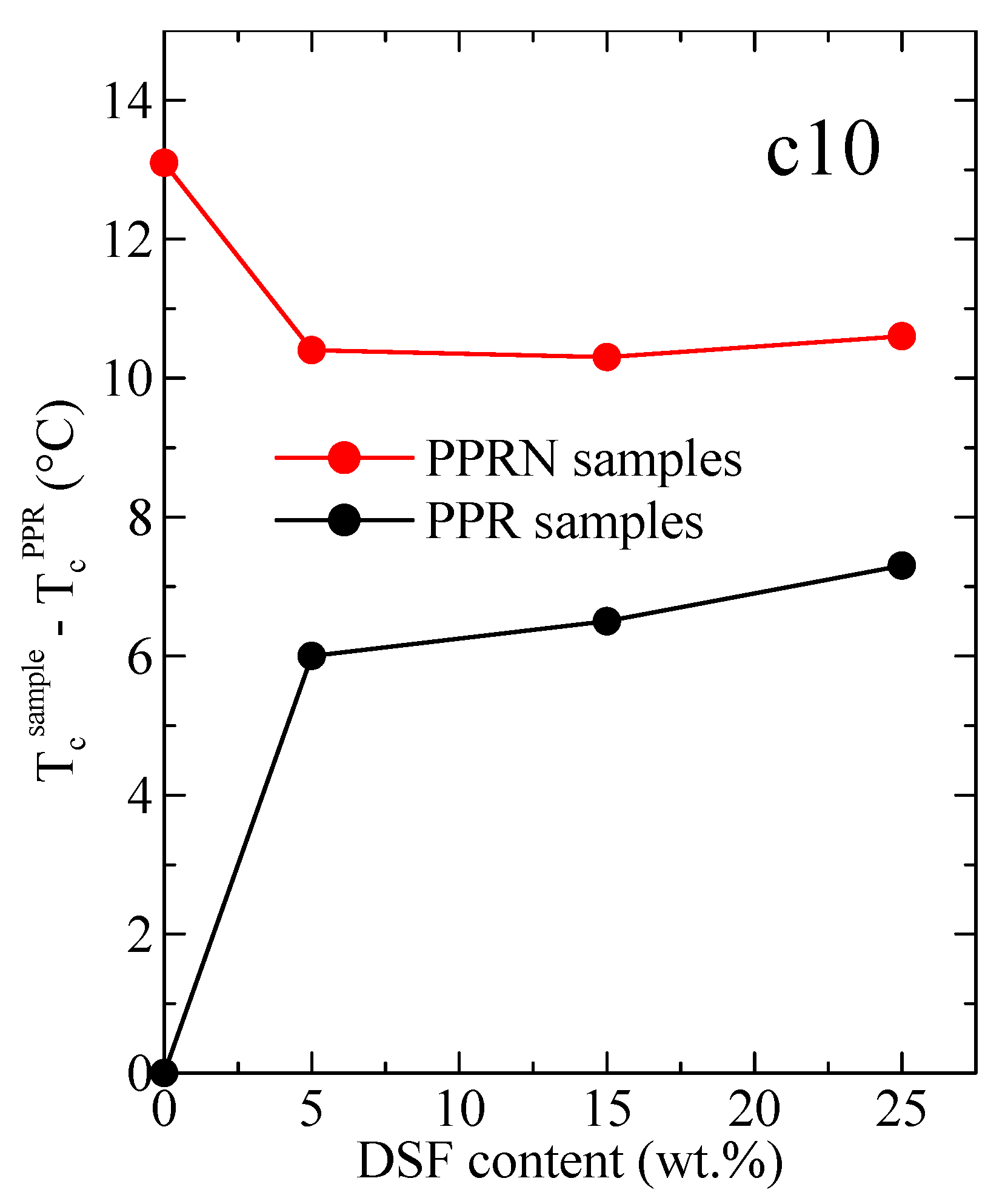
3.3. DSC Results
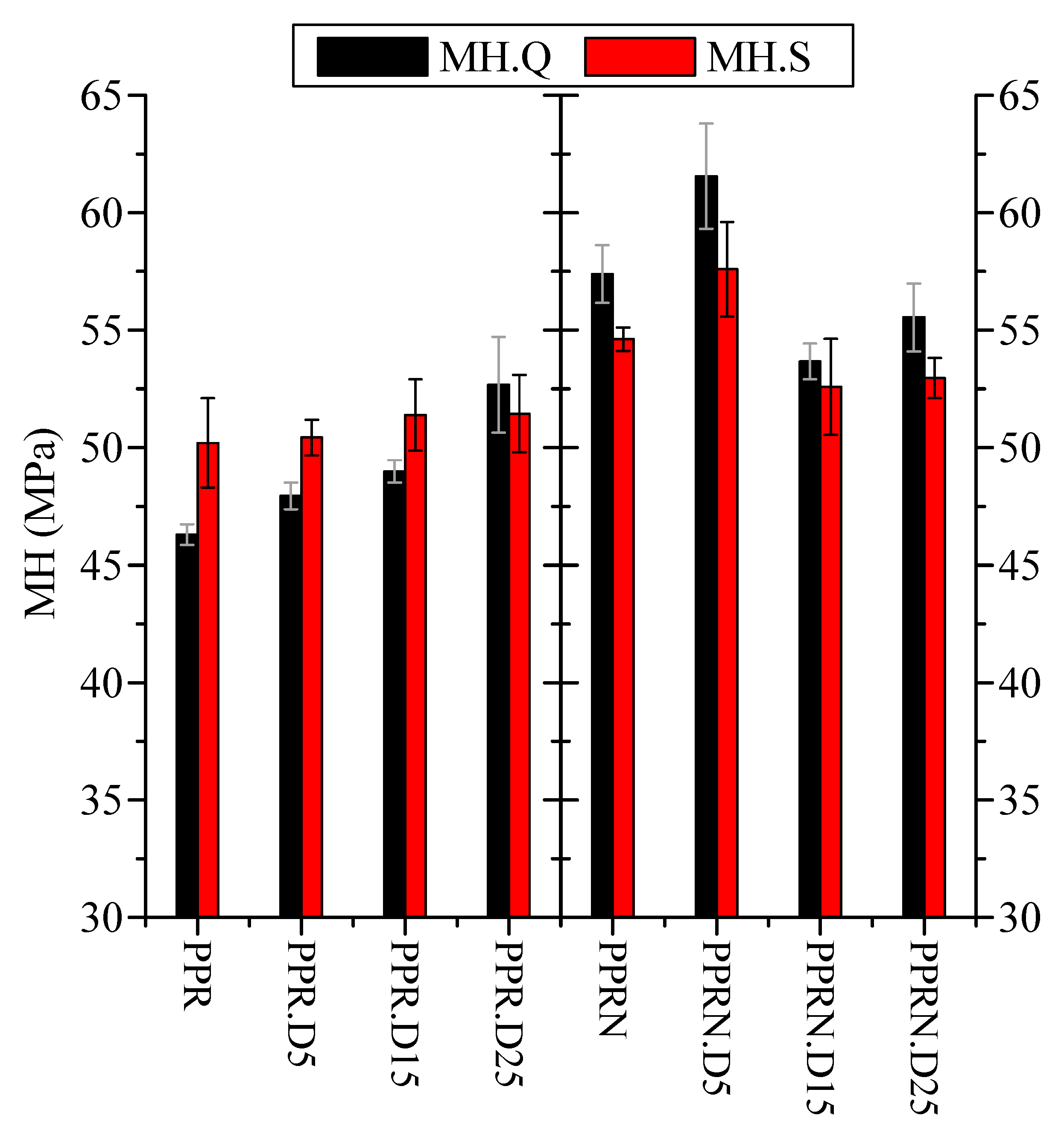
3.4. Microhardness Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- La Mantia, F.P.; Morreale, M. Green composites: A brief review. Compos. Part A 2011, 42, 579–588. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Alaimo, G.; Morreale, M. Green Composites Based on PLA and Agricultural or Marine Waste Prepared by FDM. Polymers 2021, 13, 1361. [Google Scholar] [CrossRef]
- Bogoeva-Gaceva, G.; Avella, M.; Malinconico, M.; Buzarovska, A.; Grozdanov, A.; Gentile, G.; Errico, M.E. Natural fiber eco-composites. Polym. Compos. 2007, 28, 98–107. [Google Scholar] [CrossRef]
- Vaisanen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energ. Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Hamma, A.; Kaci, M.; Pegoretti, A. Polypropylene/date stone flour composites: Effects of filler contents and EBAGMA compatibilizer on morphology, thermal, and mechanical properties. J. Appl. Polym. Sci. 2013, 128, 4314–4321. [Google Scholar] [CrossRef]
- Gachter, R.; Muller, H. Plastics Additives, 3rd ed.; Gachter, R., Muller, H., Eds.; Hanser Verlag: Munich, Germany, 1990. [Google Scholar]
- Cerrada, M.L.; Benavente, R.; Pérez, E. Effect of short glass fiber on structure and mechanical behavior of an ethylene-1-octene copolymer. Macromol. Chem. Phys. 2001, 202, 2686–2695. [Google Scholar] [CrossRef]
- Serrano-Garcia, W.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Tran Thang, Q.; Baskar, C.; Thomas, S.W.; Ramakrishna, S. Nanocomposites for electronic applications that can be embedded for textiles and wearables. Sci. China Technol. Sci. 2019, 62, 895–902. [Google Scholar] [CrossRef]
- Tran, T.Q.; Lee, J.K.Y.; Chinnappan, A.; Jayathilaka, W.A.D.M.; Ji, D.; Kumar, V.V.; Ramakrishna, S. Strong, lightweight, and highly conductive CNT/Au/Cu wires from sputtering and electroplating methods. J. Mater. Sci. Technol. 2020, 40, 99–106. [Google Scholar] [CrossRef]
- Alawar, A.; Hamed, A.M.; Al-Kaabi, K. Characterization of treated date palm tree fiber as composite reinforcement. Compos. Part B 2009, 40, 601–606. [Google Scholar] [CrossRef]
- Kaddami, H.; Dufresne, A.; Khelifi, B.; Bendahou, A.; Taourirte, M.; Raihane, M.; Issartel, N.; Sautereau, H.; Gerard, J.-F.; Sami, N. Short palm tree fibers—Thermoset matrices composites. Compos. Part A 2006, 37, 1413–1422. [Google Scholar] [CrossRef]
