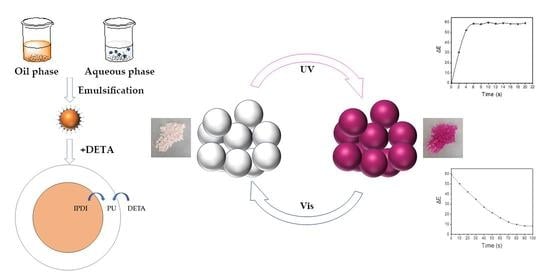
Microencapsulation of Photochromic Solution with Polyurea by Interfacial Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Photochromic Material Microcapsule
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
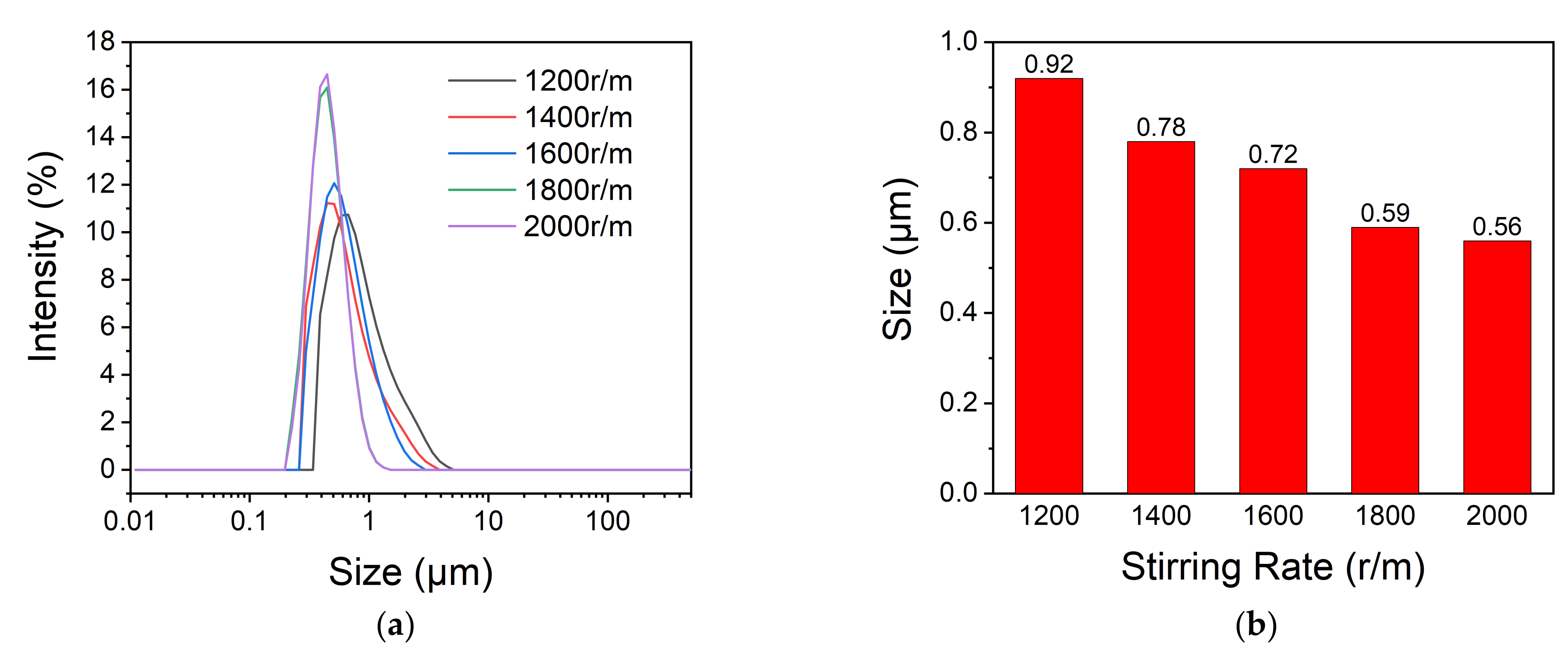
2.3.2. Particle Size and Its Distribution
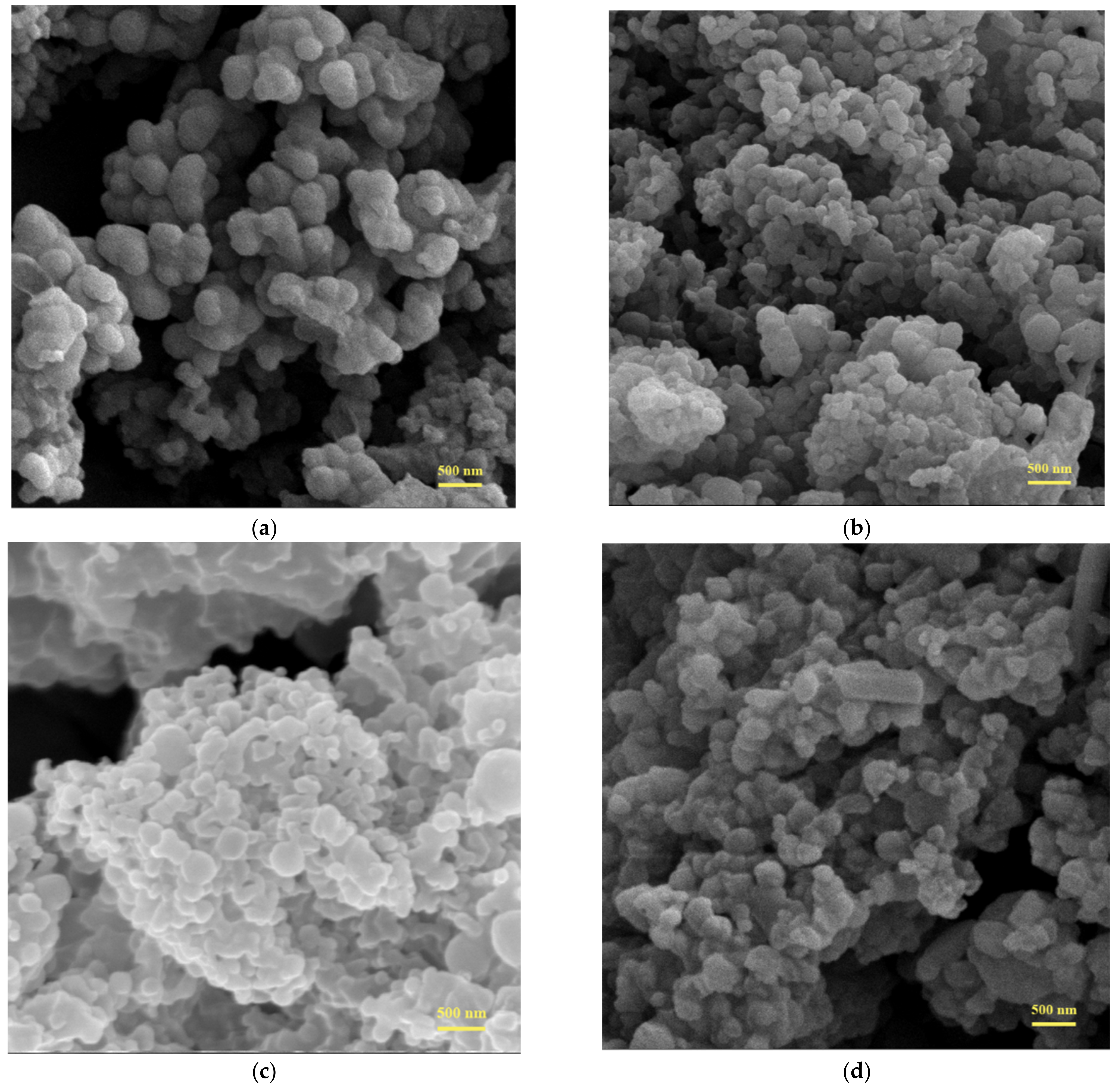
2.3.3. Surface Features and Morphology
2.3.4. Thermostability
2.3.5. Tightness Performance
2.3.6. Ultraviolet–Visible (UV-Vis) Absorbance
2.3.7. Photochromic Cycle Test
2.3.8. UV Accelerated Aging Test
2.3.9. Characterization of Color Change
3. Results and Discussion
3.1. Preparation of the Photochromic Microcapsule
3.2. FTIR
3.3. Particle Size and Its Distribution
3.4. Morphology of the Microcapsules
3.5. Thermogravimetric Analysis (TGA)
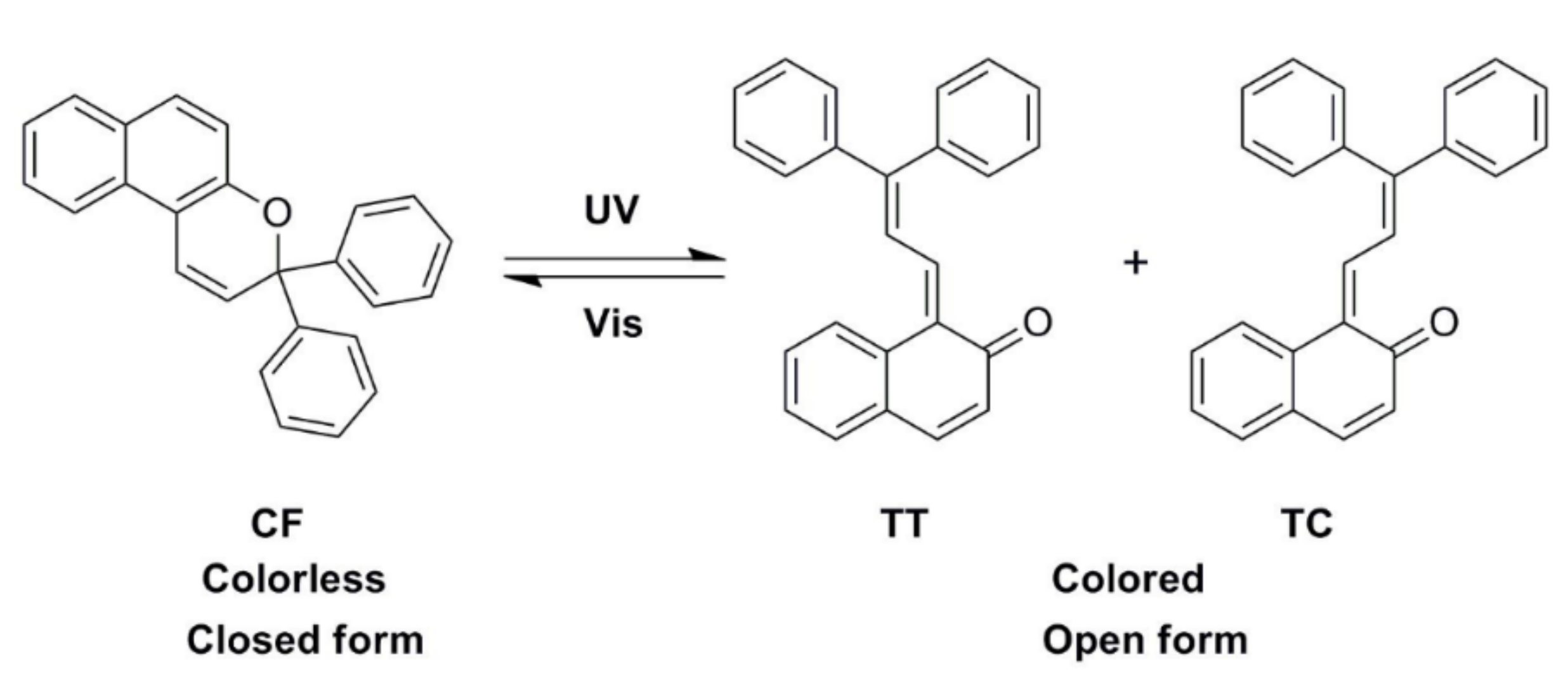
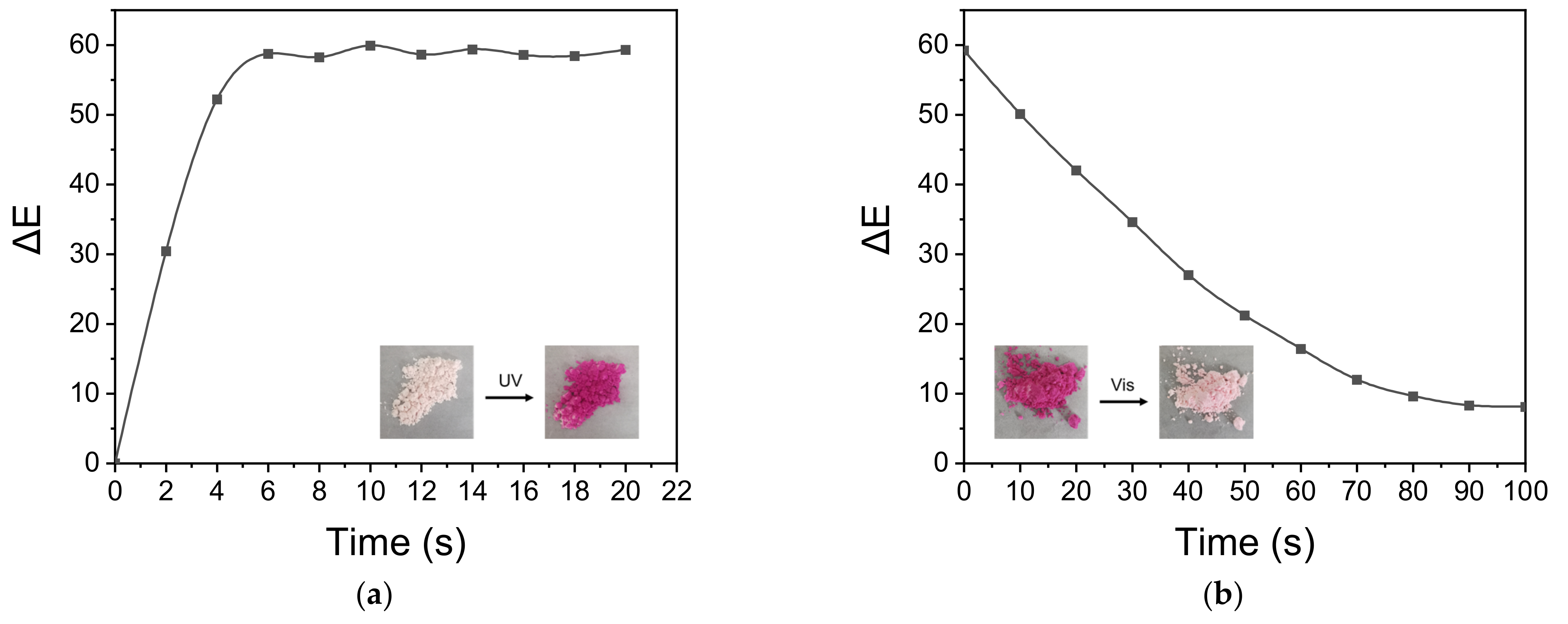
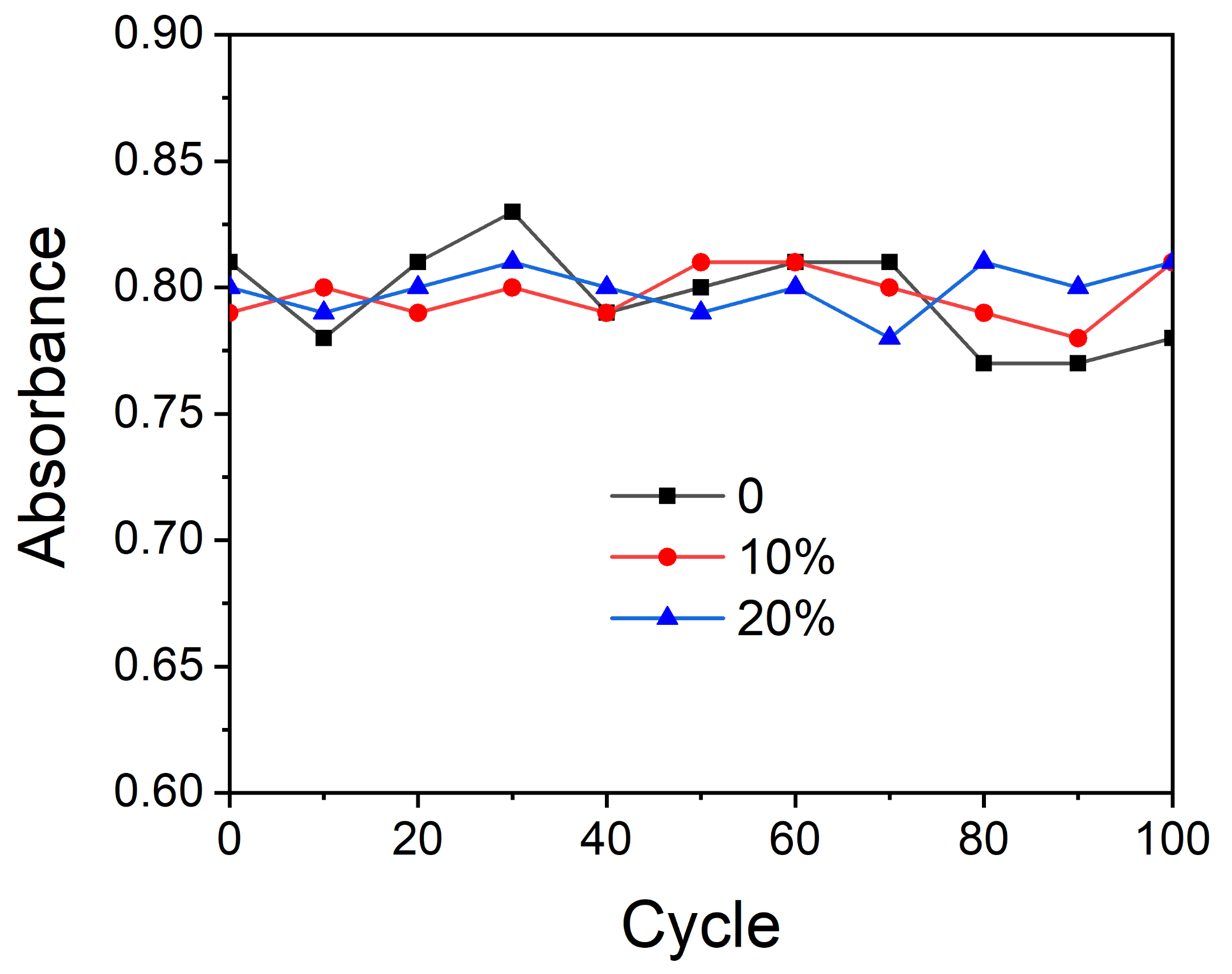
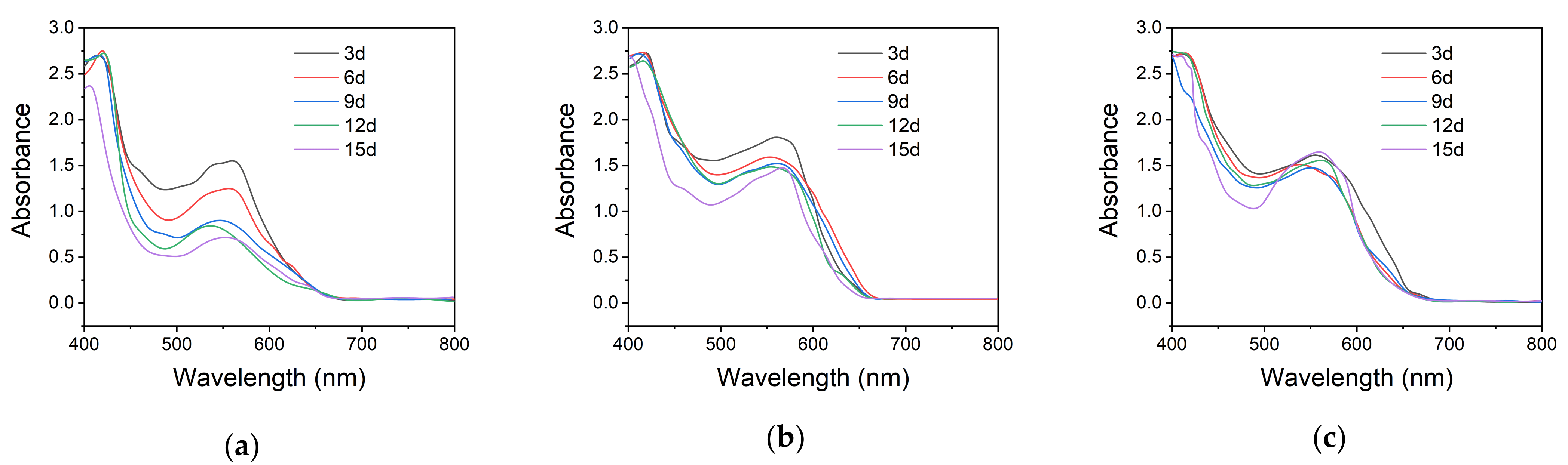
3.6. Photochromic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.S.; Xu, G.; Zhang, Z.J.; Guo, G.C. Inorganic–organic hybrid photochromic materials. Chem. Commun. 2010, 46, 361–376. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, W.; Sun, J.; Li, K.; Zhang, L. Angular photochromic LC composite film for an anti-counterfeiting label. Polymers 2018, 10, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Fan, W.; Liu, Z.; Yu, A.; Jiang, X. Advances on tungsten oxide based photochromic materials: Strategies to improve their photochromic properties. J. Mater. Chem. C 2018, 6, 191–212. [Google Scholar] [CrossRef]
