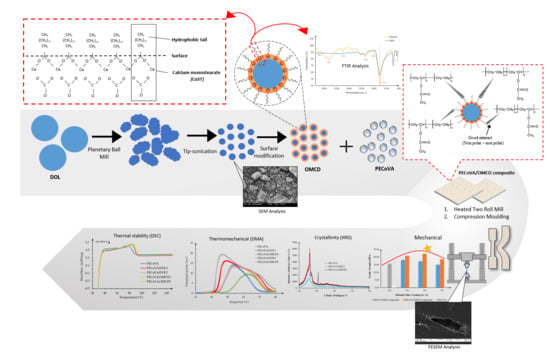
Thermomechanical Properties of the Virgin PECoVA, PECoVA/OMCD and PECoVA/DOL Composites
Dynamic mechanical analysis (DMA) measures the response of virgin PECoVA, PECoVA/OMCD and PECoVA/DOL composites to an oscillatory deformation as a function of temperature. The results of DMA are usually analyzed by considering three viscoelastic properties such as storage modulus (E’), loss modulus (E”), and damping capacity (tan δ), which were evaluated as a function of temperature. Damping capacity (tan δ) is defined as the ratio of the loss modulus to storage modulus (E”/E’) [
42]. It is used to determine the damping properties of the material which give the correlation between the elastic phase and viscous phase in a polymeric structure [
43,
44,
45]. The measurement of the damping behavior is useful to determine the occurrence of molecular mobility transitions, such as the glass transition temperature (T
g) or vicat softening temperature (T
Vicat) of the PECoVA copolymer and its composites. DMA has been increasingly used to study the relationships between the polymer structure, dispersed phase as well as the calcite structure in the properties of polymer composites systems [
42,
46,
47]. The maximum damping capacity (Tan δ
max) and the T
Vicat are given in
Table 7, while the E’ and tan δ curves analyzed by DMA are presented in
Figure 10 and Figure 12, respectively.
The previous researches proved that the thermo-mechanical properties of the given materials are strongly affected by the interfacial adhesion between the polymer–filler and the dispersion of the filler in the polymer matrix [
42,
46,
47]. When comparing the mechanical test (tensile and tear test) and DMA results of the virgin PECoVA, PECoVA/DOL and PECoVA/OMCD composites, one could agree that the treated OMCD filler gives more significant effect to the thermo-mechanical properties than the ambient mechanical properties of the host PECoVA. In fact, the OMCD influenced the thermo-mechanical properties of the PECoVA more than the DOL filler.
Figure 10 illustrates the dynamic storage modulus (E’) as a function of temperature for the virgin PECoVA, PECoVA/DOL and PECoVA/OMCD composites. The value of storage or elastic modulus (E’) signifies the stiffness of the material corresponding to deformation’s elastic response [
48,
49]. It is widely known that the interaction between polymer–filler enhanced the storage modulus value of a polymer composite, at the high temperature rubbery zone, due to the larger deviations between the thermodynamic characteristics of the matrix and filler at high temperature range [
50]. The improvements in stiffness observed in the PECoVA composites can be credited to the high stiffness behavior of the OMCD filler that will effectively constrain the movement of the PECoVA copolymer chains. It is clearly observed that the PECoVA/DOL5 composite has the lowest storage modulus, while the virgin PECoVA exhibits the intermediate storage modulus over the analyzed temperature range. Apparently, the PECoVA composites containing the OMCD (PECoVA/OMCD3 and PECoVA/OMCD5) exhibit greater storage modulus as compared to the PECoVA composites containing the pristine DOL (PECoVA/DOL3 and PECoVA/DOL5). These show that the PECoVA/OMCD3 composites are stiffer than the virgin PECoVA, PECoVA/DOL3 and PECoVA/DOL5 when conditioned at a temperature range between room temperature to 85 °C. This could be associated with good interfacial adhesion between the PECoVA and the OMCD, and good dispersion of the filler throughout the PECoVA matrix, as the OMCD had been surface modified and tip sonicated prior to mixing with the PECoVA copolymer. This caused the improvement in PECoVA/OMCD interactions that led to stiffening of the host PECoVA copolymer chains. Specifically, the physical molecular interaction via hydrogen bonds between polar groups of the PECoVA with carbonyl groups of the stearic acid, and non-polar interactions between the PE phase of the PECoVA with the organic surfactant (stearic acid), resulted in stiffening of the PECoVA matrix.
Figure 11 illustrates the proposed PECoVA–filler interactions in the PECoVA composite systems containing the DOL versus the OMCD. Initially, the DOL is comprised of crystalline particles that have affinity toward each other; they stacked and formed aggregates. Thus, the PECoVA composite with the pristine DOL contains lower degrees of filler distribution and dispersion. The presence of large aggregates causes less interaction between the PECoVA copolymer chains and the DOL particles (
Figure 3b). In contrary, the OMCD contains smaller size particles with organophilic surface. This facilitates the dispersion of OMCD particles to occupy the free volume between the PECoVA chains (please refer to the XRD result where diffraction signals of OMCD can be detected in the PECoVA/OMCD composite). A more homogeneous composite was obtained when the OMCD was employed as filler (FESEM result—
Figure 3c). More interactions between the matrix and filler are obtained due to: (i) Hydrogen bond between the vinyl acetate phase of the PECoVA and hydroxyl group of the dolomite surface (please refer to the FTIR and DMA results); (ii) non-polar–non-polar interactions between the hydrocarbon tail of the surface modifier’s chains (stearic acid) and polyethylene phase of the PECoVA (please refer to the XRD result (crystallinity index). This contributes to the excellent reinforcing capability of the OMCD towards the PECoVA matrix (please refer to tensile and tear results). Without the surface modification and tip sonication process, the resultant PECoVA composite would not achieve an appreciable enhancement in the storage modulus over the virgin PECoVA. Poor dispersion of DOL would not encourage significant polymer–filler interactions. The storage modulus data of the PECoVA/DOL5 sample proved this statement.