- Al-Khanbashi, A.; Al-Kaabi, K.; Hammami, A. Date palm fibers as polymeric matrix reinforcement: Fiber characterization. Polym. Compos. 2005, 26, 486–497. [Google Scholar] [CrossRef]
- Bendahou, A.; Kaddami, H.; Sautereau, H.; Raihane, M.; Erchiqui, F.; Dufresne, A. Short palm tree fibers polyolefin composites: Effect of filler content and coupling agent on physical properties. Macromol. Mater. Eng. 2008, 293, 140–148. [Google Scholar] [CrossRef]
- Karian, H.G. Handbook of Polypropylene and Polypropylene Composites, 2nd ed.; Karian, H.G., Ed.; Marcel Dekker: New York, NY, USA, 2009. [Google Scholar]
- Arranz-Andrés, J.; Suárez, I.; Benavente, R.; Pérez, E. Characterization and properties of ethylene-propylene copolymers synthesized with homogeneous and supported metallocene catalyst in the whole range of compositions. Macromol. Res. 2011, 19, 351–363. [Google Scholar] [CrossRef]
- Pérez, E.; Gómez-Elvira, J.M.; Benavente, R.; Cerrada, M.L. Tailoring the formation rate of the mesophase in random propylene-co-1-pentene copolymers. Macromolecules 2012, 45, 6481–6490. [Google Scholar] [CrossRef]
- Danyadi, L.; Renner, K.; Szabo, Z.; Nagy, G.; Moczo, J.; Pukanszky, B. Wood flour filled PP composites: Adhesion, deformation, failure. Polym. Adv. Technol. 2006, 17, 967–974. [Google Scholar] [CrossRef]
- Vardai, R.; Lummerstorfer, T.; Pretschuh, C.; Jerabek, M.; Gahleitner, M.; Pukanszky, B.; Renner, K. Impact modification of PP/wood composites: A new approach using hybrid fibers. Express Polym. Lett. 2019, 13, 223–234. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, H.; Njuguna, J.; Abhyankar, H. Recent Development of Flax Fibres and Their Reinforced Composites Based on Different Polymeric Matrices. Materials 2013, 6, 5171–5198. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part A 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Peltola, H.; Paakkonen, E.; Jetsu, P.; Heinemann, S. Wood based PLA and PP composites: Effect of fibre type and matrix polymer on fibre morphology, dispersion and composite properties. Compos. Part A 2014, 61, 13–22. [Google Scholar] [CrossRef]