- Cheng, T.; Lin, T.; Brady, R.; Wang, X. Fast response photochromic textiles from hybrid silica surface coating. Fibers Polym. 2008, 9, 301–306. [Google Scholar] [CrossRef]
- Ma, L.; Li, C.; Yan, Q.; Wang, S.; Miao, W.; Cao, D. Unsymmetrical photochromic bithienylethene-bridge tetraphenylethene molecular switches: Synthesis, aggregation-induced emission and information storage. Chin. Chem. Lett. 2020, 31, 361–364. [Google Scholar] [CrossRef]
- Torres-Pierna, H.; Ruiz-Molina, D.; Roscini, C. Highly transparent photochromic films with a tunable and fast solution-like response. Mater. Horiz. 2020, 7, 2749–2759. [Google Scholar] [CrossRef]
- Tsujioka, T.; Nakanishi, Y.; Nishimura, R.; Uchida, K. Measurement of glass-transition temperature of thermoreversible photochromic materials based on mechanochemical amorphization. Dye. Pigment. 2021, 186, 109069. [Google Scholar] [CrossRef]
- Abdelghany, A.M. Photochromic behavior of tungsten ions in sodium metaphosphate glass and effect of oxidizing condition assessed by spectroscopic analysis. J. Non-Cryst. Solids 2021, 552, 120460. [Google Scholar] [CrossRef]
- Vyasamudri, S.; Yang, D. Regiodivergent synthesis of Bis(4-oxycoumarin)-based dioxabicycles: Exploration of [4 + 4] (heterocyclo)reversion/addition and 1,5-hydrogen shift photochromism. Org. Lett. 2020, 22, 3166–3170. [Google Scholar] [CrossRef]