The graph in
Figure 12 reveals the damping capacity (Tan δ) of the virgin PECoVA, PECoVA/DOL and PECoVA/OMCD composites in the range of room temperature until 90 °C. It is noticed that when temperature increased, the Tan δ increased to the maximum value (Tan δ
Max) in the transition region and decreased in the rubbery region. It is related to the movements of molecules and small groups within the copolymer structure of PECoVA [
49,
50]. The Tan δ
Max and T
Vicat of the virgin PECoVA were 14.0920 and 58.52 °C, respectively. This peak transition ranging from 45 to 58 °C corresponds to Vicat softening temperature for PECoVA [
51]. Furthermore, the virgin PECoVA possesses a damping peak between the PECoVA/DOL and PECoVA/OMCD composites, indicating the intermediate degree of molecular mobility as compared to both types of the composites. All of the OMCD-filled PECoVA composites (PECoVA/OMCD3 and PECoVA/OMCD5) show significantly higher T
Vicat with comparatively lower Tan δ, while the increase of DOL filler loading (from 1 to 5 wt%) in the PECoVA shifted up the Tan δ
Max and simultaneously reduced the T
Vicat of PECoVA/DOL composites. These are due to the integration of OMCD filler particles that decreases the viscoelastic damping factor of the PECoVA matrix. The decrease of Tan δ
Max proposes that the process for energy dissipation becomes slower by the addition of OMCD filler. This was due to the presence of the OMCD filler that restricted the molecular mobility of the PECoVA chains. PECoVA/DOL composites show comparatively lower T
Vicat, which can confirm the weaker interaction of the DOL surface with PECoVA chains. The DMA results show that the addition of 3 wt% of OMCD successfully lowered the Tan δ
Max and enhanced the T
Vicat of PECoVA composites to 9.5569 and 67.91 °C, respectively. However, further increase in the OMCD loading to 5 wt% will increase the Tan δ
Max of the composites, suggesting the weakening of the matrix–filler interaction due to poor dispersion of filler in the PECoVA matrix. Thus, it is believed that the incorporation of 3 wt% filler loading into the virgin PECoVA is already enough for the composite to have the optimum interfacial adhesion, because the strong bonded interface of the composite will reflect a low magnitude of Tan δ
Max and higher T
Vicat [
45]. Other than the DMA results, better dispersion of OMCD particles in the composites obtained after the combination of sonication and surface treatment can be also proven by DSC analysis.
The PECoVA/OMCD3 composite indicates a lower Tan δ
Max that appears at a higher temperature than that of PECoVA/OMCD5, virgin PECoVA PECoVA/DOL3 and PECoVA/DOL5. The main reason was due to the existence of more restricted PECoVA chain mobility in the PECoVA/OMCD3 composite, caused by greater PECoVA–OMCD interactions. Previous research also implied that this Tan δ
Max that shifted to a somewhat broader peak with a higher temperature was due to more molecular mobility restriction forced by dispersed OMCD in the PECoVA molecular chains [
52]. The results also indicated that the DOL loading brought significant influence on the damping behavior of the PECoVA composites. When the filler loading increased from 3 to 5 wt% (DOL), an increase in Tan δ
Max intensity was perceived. This was due to weaker matrix–filler interactions as a result of reduced quality of DOL dispersion in the PECoVA matrix.
An anomalous behavior was observed when the PECoVA was added with (3 and 5 wt%) DOL filler. The presence of a more intense and broader peak can be observed through the DMA curve. This may be because the hydrophilic DOL filler, that induced the phase separation in the PECoVA system at the weaker interphase site between the VA and PE molecules, resulted in a more isolated PE structure.
From the obtained results, it is clear that the PECoVA composites containing the OMCD filler had better tensile and tear properties as compared to the PECoVA composites containing the pristine DOL. As proved through the SEM and XRD results, this was due to the homogeneous distribution, improved filler dispersion and good interfacial adhesion of OMCD inside the PECoVA matrix as a result of tip sonication and surface treatment process. Without these processes, the dolomite could not serve as an efficient reinforcing filler. As proved through the mechanical data, the tensile strength and toughness of the PECoVA/DOL composite showed a decreasing trend when the DOL content increased from 1 to 5 wt%. In contrast, the tensile strength of the PECoVA/OMCD composite increased more significantly when 1 wt% filler was employed and further increased when the filler content increased to 3 wt%.
In this study, the DMA analysis was conducted in the range of room temperature to 85 °C. Based on the supplier datasheet, the primary relaxation temperature (Tα) which corresponds to glass transition temperature (Tg) of this PECoVA copolymer is around 70 °C. However, due to the unavailability of liquid nitrogen during this DMA measurement, the experiment could not be started from minus degree Celsius, thus the presence of Tα was not observed.