Modeling and Simulation of Fabricated Graphene Nanoplates/Polystyrene Nanofibrous Membrane for DCMD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication Process
- Solution injection rate = 0.6 mL/h.
- Tip to collector distance = 18 cm.
- Potential difference = 28 kV.
- Solution volume = 3.5 mL.
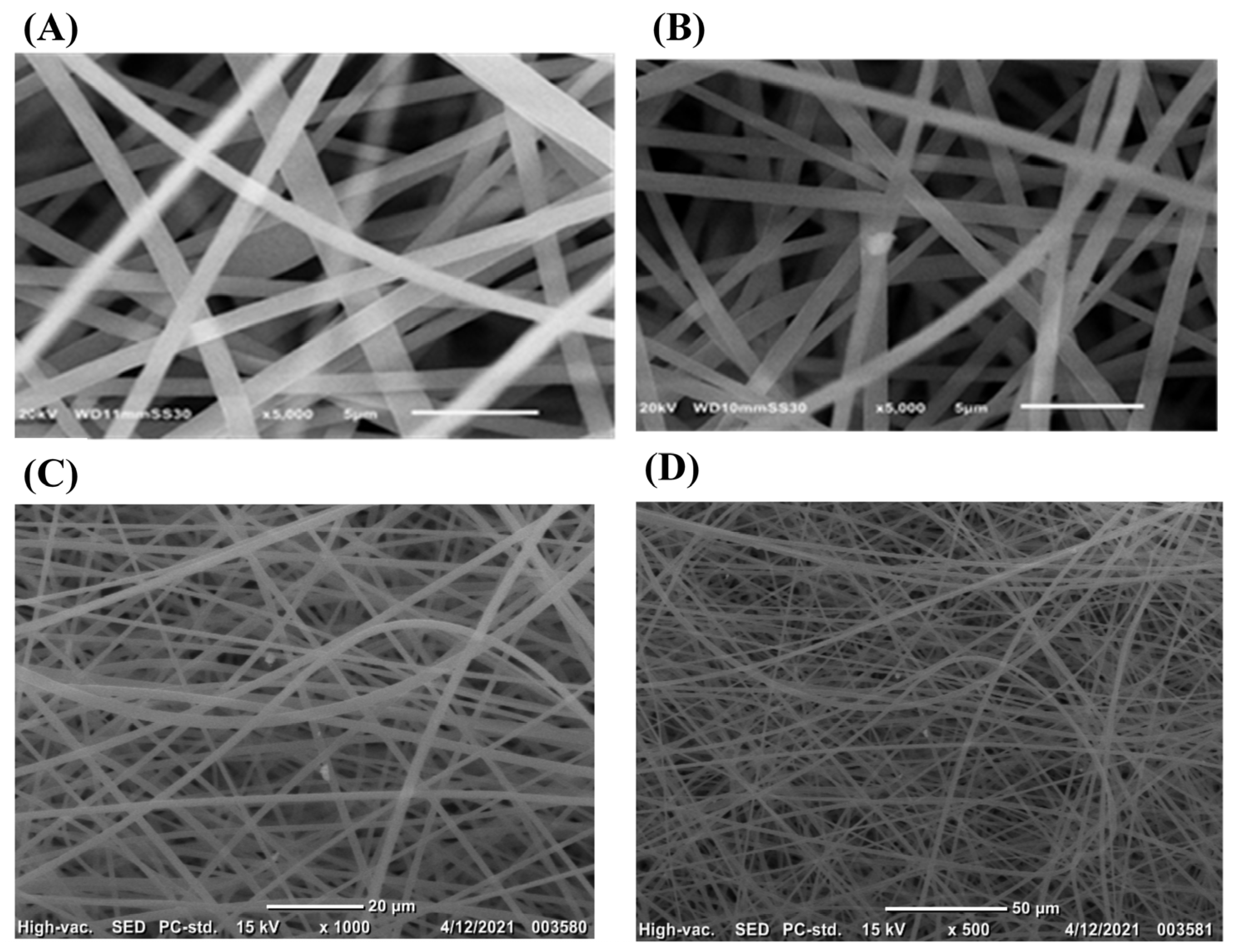
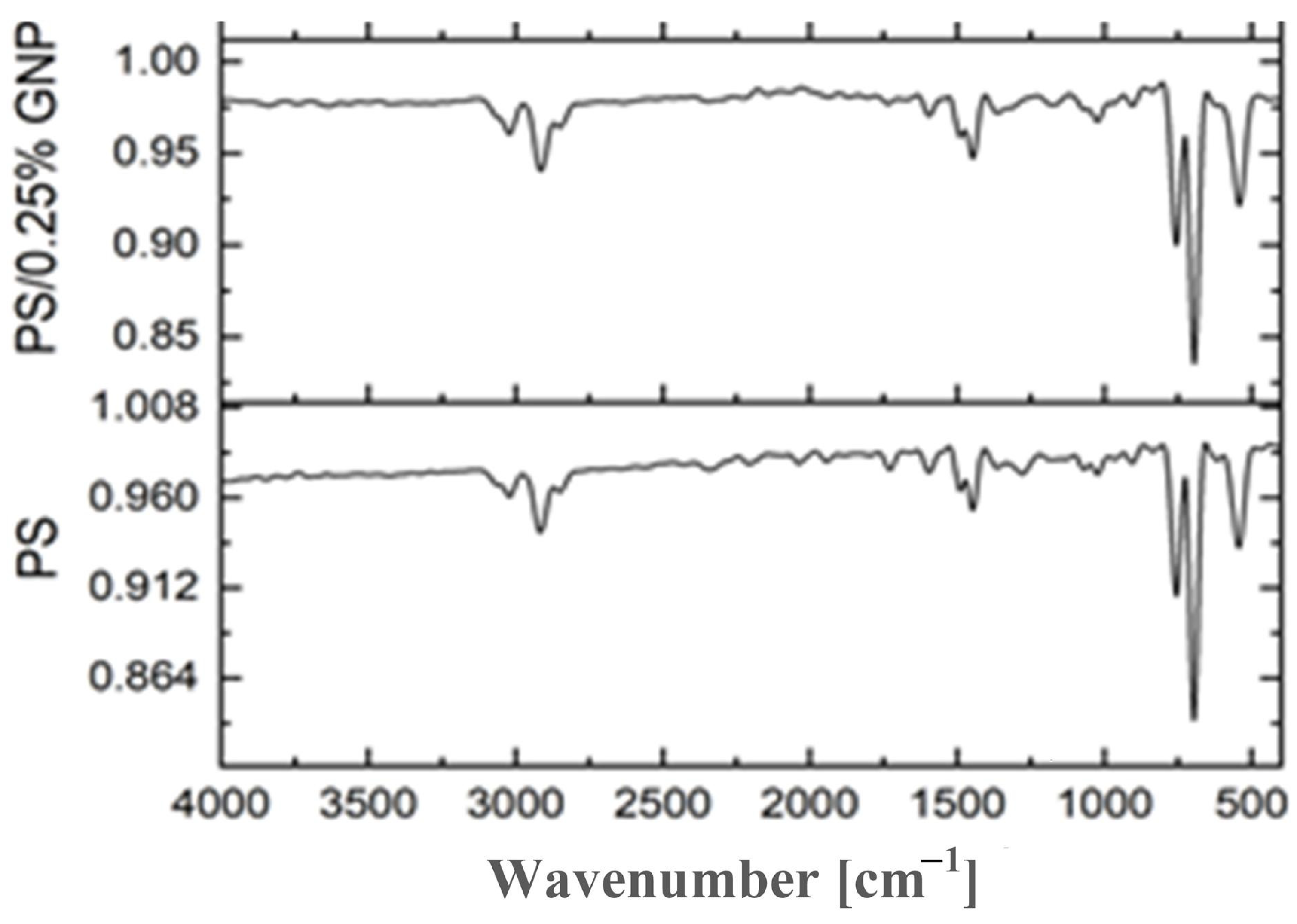
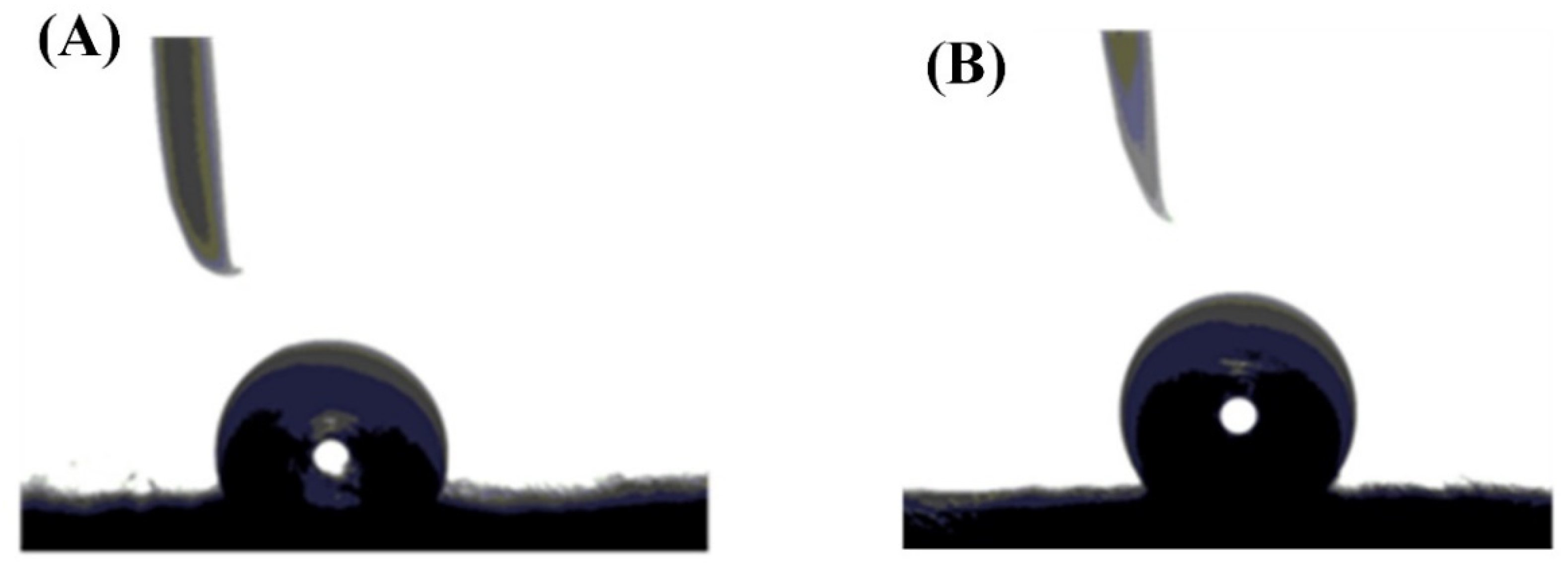
2.2. Characterization
- Scanning Electron Microscope (SEM).
- Fourier-Transform Infra-Red (FTIR).
- Contact Angle.
- Porosity.
3. Mathematical Model
3.1. Mass Conservation
3.2. Momentum Conservation
3.3. Energy Conservation
3.4. Permeate Flux
3.5. Thermal Efficiency
3.6. Temperature Polarization Coefficient
3.7. Porosity
4. Numerical Solution
- Laminar fully developed flow.
- Temperature-independent properties for water.
- The side walls are adiabatic.
- Exclude any chemical reactions.
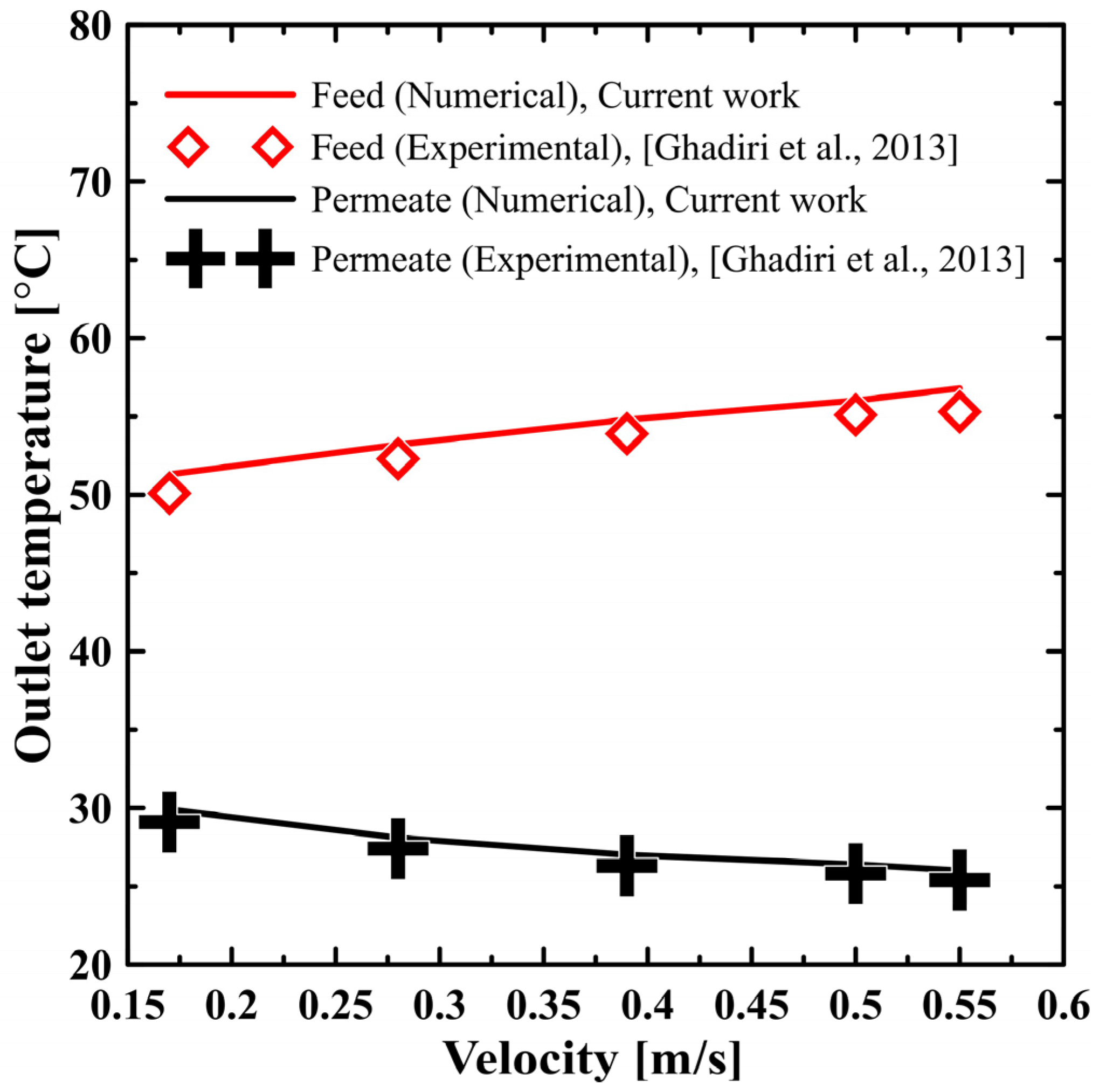
5. Result and Discussion
5.1. Characterization
5.2. Performance on DCMD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Rabie, M.; Salem, M.S.; Ali, A.Y.; El-Shazly, A.; Elkady, M.; Ookawara, S. Modeling of an integrated air gap membrane distillation unit utilizing a flat plate solar collector. Energy Rep. 2020, 6, 1591–1596. [Google Scholar] [CrossRef]