- Helmy, S.; Leibfarth, F.A.; Oh, S.; Poelma, J.E.; Hawker, C.J.; de Alaniz, J.R. Photoswitching using visible light: A new class of organic photochromic molecules. J. Am. Chem. Soc. 2014, 136, 8169–8172. [Google Scholar] [CrossRef] [Green Version]
- Ge, B.; Han, S.; Wei, Q.; Li, J.; Wang, G. Coordination-driven strategy towards crystalline hybrid photochromic materialsvia the marriage of a non-photochromic extended dipyridine unit and zincophosphate. J. Mater. Chem. C 2019, 7, 3920–3923. [Google Scholar] [CrossRef]
- Fu, Z.; Sun, B.; Chen, J.; Yuan, L. Preparation and photochromism of carboxymethyl chitin derivatives containing spirooxazine moiety. Dye. Pigment. 2008, 76, 515–518. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, G.; Zhang, K. Photochromic behavior of naphthopyran in styrene-butadiene-styrene elastomer thin films: Effect of stretching of film and linker. J. Appl. Polym. Sci. 2013, 127, 1794–1802. [Google Scholar] [CrossRef]
- Dridi, H.; Boulmier, A.; Bolle, P.; Dolbecq, A.; Rebilly, J.; Banse, F.; Ruhlmann, L.; Serier-Brault, H.; Dessapt, R.; Mialane, P.; et al. Directing the solid-state photochromic and luminescent behaviors of spiromolecules with Dawson and Anderson polyoxometalate units. J. Mater. Chem. C 2020, 8, 637–649. [Google Scholar] [CrossRef]
