Polymer for Internal Hydrophobization of Cement-Based Materials: Design, Synthesis, and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Design
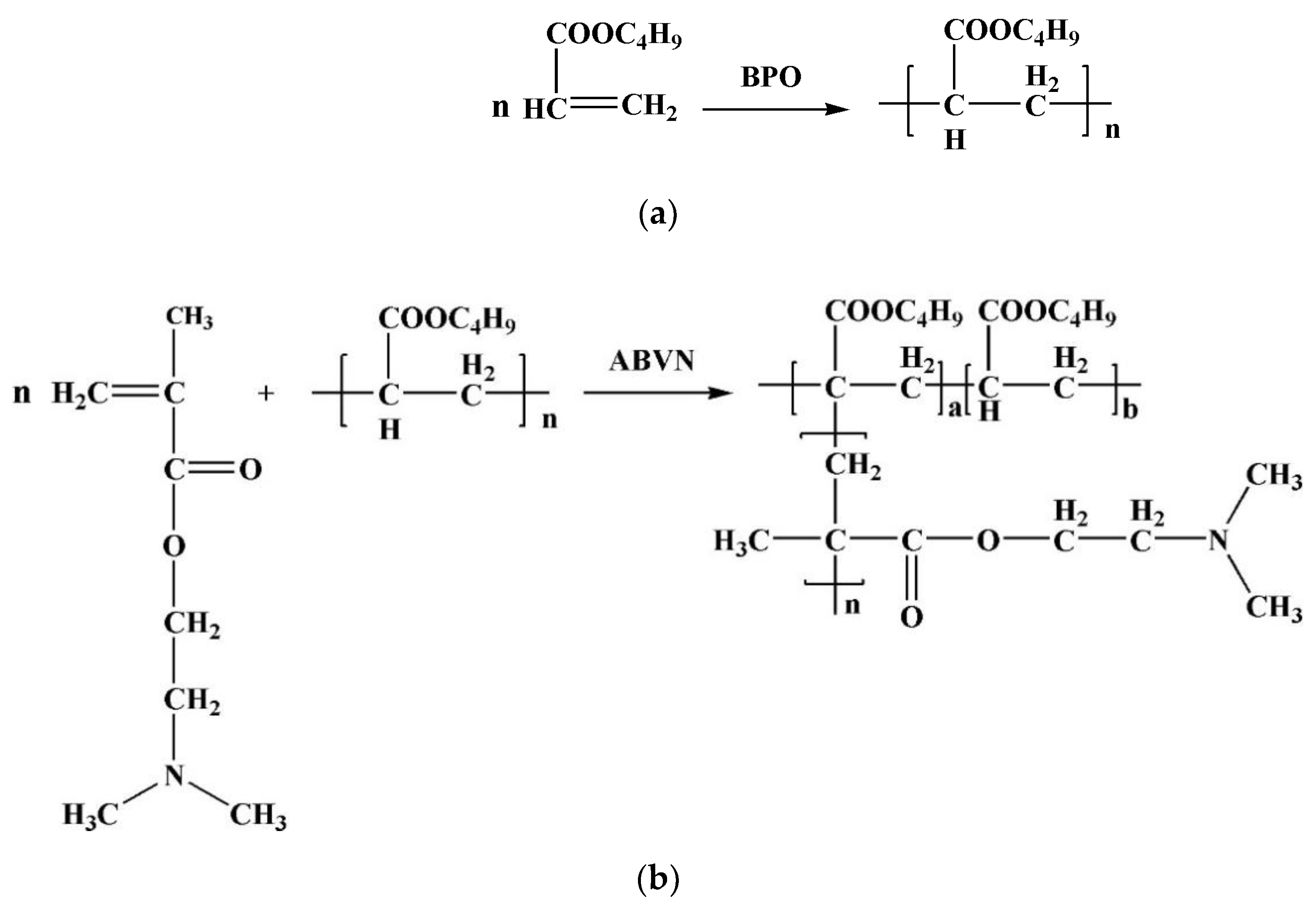