- Rabie, M.; Ali, A.Y.; Abo-Zahhad, E.M.; Elqady, H.I.; Elkady, M.; Ookawara, S.; El-Shazly, A.; Salem, M.S.; Radwan, A. Thermal analysis of a hybrid high concentrator photovoltaic/membrane distillation system for isolated coastal regions. Sol. Energy 2021, 215, 220–239. [Google Scholar] [CrossRef]
- Foureaux, A.F.; Moreira, V.R.; Lebron, Y.; Santos, L.; Amaral, M. Direct contact membrane distillation as an alternative to the conventional methods for value-added compounds recovery from acidic effluents: A review. Sep. Purif. Technol. 2020, 236, 116251. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Rabie, M.; El-Shazly, A.; Bassyouni, M.; Abdel-Hamid, S.; El Kady, M. Numerical investigation of fabricated MWCNTs/polystyrene nanofibrous membrane for DCMD. Polymers 2021, 13, 160. [Google Scholar] [CrossRef]
- Ashoor, B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S.W. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- Ullah, R.; Khraisheh, M.; Esteves, R.J.; McLeskey, J.T.; AlGhouti, M.; Gad-El-Hak, M.; Tafreshi, H.V. Energy efficiency of direct contact membrane distillation. Desalination 2018, 433, 56–67. [Google Scholar] [CrossRef]