- Fan, J.; Bao, B.; Wang, Z.; Xu, R.; Wang, W.; Yu, D. High tri-stimulus response photochromic cotton fabrics based on spiropyran dye by thiol-ene click chemistry. Cellulose 2020, 27, 493–510. [Google Scholar] [CrossRef]
- Huo, Z.; Li, G.; Xiao, X. Applications of organic photochromic materials in rapid visual detection. Prog. Chem. 2017, 29, 252–261. [Google Scholar]
- He, Z.; Wang, W.; Fan, J.; Bao, B.; Qin, X.; Yu, D. Photochromic microcapsules anchored on cotton fabric by layer-by-layer self-assembly method with erasable property. React. Funct. Polym. 2020, 157, 104762. [Google Scholar] [CrossRef]
- Irie, M.; Sayo, K. Solvent effects on the photochromic reactions of diarylethene derivatives. J. Phys. Chem. B 1992, 96, 7671–7674. [Google Scholar] [CrossRef]
- Mallo, N.; Brown, P.T.; Iranmanesh, H.; MacDonald, T.S.C.; Teusner, M.J.; Harper, J.B.; Ball, G.E.; Beves, J.E. Photochromic switching behaviour of donor-acceptor Stenhouse adducts in organic solvents. Chem. Commun. 2016, 52, 13576–13579. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Castillo-Muzquiz, A.; Biewer, M.C. Studying monolayer/solvent interactions with a photochromic compound in a self-assembled monolayer. Tetrahedron Lett. 2002, 43, 5933–5935. [Google Scholar] [CrossRef]
- Brown, E.N.; White, S.R.; Sottos, N.R. Microcapsule induced toughening in a self-healing polymer composite. J. Mater. Sci. 2004, 39, 1703–1710. [Google Scholar] [CrossRef]
