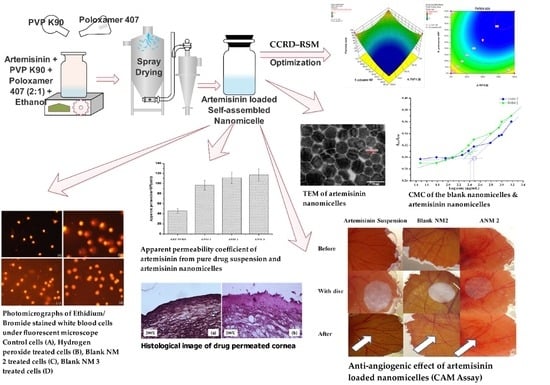
Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compatibility Study
2.3. Preliminary Screening for Nanomicelles Formation Capacity
2.4. Design of Experiment (DoE)
2.5. Preparation of Artemisinin Loaded Nanomicelles
2.6. Determination of the Critical Micellar Concentration (CMC)
2.7. Characterization of Artemisinin-Loaded Nanomicelles
2.7.1. Transparency
2.7.2. Cloud Point
2.7.3. Particle Size and Zeta Potential
2.7.4. Transmission Electron Microscopy (TEM)
2.7.5. Drug Content
2.8. In Vitro Hemolytic Potential
2.9. Evaluation of the Genotoxicity of the Nanomicelles (Alkaline Comet Assay)
2.10. In Vitro Drug Release
2.11. In Vitro Trans-Corneal Permeation Studies
2.12. Apparent Permeability Coefficient
2.13. Histological Examination of the Drug-Permeated Cornea
2.14. Evaluation of the Anti-Angiogenic Effect of Artemisinin Nanomicelles Using a Chorioallantoic Membrane Assay (CAM Assay)
2.15. Stability Studies
2.16. Statistical Analysis
3. Results and Discussions
3.1. Compatibility Studies (FTIR)
3.2. Preliminary Screening for Nanomicelles Formation Capacity
3.3. Experimental Design
3.4. Preparation of Artemisinin-Loaded Nanomicelles
3.5. Determination of Critical Micellar Concentration
3.6. Characterization of Artemisinin-Loaded Nanomicelles
3.6.1. Transparency
3.6.2. Cloud Point Measurement
3.6.3. Particle Size and Zeta Potential
3.6.4. Morphology and Drug Content
3.7. In Vitro Hemolytic Potential
3.8. Evaluation of the Genotoxicity of the Nanomicelles by the Alkaline Comet Assay
3.9. In Vitro Drug Release
3.10. In Vitro Trans-Corneal Permeation of All the Nanomicelles
3.11. Apparent Permeability Coefficient (Papp)
3.12. Histological Examination of the Drug-Permeated Cornea
3.13. Anti-Angiogenic Effect of Artemisinin-Loaded Nanomicelles
3.14. Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stahl, A. The Diagnosis and Treatment of Age-Related Macular Degeneration. Dtsch. Arztebl. Int. 2020, 117, 513–520. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Rodrigues, G.A.; Lutz, D.; Shen, J.; Yuan, X.; Shen, H.; Cunningham, J.; Rivers, H.M. Topical Drug Delivery to the Posterior Segment of the Eye: Addressing the Challenge of Preclinical to Clinical Translation. Pharm. Res. 2018, 35, 245. [Google Scholar] [CrossRef]
- Gokulgandhi, M.R.; Vadlapudi, A.D.; Mitra, A.K. Ocular toxicity from systemically administered xenobiotics. Expert Opin. Drug Metab. Toxicol. 2012, 8, 1277–1291. [Google Scholar] [CrossRef][Green Version]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Sapino, S.; Chirio, D.; Peira, E.; Abellán Rubio, E.; Brunella, V.; Jadhav, S.A.; Chindamo, G.; Gallarate, M. Ocular Drug Delivery: A Special Focus on the Thermosensitive Approach. Nanomaterials 2019, 9, 884. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
- Swetledge, S.; Jung, J.P.; Carter, R.; Sabliov, C. Distribution of polymeric nanoparticles in the eye: Implications in ocular disease therapy. J. Nanobiotech. 2021, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef]