- Bouza, R.; Marco, C.; Ellis, G.; Martin, Z.; Gomez, M.A.; Barral, L. Analysis of the isothermal crystallization of polypropylene/wood flour composites. J. Therm. Anal. Calorim. 2008, 94, 119–127. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Q.; Li, S.; Ou, R.; Wang, Q. Toughness and crystallization enhancement in wood fiber-reinforced polypropylene composite through controlling matrix nucleation. J. Mater. Sci. 2018, 53, 6542–6551. [Google Scholar] [CrossRef]
- Shin, Y.W.; Uozumi, T.; Terano, M.; Nitta, K.H. Synthesis and characterization of ethylene-propylene random copolymers with isotactic propylene sequence. Polymer 2001, 42, 9611–9615. [Google Scholar] [CrossRef]
- Gahleitner, M.; Jaaskelainen, P.; Ratajski, E.; Paulik, C.; Reussner, J.; Wolfschwenger, J.; Neissl, W. Propylene-ethylene random copolymers: Comonomer effects on crystallinity and application properties. J. Appl. Polym. Sci. 2005, 95, 1073–1081. [Google Scholar] [CrossRef]
- Yu, L.; Wu, T.; Chen, T.; Yang, F.; Xiang, M. Polypropylene random copolymer in pipe application: Performance improvement with controlled molecular weight distribution. Thermochim. Acta 2014, 578, 43–52. [Google Scholar] [CrossRef]
- Caveda, S.; Pérez, E.; Blázquez-Blázquez, E.; Peña, B.; van Grieken, R.; Suárez, I.; Benavente, R. Influence of structure on the properties of polypropylene copolymers and terpolymers. Polym. Test. 2017, 62, 23–32. [Google Scholar] [CrossRef]
- Bodirlau, R.; Spiridon, I.; Teaca, C.A. Chemical investigation of wood tree species in temperate forest in East-Northern Romania. Bioresources 2007, 2, 41–57. [Google Scholar] [CrossRef]
- Madhu, P.; Sanjay, M.R.; Jawaid, M.; Siengchin, S.; Khan, A.; Pruncu, C.I. A new study on effect of various chemical treatments on Agave Americana fiber for composite reinforcement: Physico-chemical, thermal, mechanical and morphological properties. Polym. Test. 2020, 85, 106437. [Google Scholar] [CrossRef]
- Hong, C.K.; Hwang, I.; Kim, N.; Park, D.H.; Hwang, B.S.; Nah, C. Mechanical properties of silanized jute-polypropylene composites. J. Ind. Eng. Chem. 2008, 14, 71–76. [Google Scholar] [CrossRef]
- Mansel, S.; Pérez, E.; Benavente, R.; Pereña, J.M.; Bello, A.; Röll, W.; Kirsten, R.; Beck, S.; Brintzinger, H.H. Synthesis and properties of elastomeric poly(propylene). Macromol. Chem. Phys. 1999, 200, 1292–1297. [Google Scholar] [CrossRef][Green Version]