- Chu, L.Y.; Yamaguchi, T.; Nakao, S. A molecular-recognition microcapsule for environmental stimuli-responsive controlled release. Adv. Mater. 2002, 14, 386–389. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.; Wu, H.; Lv, F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 2009, 136, 71–74. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Liao, L.P.; Wang, S.J.; Li, W.J. Self-healing coatings containing microcapsule. Appl. Surf. Sci. 2012, 258, 1915–1918. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, X.; Yang, Z.; Ji, H. Sunscreen enhancement of octyl methoxycinnamate microcapsules by using two biopolymers as wall materials. Polymers 2021, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Bayés-García, L.; Ventolà, L.; Cordobilla, R.; Benages, R.; Calvet, T.; Cuevas-Diarte, M.A. Phase change materials (PCM) microcapsules with different shell compositions: Preparation, characterization and thermal stability. Sol. Energy Mater. Sol. Cells 2010, 94, 1235–1240. [Google Scholar] [CrossRef]
- Jia, J.; Wang, C.; Chen, K.; Yin, Y. Drug release of yolk/shell microcapsule controlled by pH-responsive yolk swelling. Chem. Eng. J. 2017, 327, 953–961. [Google Scholar] [CrossRef]
- Cam, M.E.; Zhang, Y.; Edirisinghe, M. Electrosprayed microparticles: A novel drug delivery method. Expert Opin. Drug Deliv. 2019, 16, 895–901. [Google Scholar] [CrossRef]