- Bose, A.; Roy Burman, D.; Sikdar, B.; Patra, P. Nanomicelles: Types, properties and applications in drug delivery. IET Nanobiotech. 2021, 15, 19–27. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Sugumaran, A.; Ponnusamy, C.; Kandasamy, P.; Krishnaswami, V.; Palanichamy, R.; Kandasamy, R.; Lakshmanan, M.; Natesan, S. Development and evaluation of camptothecin loaded polymer stabilized nanoemulsion: Targeting potential in 4T1-breast tumour xenograft model. Eur. J. Pharm. Sci. 2018, 116, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P. Antimalarial and anticancer properties of artesunate and other artemisinins: Current development. Mon. Chem. Chem. Mon. 2021, 152, 387–400. [Google Scholar] [CrossRef]
- Cheong, D.H.J.; Tan, D.W.S.; Wong, F.W.S.; Tran, T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol. Res. 2020, 158, 104901. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Shi, Y.; Xu, C.; Zhang, C.; Wong, Y.K.; Lee, Y.M.; Krishna, S.; He, Y.; Lim, T.K.; et al. Mechanistic Investigation of the Specific Anticancer Property of Artemisinin and Its Combination with Aminolevulinic Acid for Enhanced Anticolorectal Cancer Activity. ACS Cent. Sci. 2017, 3, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-W.; Xie, L.-K. Potential applications of artemisinins in ocular diseases. Int. J. Ophthalmol. 2019, 12, 1793–1800. [Google Scholar] [CrossRef]
- Chong, C.-M.; Zheng, W. Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of ERK/CREB signaling. Redox Biol. 2016, 9, 50–56. [Google Scholar] [CrossRef]
- Ponnusamy, C.; Sugumaran, A.; Krishnaswami, V.; Kandasamy, R.; Natesan, S. Design and development of artemisinin and dexamethasone loaded topical nanodispersion for the effective treatment of age-related macular degeneration. IET Nanobiotech. 2019, 13, 868–874. [Google Scholar] [CrossRef]
- Li, H.; Hu, D.; Liang, F.; Huang, X.; Zhu, Q. Influence factors on the critical micelle concentration determination using pyrene as a probe and a simple method of preparing samples. R. Soc. Open Sci. 2020, 7, 192092. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Talbiersky, P.; Korth, H.-G.; Sustmann, R.; Boese, R.; Bläser, D.; Rehage, H. A new pyrene-based fluorescent probe for the determination of critical micelle concentrations. J. Phys. Chem. B 2007, 111, 12985–12992. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.; Ray, S.; Ghosal, S.K.; Bhadra, R.; Moulik, S.P. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol. Pharm. Bull. 2004, 27, 1993–1999. [Google Scholar] [CrossRef][Green Version]
- Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: Design and optimization. Int. J. Pharm. 2009, 380, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, C.; Krishnaswami, V.; Sugumaran, A.; Natesan, S. Simultaneous estimation of artemisinin and dexamethasone in nanodispersions and assessment of Ex-vivo corneal transport study by RP-HPLC. Curr. Pharm. Anal. 2014, 10, 44–50. [Google Scholar] [CrossRef]
- Amin, K.; Dannenfelser, R.-M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Sugumaran, A.; Ponnusamy, C.; Jeevanesan, V.; Girija, G.; Palanichamy, R. Development and evaluation of magnetic microemulsion: Tool for targeted delivery of camptothecin to BALB/c mice-bearing breast cancer. J. Drug Target. 2014, 22, 913–926. [Google Scholar] [CrossRef]
- Subramanian, N.; Abimanyu, S.; Vinoth, J.; Sekar, P.C. Biodegradable Chitosan Magnetic Nanoparticle Carriers for Sub-Cellular Targeting Delivery of Artesunate for Efficient Treatment of Breast Cancer. AIP Conf. Proc. 2010, 1311, 416–424. [Google Scholar] [CrossRef]