- Prieto, O.; Pereña, J.M.; Benavente, R.; Pérez, E.; Cerrada, M.L. Viscoelastic relaxation mechanisms of conventional polypropylene toughened by a plastomer. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1878–1888. [Google Scholar] [CrossRef]
- Krache, R.; Benavente, R.; López-Majada, J.M.; Perena, J.M.; Cerrada, M.L.; Pérez, E. Competition between α, β, and γ polymorphs in a β-nucleated metallocenic isotactic polypropylene. Macromolecules 2007, 40, 6871–6878. [Google Scholar] [CrossRef]
- Bond, E.B.; Spruiell, J.E.; Lin, J.S. A WAXD/SAXS/DSC study on the melting behavior of Ziegler-Natta and metallocene catalyzed isotactic polypropylene. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 3050–3064. [Google Scholar] [CrossRef]
- Fonseca, C.; Pereña, J.M.; Benavente, R.; Cerrada, M.L.; Bello, A.; Pérez, E. Microhardness and thermal study of the annealing effects in vinyl alcohol-ethylene copolymers. Polymer 1995, 36, 1887–1892. [Google Scholar] [CrossRef]
- Baltá-Calleja, F.J. Microhardness relating to crystalline polymers. Adv. Polym. Sci. 1985, 66, 117–148. [Google Scholar]
- Natta, G.; Corradini, P. Structure and properties of isotactic polypropylene. Stereoregul. Polym. Stereospecif. Polym. 1960, 15, 40–51. [Google Scholar] [CrossRef]
- Miller, R.L. On the existence of near-range order in isotactic polypropylenes. Polymer 1960, 1, 135–143. [Google Scholar] [CrossRef]
- Turner-Jones, A.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Makromol. Chem. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Brückner, S.; Meille, S.V.; Petraccone, V.; Pirozzi, B. Polymorphism in isotactic polypropylene. Prog. Polym. Sci. 1991, 16, 361–404. [Google Scholar] [CrossRef]
- Lotz, B.; Wittmann, J.C.; Lovinger, A.J. Structure and morphology of poly(propylenes): A molecular analysis. Polymer 1996, 37, 4979–4992. [Google Scholar] [CrossRef]
- Varga, J. Supermolecular structure of isotactic polypropylene. J. Mater. Sci. 1992, 27, 2557–2579. [Google Scholar] [CrossRef]
- Polo-Corpa, M.J.; Benavente, R.; Velilla, T.; Quijada, R.; Perez, E.; Cerrada, M.L. Development of the mesomorphic phase in isotactic propene/higher alpha-olefin copolymers at intermediate comonomer content and its effect on properties. Eur. Polym. J. 2010, 46, 1345–1354. [Google Scholar] [CrossRef][Green Version]