- Rabie, M.; Elkady, M.; El-Shazly, A. Effect of channel height on the overall performance of direct contact membrane distillation. Appl. Therm. Eng. 2021, 196, 117262. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, H.; Hu, Z.; Hou, S. A solar driven hybrid photovoltaic module/direct contact membrane distillation system for electricity generation and water desalination. Energy Convers. Manag. 2020, 221, 113146. [Google Scholar] [CrossRef]
- Niknejad, A.S.; Bazgir, S.; Kargari, A. Desalination by direct contact membrane distillation using a superhydrophobic nanofibrous poly (methyl methacrylate) membrane. Desalination 2021, 511, 115108. [Google Scholar] [CrossRef]
- Li, B.; Yun, Y.; Wang, M.; Li, C.; Yang, W.; Li, J.; Liu, G. Superhydrophobic polymer membrane coated by mineralized β-FeOOH nanorods for direct contact membrane distillation. Desalination 2021, 500, 114889. [Google Scholar] [CrossRef]
- Park, D.; Norouzi, E.; Park, C. Experimentally-validated computational simulation of direct contact membrane distillation performance. Int. J. Heat Mass Transf. 2019, 129, 1031–1042. [Google Scholar] [CrossRef]
- Ismail, M.; Mohamed, A.; Poggio, D.; Pourkashanian, M. Direct contact membrane distillation: A sensitivity analysis and an outlook on membrane effective thermal conductivity. J. Membr. Sci. 2021, 624, 119035. [Google Scholar] [CrossRef]