- Feczkó, T.; Varga, O.; Kovács, M.; Vidóczy, T.; Voncina, B. Preparation and characterization of photochromic poly (methyl methacrylate) and ethyl cellulose nanocapsules containing a spirooxazine dye. J. Photochem. Photobiol. A Chem. 2011, 222, 293–298. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, Y.; Du, Y.; Chen, J.; Hou, X.; Meng, J. Preparation and application of melamine-formaldehyde photochromic microcapsules. Sens. Actuators B Chem. 2013, 188, 502–512. [Google Scholar] [CrossRef]
- Di Credico, B.; Griffini, G.; Levi, M.; Turri, S. Microencapsulation of a UV-responsive photochromic dye by means of novel UV-screening polyurea-based shells for smart coating applications. ACS Appl. Mater. Interfaces 2013, 5, 6628–6634. [Google Scholar] [CrossRef]
- Tsuru, Y.; Kohri, M.; Taniguchi, T.; Kishikawa, K.; Karatsu, T.; Hayashi, M. Preparation of photochromic liquid core nanocapsules based on theoretical design. J. Colloid Interface Sci. 2019, 547, 318–329. [Google Scholar] [CrossRef]
- Scher, H.B.; Rodson, M.; Lee, K.S. Microencapsulation of pesticides by interfacial polymerization utilizing isocyanate or aminoplast chemistry. Pestic. Sci. 1998, 54, 394–400. [Google Scholar] [CrossRef]
- Sun, S.; Gao, Y.; Han, N.; Zhang, X.; Li, W. Reversible photochromic energy storage polyurea microcapsules via in-situ polymerization. Energy 2021, 219, 119630. [Google Scholar] [CrossRef]
- Kulkarni, A.; Dasari, H. Current Status of Methods Used in Degradation of Polymers: A Review. In Proceedings of the MATEC Web of Conferences, Manipal, India, 21–23 December 2017; EDP Sciences: Les Ulis, France, 2018; Volume 144, p. 02023. [Google Scholar]