- Poon, B.; Rogunova, M.; Hiltner, A.; Baer, E.; Chum, S.P.; Galeski, A.; Piorkowska, E. Structure and properties of homogeneous copolymers of propylene and 1-hexene. Macromolecules 2005, 38, 1232–1243. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Elvira, J.M.; Barranco-García, R.; Pérez, E.; Cerrada, M.L. Trigonal δ form as a tool for tuning mechanical behavior in poly(propylene-co-1-pentene-co-1-heptene) terpolymers. Polymer 2016, 99, 112–121. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, C.; Tang, C.; Zhang, Q.; Du, R.; Fu, Q.; Li, L. Rheologically determined negative influence of increasing nucleating agent content on the crystallization of isotactic polypropylene. Polymer 2009, 50, 696–706. [Google Scholar] [CrossRef]
- Horvath, Z.; Menyhard, A.; Doshev, P.; Gahleitner, M.; Friel, D.; Varga, J.; Pukanszky, B. Improvement of the impact strength of ethylene-propylene random copolymers by nucleation. J. Appl. Polym. Sci. 2016, 133, 43823. [Google Scholar] [CrossRef]
- Hosier, I.L.; Alamo, R.G.; Esteso, P.; Isasi, J.R.; Mandelkern, L. Formation of the alpha and gamma polymorphs in random metallocene-propylene copolymers. Effect of concentration and type of comonomer. Macromolecules 2003, 36, 5623–5636. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Pérez, E.; Benavente, R.; Ressia, J.; Sarmoria, C.; Vallés, E.M. Gamma polymorph and branching formation as inductors of resistance to electron beam irradiation in metallocene isotactic polypropylene. Polym. Degrad. Stab. 2010, 95, 462–469. [Google Scholar] [CrossRef]
- Barranco-García, R.; López-Majada, J.M.; Martínez, J.C.; Gómez-Elvira, J.M.; Pérez, E.; Cerrada, M.L. Confinement of iPP crystallites within mesoporous SBA-15 channels in extruded iPP-SBA-15 nanocomposites studied by Small Angle X-ray scattering. Micropor. Mesopor. Mat. 2018, 272, 209–216. [Google Scholar] [CrossRef]
- Lorenzo, V.; Perena, J.M.; Fatou, J.M. Relationships between mechanical-properties and microhardness of polyethylenes. Angew. Makromol. Chem. 1989, 172, 25–35. [Google Scholar] [CrossRef]
- Baltá-Calleja, F.J.; Rueda, D.R.; Porter, R.S.; Mead, W.T. New aspects of the microhardness of ultraoriented polyethylene. J. Mater. Sci. 1980, 15, 765–772. [Google Scholar] [CrossRef]
- Engel, P.A.; Derwin, M.D. Indentation Test for Polymer-Film-Coated Computer Board Substrate. In Microindentation Techniques in Materials Science and Engineering; Blau, P.T., Lawn, B.R., Eds.; Special Technical Publication 889; American Society for Testing and Materials: Philadelphia, PA, USA, 1986; pp. 272–285. [Google Scholar]