- Anvari, A.; Yancheshme, A.A.; Kekre, K.M.; Ronen, A. State-of-the-art methods for overcoming temperature polarization in membrane distillation process: A review. J. Membr. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Ve, Q.L.; Koirala, R.; Bawahab, M.; Faqeha, H.; Do, M.C.; Nguyen, Q.L.; Date, A.; Akbarzadeh, A. Experimental investigation of the effect of the spacer and operating conditions on mass transfer in direct contact membrane distillation. Desalination 2021, 500, 114839. [Google Scholar] [CrossRef]
- Tibi, F.; Charfi, A.; Cho, J.; Kim, J. Fabrication of polymeric membranes for membrane distillation process and application for wastewater treatment: Critical review. Process Saf. Environ. Prot. 2020, 141, 190–201. [Google Scholar] [CrossRef]
- Ravi, J.; Othman, M.H.D.; Matsuura, T.; Bilad, M.R.; El-Badawy, T.; Aziz, F.; Ismail, A.; Rahman, M.A.; Jaafar, J. Polymeric membranes for desalination using membrane distillation: A review. Desalination 2020, 490, 114530. [Google Scholar] [CrossRef]
- Abdelrazeq, H.; Khraisheh, M.; Al Momani, F.; McLeskey, J.T.; Hassan, M.K.; Gad-El-Hak, M.; Tafreshi, H.V. Performance of electrospun polystyrene membranes in synthetic produced industrial water using direct-contact membrane distillation. Desalination 2020, 493, 114663. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Rabie, M.; El-Shazly, A.H.; Bassyouni, M.; El-Moneim, A.A.; El-Kady, M.F. Investigation of different membrane porosities on the permeate flux of direct contact membrane distillation. Key Eng. Mater. 2021, 889, 85–90. [Google Scholar] [CrossRef]
- Suwwan, D.; Hashaikeh, R.; Janajreh, I. Low energy direct contact membrane desalination: Conjugated heat and high fidelity flow simulation. Energy Procedia 2015, 75, 1722–1727. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Z.; Long, R.; Liu, Z.; Liu, W. Analysis of temperature and concentration polarizations for performance improvement in direct contact membrane distillation. Int. J. Heat Mass Transf. 2019, 145, 118724. [Google Scholar] [CrossRef]
- Dutta, N.; Singh, B.; Subbiah, S.; Muthukumar, P. Performance analysis of a single and multi-staged direct contact membrane distillation module integrated with heat recovery units. Chem. Eng. J. Adv. 2020, 4, 100055. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Ji, S.; Li, Z.; Chen, P. Review of thermal efficiency and heat recycling in membrane distillation processes. Desalination 2015, 367, 223–239. [Google Scholar] [CrossRef]