- Danafar, H.; Jaberizadeh, H.; Andalib, S. In vitro and in vivo delivery of gliclazide loaded mPEG-PCL micelles and its kinetic release and solubility study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1625–1636. [Google Scholar] [CrossRef]
- Begum, G.; Leigh, T.; Courtie, E.; Moakes, R.; Butt, G.; Ahmed, Z.; Rauz, S.; Logan, A.; Blanch, R.J. Rapid assessment of ocular drug delivery in a novel ex vivo corneal model. Sci. Rep. 2020, 10, 11754. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, W.; Ju, C.; Liu, Z.; Pan, H.; Zhang, H.; Nie, S. Evaluation of Pharmasolve corneal permeability enhancement and its irritation on rabbit eyes. Drug Deliv. 2009, 16, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Pescina, S.; Govoni, P.; Potenza, A.; Padula, C.; Santi, P.; Nicoli, S. Development of a Convenient ex vivo Model for the Study of the Transcorneal Permeation of Drugs: Histological and Permeability Evaluation. J. Pharm. Sci. 2014, 104, 63–71. [Google Scholar] [CrossRef]
- Velpandian, T.; Bankoti, R.; Humayun, S.; Ravi, A.K.; Kumari, S.S.; Biswas, N.R. Comparative evaluation of possible ocular photochemical toxicity of fluoroquinolones meant for ocular use in experimental models. Indian J. Exp. Biol. 2006, 44, 387–391. [Google Scholar]
- Ansari, M.; Haneef, M.; Murtaza, G.; Dyspersja, S. Solid Dispersions of Artemisinin in Polyvinyl Pyrrolidone and Polyethylene Glycol. Adv. Clin. Experimetal Med. 2010, 19, 745–754. [Google Scholar]
- Garg, A.K.; Sachdeva, R.K.; Kapoor, G. Comparison of crystalline and amorphous carriers to improve the dissolution profile of water insoluble drug itraconazole. Int. J. Pharm. Bio. Sci. 2013, 4, 934–948. [Google Scholar]
- Vyas, V.; Sancheti, P.; Karekar, P.; Shah, M.; Pore, Y. Physicochemical characterization of solid dispersion systems of tadalafil with poloxamer 407. Acta Pharm. 2009, 59, 453–461. [Google Scholar] [CrossRef]
- Zhang, Y.; Lam, Y.M. Controlled synthesis and association behavior of graft Pluronic in aqueous solutions. J. Colloid Interface Sci. 2007, 306, 398–404. [Google Scholar] [CrossRef]
- Saluja, H.; Mehanna, A.; Panicucci, R.; Atef, E. Hydrogen Bonding: Between Strengthening the Crystal Packing and Improving Solubility of Three Haloperidol Derivatives. Molecules 2016, 21, 719. [Google Scholar] [CrossRef]
- Li, G.; Zhong, M.; Zhou, Z.; Zhong, Y.; Ding, P.; Huang, Y. Formulation optimization of chelerythrine loaded O-carboxymethylchitosan microspheres using response surface methodology. Int. J. Biol. Macromol. 2011, 49, 970–978. [Google Scholar] [CrossRef]
- Singh, B.; Chakkal, S.K.; Ahuja, N. Formulation and optimization of controlled release mucoadhesive tablets of atenolol using response surface methodology. AAPS PharmSciTech 2006, 7, E19–E28. [Google Scholar] [CrossRef]
- El-Kamel, A.H. In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. Int. J. Pharm. 2002, 241, 47–55. [Google Scholar] [CrossRef]
- Kim, H.; Csaky, K.G. Nanoparticle-integrin antagonist C16Y peptide treatment of choroidal neovascularization in rats. J. Control. Release 2010, 142, 286–293. [Google Scholar] [CrossRef]