- Cerrada, M.L.; Benavente, R.; Pérez, E. Crystalline structure and viscoelastic behavior in composites of a metallocenic ethylene-1-octene copolymer and glass fiber. Macromol. Chem. Phys. 2002, 203, 718–726. [Google Scholar] [CrossRef]
- López-Majada, J.M.; Palza, H.; Guevara, J.L.; Quijada, R.; Martínez, M.C.; Benavente, R.; Pereña, J.M.; Pérez, E.; Cerrada, M.L. Metallocene copolymers of propene and 1-hexene: The influence of the comonomer content and thermal history on the structure and mechanical properties. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1253–1267. [Google Scholar] [CrossRef]





| Cellulose | Lignin | Hemicellulose | Ash | Extractable | Water |
|---|---|---|---|---|---|
| 42.6 | 16.3 | 19.2 | 2.2 | 14.8 | 4.9 |
| Sample Code | Composition (wt.%) | ||
|---|---|---|---|
| PPR | DSF | Millad | |
| PPR | 100 | 0 | 0 |
| PPR-D5 | 95 | 5 | 0 |
| PPR-D15 | 85 | 15 | 0 |
| PPR-D25 | 75 | 25 | 0 |
| PPRN | 99.8 | 0 | 0.2 |
| PPRN-D5 | 94.8 | 5 | 0.2 |
| PPRN-D15 | 84.8 | 15 | 0.2 |
| PPRN-D25 | 74.8 | 25 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benarab, A.; Blázquez-Blázquez, E.; Krache, R.; Benavente, R.; Cerrada, M.L.; Pérez, E. Composites of a Polypropylene Random Copolymer and Date Stone Flour: Crystalline Details and Mechanical Response. Polymers 2021, 13, 2957. https://doi.org/10.3390/polym13172957
Benarab A, Blázquez-Blázquez E, Krache R, Benavente R, Cerrada ML, Pérez E. Composites of a Polypropylene Random Copolymer and Date Stone Flour: Crystalline Details and Mechanical Response. Polymers. 2021; 13(17):2957. https://doi.org/10.3390/polym13172957
Chicago/Turabian StyleBenarab, Amina, Enrique Blázquez-Blázquez, Rachida Krache, Rosario Benavente, María L. Cerrada, and Ernesto Pérez. 2021. "Composites of a Polypropylene Random Copolymer and Date Stone Flour: Crystalline Details and Mechanical Response" Polymers 13, no. 17: 2957. https://doi.org/10.3390/polym13172957
APA StyleBenarab, A., Blázquez-Blázquez, E., Krache, R., Benavente, R., Cerrada, M. L., & Pérez, E. (2021). Composites of a Polypropylene Random Copolymer and Date Stone Flour: Crystalline Details and Mechanical Response. Polymers, 13(17), 2957. https://doi.org/10.3390/polym13172957