- Janajreh, I.; Hussain, M.N.; Hashaikeh, R.; Ahmed, R. Thermal efficiency enhancement of the direct contact membrane distillation: Conductive layer integration and geometrical undulation. Appl. Energy 2020, 227, 7–17. [Google Scholar] [CrossRef]
- Ahmed, F.; El Kadi, K.; Hashaikeh, R.; Janajreh, I. Low energy membrane distillation: A numerical study on the role of conductive spacers. Energy Procedia 2017, 142, 4056–4063. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, R.; Field, R.W. Direct contact membrane distillation: An experimental and analytical investigation of the effect of membrane thickness upon transmembrane flux. J. Membr. Sci. 2014, 470, 257–265. [Google Scholar] [CrossRef]
- Ghadiri, M.; Fakhri, S.; Shirazian, S. Modeling and CFD simulation of water desalination using nanoporous membrane contactors. Ind. Eng. Chem. Res. 2013, 52, 3490–3498. [Google Scholar] [CrossRef]
- Salem, M.S.A.; El-Shazly, A.H.; El-Marghany, M.R.; Sabry, M.N.; Nady, N. Effect of adding functionalized graphene on the performance of PVDF membrane in direct contact membrane distillation. Key Eng. Mater. 2019, 801, 337–342. [Google Scholar] [CrossRef]