- Paradkar, A.; Ambike, A.; Mahadik, K. Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int. J. Pharm. 2004, 271, 281–286. [Google Scholar] [CrossRef]
- Ahuja, M.; Dhake, A.S.; Sharma, S.K.; Majumdar, D.K. Diclofenac-loaded Eudragit S100 nanosuspension for ophthalmic delivery. J. Microencapsul. 2011, 28, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Srirangam, R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: A natural bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef]
- Toropainen, E.; Ranta, V.P.; Talvitie, A.; Suhonen, P.; Urtti, A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest. Ophthalmol. Vis. Sci. 2001, 42, 2942–2948. [Google Scholar] [PubMed]
- Naguib, S.S.; Hathout, R.M.; Mansour, S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017, 24, 99–108. [Google Scholar] [CrossRef]











| Nanomicelles | Composition (%) | ||
|---|---|---|---|
| Polyvinyl Pyrolidone K90 | Poloxamer 407 | Artemisinin | |
| ANM 1 | 5 | 2.5 | 0.05 |
| ANM 2 | 8 | 4 | 0.05 |
| ANM 3 | 10 | 5 | 0.05 |
| BNM 1 | 5 | 2.5 | - |
| BNM 2 | 8 | 4 | - |
| BNM 3 | 10 | 5 | - |
| Run | Factor 1 A: Poloxamer 407 | Factor 2 B: PVP K 90 | Response 1 Particle Size nm (PDI) | Response 2 Transparency % |
|---|---|---|---|---|
| 1 | 0.00 | 0.00 | 44 (0.36) | 96 |
| 2 | −1.00 | 1.00 | 106 (0.54) | 44 |
| 3 | 1.00 | 1.00 | 51 (0.42) | 94 |
| 4 | 0.00 | 0.00 | 44 (0.36) | 96 |
| 5 | 0.00 | 0.00 | 44 (0.36) | 96 |
| 6 | 0.00 | 1.41 | 98 (0.43) | 46 |
| 7 | −1.00 | −1.00 | 41 (0.35) | 99 |
| 8 | 1.00 | −1.00 | 78 (0.33) | 44 |
| 9 | −1.41 | 0.00 | 156 (0.41) | 33 |
| 10 | 1.41 | 0.00 | 143 (0.56) | 38 |
| 11 | 0.00 | −1.41 | 139 (0.86) | 37 |
| 12 | 0.00 | 0.00 | 44 (0.36) | 96 |
| 13 | 0.00 | 0.00 | 44 (0.36) | 96 |
| Formulation Code | Particle Size (nm) | PDI | Zeta Potential (mV) | Transparency (%) | Cloud Point (°C) |
|---|---|---|---|---|---|
| BNM 1 | 32 ± 0.7 | 0.31 ± 0.13 | −4.0 ± 1.6 | 99 ± 1.1 | 69 ± 1 |
| BNM 2 | 34 ± 0.3 | 0.30 ± 0.11 | −7.0 ± 1.1 | 97 ± 1.4 | 68 ± 1 |
| BNM 3 | 39 ± 0.6 | 0.34 ± 0.21 | −10.0 ± 1.3 | 95 ± 1.8 | 69 ± 2 |
| ANM 1 | 41 ±0.9 | 0.35 ± 0.11 | −5.0 ± 1.2 | 99 ± 1.3 | 70 ± 1 |
| ANM 2 | 44 ± 1.1 | 0.36 ± 0.12 | −9.2 ± 1.4 | 96 ± 1.9 | 69 ± 2 |
| ANM 3 | 51 ± 2.1 | 0.42± 0.13 | −12.0 ± 2.8 | 94 ± 2.1 | 68 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnusamy, C.; Sugumaran, A.; Krishnaswami, V.; Palanichamy, R.; Velayutham, R.; Natesan, S. Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin. Polymers 2021, 13, 3038. https://doi.org/10.3390/polym13183038
Ponnusamy C, Sugumaran A, Krishnaswami V, Palanichamy R, Velayutham R, Natesan S. Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin. Polymers. 2021; 13(18):3038. https://doi.org/10.3390/polym13183038
Chicago/Turabian StylePonnusamy, Chandrasekar, Abimanyu Sugumaran, Venkateshwaran Krishnaswami, Rajaguru Palanichamy, Ravichandiran Velayutham, and Subramanian Natesan. 2021. "Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin" Polymers 13, no. 18: 3038. https://doi.org/10.3390/polym13183038
APA StylePonnusamy, C., Sugumaran, A., Krishnaswami, V., Palanichamy, R., Velayutham, R., & Natesan, S. (2021). Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin. Polymers, 13(18), 3038. https://doi.org/10.3390/polym13183038