- Muasher, M.; Sain, M. The efficacy of photostabilizers on the color change of wood filled plastic composites. Polym. Degrad. Stab. 2006, 91, 1156–1165. [Google Scholar] [CrossRef]
- Lu, K.; Ge, X.; Wei, Z.; Li, G.; Yang, X.; Liu, H. Synergistically enhanced ultraviolet aging resistance and interfacial adhesion of poly (p-phenylene benzobisoxazole) fiber with soluble naphthalimide sizing. Polym. Compos. 2021, 42, 2122–2134. [Google Scholar] [CrossRef]
- Entelis, S.G.; Nesterov, O.V. Kinetics and mechanism of the reactions of isocyanates with compounds containing “active” hydrogen. Russ. Chem. Rev. 1966, 35, 917–930. [Google Scholar] [CrossRef]
- Chamontin, K.; Lokshin, V.; Rossollin, V.; Samat, A.; Guglielmetti, R. Synthesis and reactivity of formyl-substituted photochromic 3, 3-diphenyl-[3H]-naphtho [2, 1-b] pyrans. Tetrahedron 1999, 55, 5821–5830. [Google Scholar] [CrossRef]
- Haddad, R.A.; Alsayed, R.; Ahmed, D.S.; Bufaroosha, M.; Salih, N.; Mohammed, S.A.; Yousif, E. Environmental performance of coordination complexes as PVC photostabilizers. Mater. Today Proc. 2021, 42, 2849–2852. [Google Scholar] [CrossRef]
- Cui, Z.H.; Xia, G.; Chen, W.G.; Jiang, H.; Yang, L.; Zuo, Z.W. Synthesis of novel multifunctional photostabilizers containing UVA and HALS moieties and their effects on polymers and dyes. J. Vinyl Addit. Technol. 2020, 26, 259–267. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Guo, L.; Ma, Y.; Wang, Z.; Niu, Q. The effect of a hindered amine light stabilizer on the aging behavior of moisture-curable polyurethane as a cultural relics consolidant. Polimery 2020, 65, 297–303. [Google Scholar] [CrossRef]




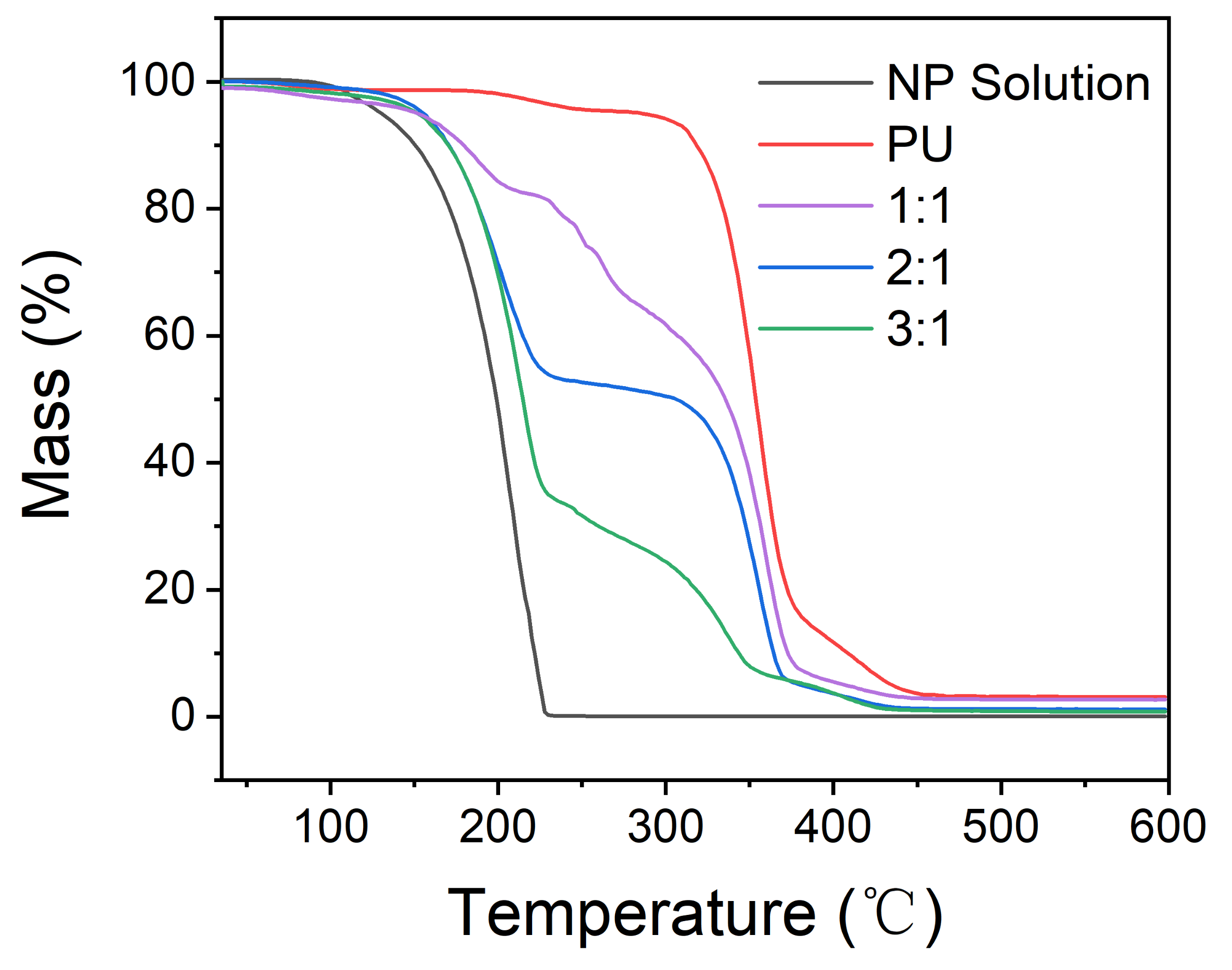

| Samples | T10% (°C) | Char Yield (%) |
|---|---|---|
| NP Solution | 149 | 0.04 |
| PU | 317 | 3.10 |
| Core/shell ratio of 1:1 | 178 | 2.69 |
| Core/shell ratio of 2:1 | 171 | 1.12 |
| Core/shell ratio of 3:1 | 169 | 0.79 |
| Content of HALS 770 (%) | ||||
|---|---|---|---|---|
| 0 | 10 | 20 | ||
| Before UV light irradiation | L | 84 | 84 | 82 |
| a | 6 | 7 | 7 | |
| b | 6 | 6 | 6 | |
| After UV light irradiation | L | 67 | 71 | 73 |
| a | 33 | 32 | 34 | |
| b | −12 | −9 | −16 | |
| ΔE | 36.63 | 31.92 | 35.97 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, X.; Yan, Y.; Chen, Z. Microencapsulation of Photochromic Solution with Polyurea by Interfacial Polymerization. Polymers 2021, 13, 3049. https://doi.org/10.3390/polym13183049
Zhang Y, Zhang X, Yan Y, Chen Z. Microencapsulation of Photochromic Solution with Polyurea by Interfacial Polymerization. Polymers. 2021; 13(18):3049. https://doi.org/10.3390/polym13183049
Chicago/Turabian StyleZhang, Yuhua, Xi Zhang, Yurong Yan, and Zhonghua Chen. 2021. "Microencapsulation of Photochromic Solution with Polyurea by Interfacial Polymerization" Polymers 13, no. 18: 3049. https://doi.org/10.3390/polym13183049