- Ding, P.; Qu, B. Synthesis and characterization of polystyrene/layered double-hydroxide nanocomposites viain situ emulsion and suspension polymerization. J. Appl. Polym. Sci. 2006, 101, 3758–3766. [Google Scholar] [CrossRef]
- Boubakri, A.; Hafiane, A.; Bouguecha, S.A.T. Nitrate removal from aqueous solution by direct contact membrane distillation using two different commercial membranes. Desalination Water Treat. 2015, 56, 2723–2730. [Google Scholar] [CrossRef]
- Santoro, S.; Vidorreta, I.; Coelhoso, I.; Lima, J.C.; Desiderio, G.; Lombardo, G.; Drioli, E.; Mallada, R.; Crespo, J.; Criscuoli, A.; et al. Experimental evaluation of the thermal polarization in direct contact membrane distillation using electrospun nanofiber membranes doped with molecular probes. Molecules 2019, 24, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Termpiyakul, P.; Jiraratananon, R.; Srisurichan, S. Heat and mass transfer characteristics of a direct contact membrane distillation process for desalination. Desalination 2005, 177, 133–141. [Google Scholar] [CrossRef]

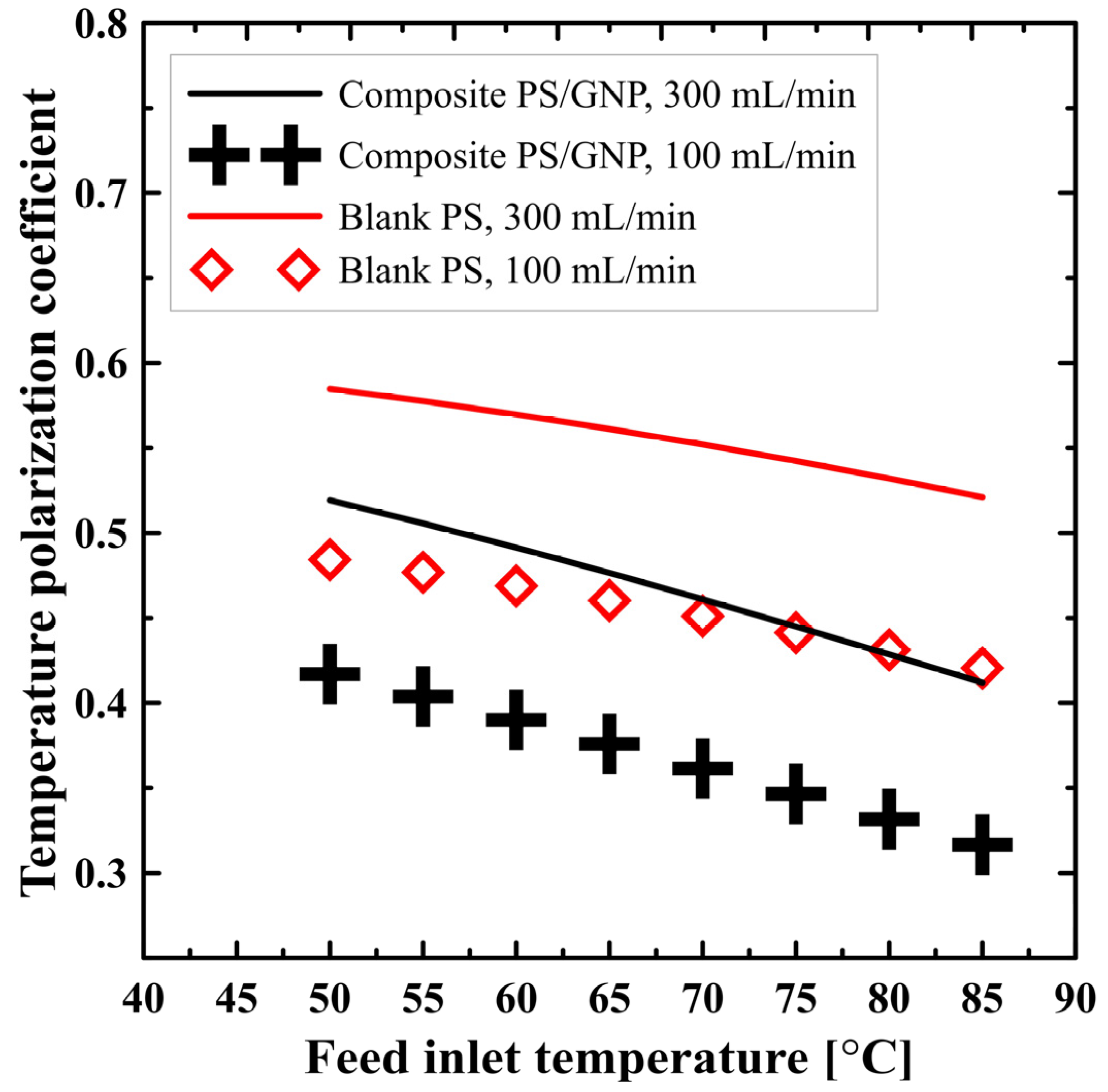
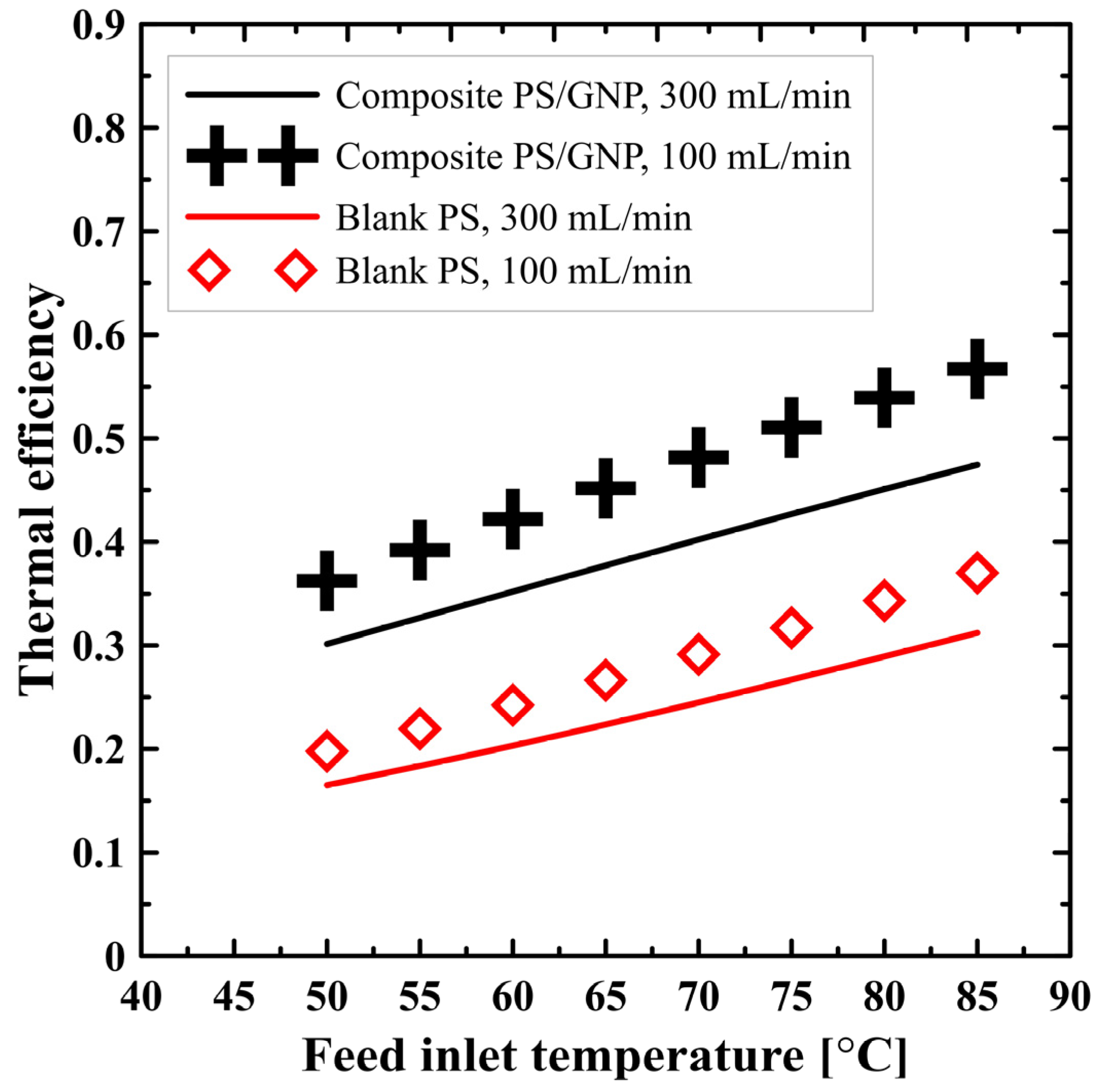
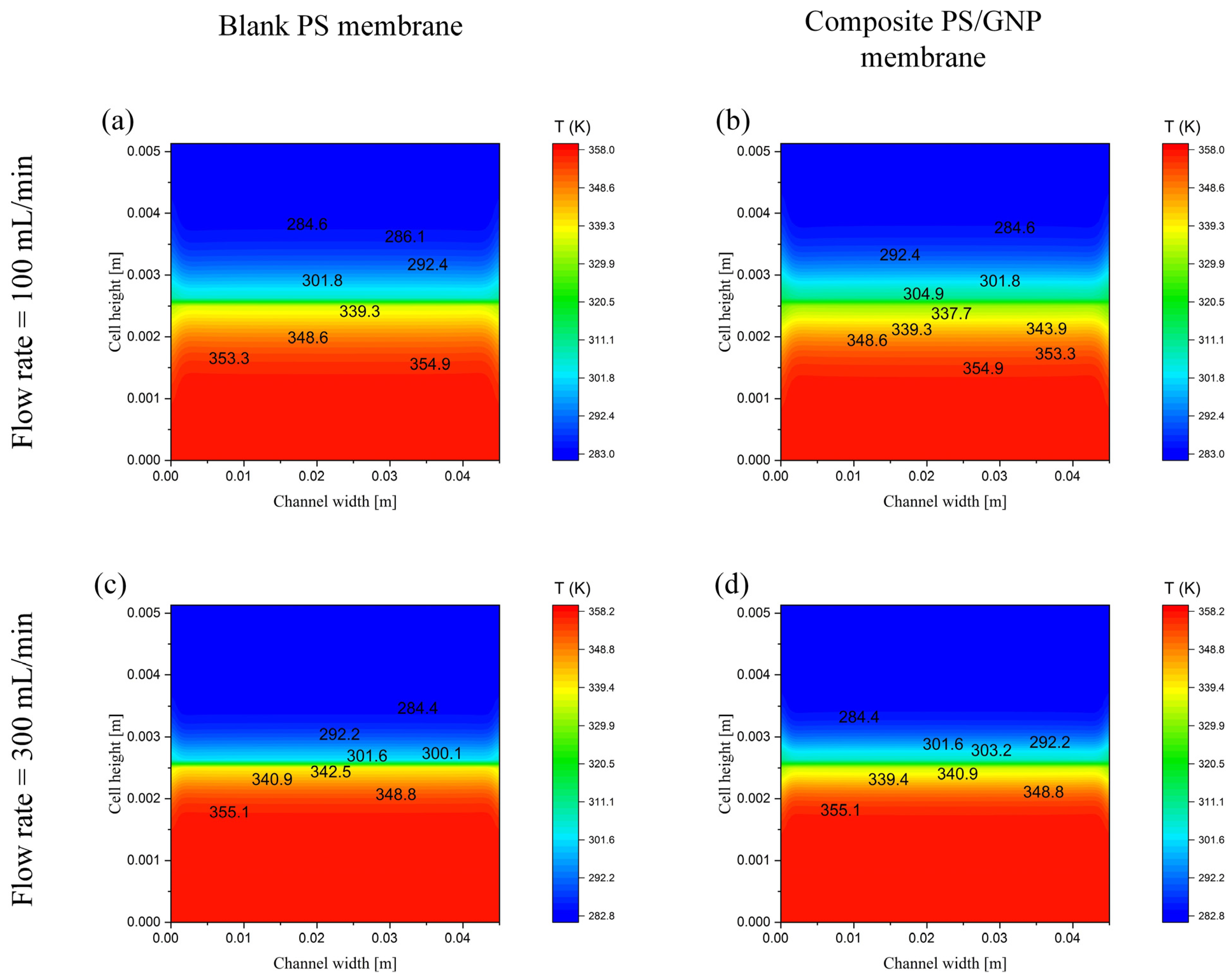
| Case | Number of Elements | Permeate Flux [kg/m2 h] | % Error |
|---|---|---|---|
| 1 | 50,000 | 12.30483 | 2.2 |
| 2 | 100,000 | 12.25667 | 1.8 |
| 3 | 200,000 | 12.20851 | 1.4 |
| 4 | 300,000 | 12.14831 | 0.9 |
| 5 | 400,000 | 12.052 | 0.1001 |
| 6 | 500,000 | 12.03995 | Datum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, A.; Al-Qahatani, A.; Alquraish, M.; Bailey, C.; El-Shazly, A.; El-Mofty, S. Modeling and Simulation of Fabricated Graphene Nanoplates/Polystyrene Nanofibrous Membrane for DCMD. Polymers 2021, 13, 2987. https://doi.org/10.3390/polym13172987
Abdullah A, Al-Qahatani A, Alquraish M, Bailey C, El-Shazly A, El-Mofty S. Modeling and Simulation of Fabricated Graphene Nanoplates/Polystyrene Nanofibrous Membrane for DCMD. Polymers. 2021; 13(17):2987. https://doi.org/10.3390/polym13172987
Chicago/Turabian StyleAbdullah, Ahmad, Abdulaziz Al-Qahatani, Mohammed Alquraish, Colin Bailey, Ahmed El-Shazly, and Salah El-Mofty. 2021. "Modeling and Simulation of Fabricated Graphene Nanoplates/Polystyrene Nanofibrous Membrane for DCMD" Polymers 13, no. 17: 2987. https://doi.org/10.3390/polym13172987
APA StyleAbdullah, A., Al-Qahatani, A., Alquraish, M., Bailey, C., El-Shazly, A., & El-Mofty, S. (2021). Modeling and Simulation of Fabricated Graphene Nanoplates/Polystyrene Nanofibrous Membrane for DCMD. Polymers, 13(17), 2987. https://doi.org/10.3390/polym13172987