2.3. Synthesis
2.3.1. Synthesis of PBA
2.3.2. Synthesis of PBA-g-PDMAEMA
2.4. Structural Characterizations
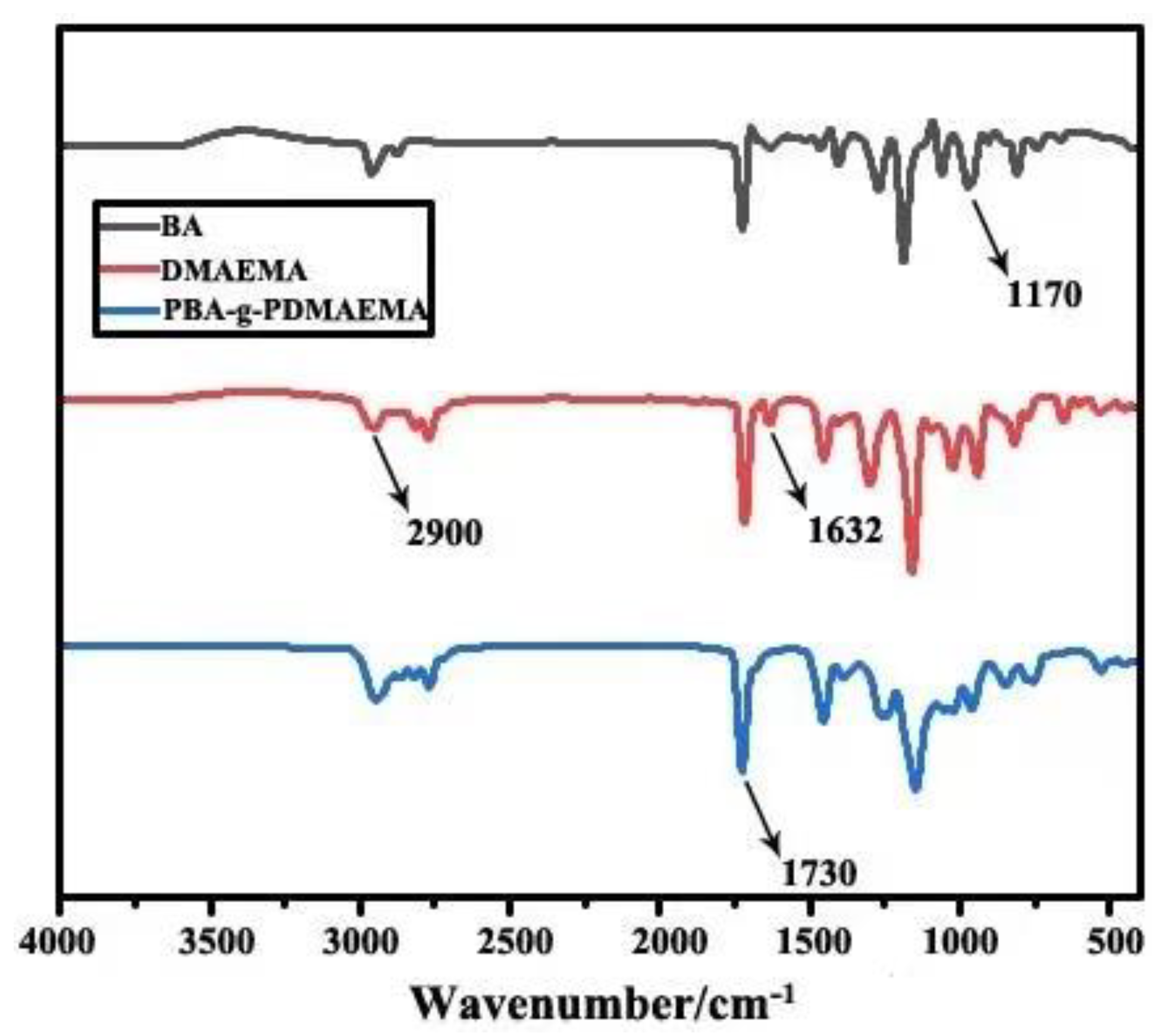
2.4.1. IR Analysis
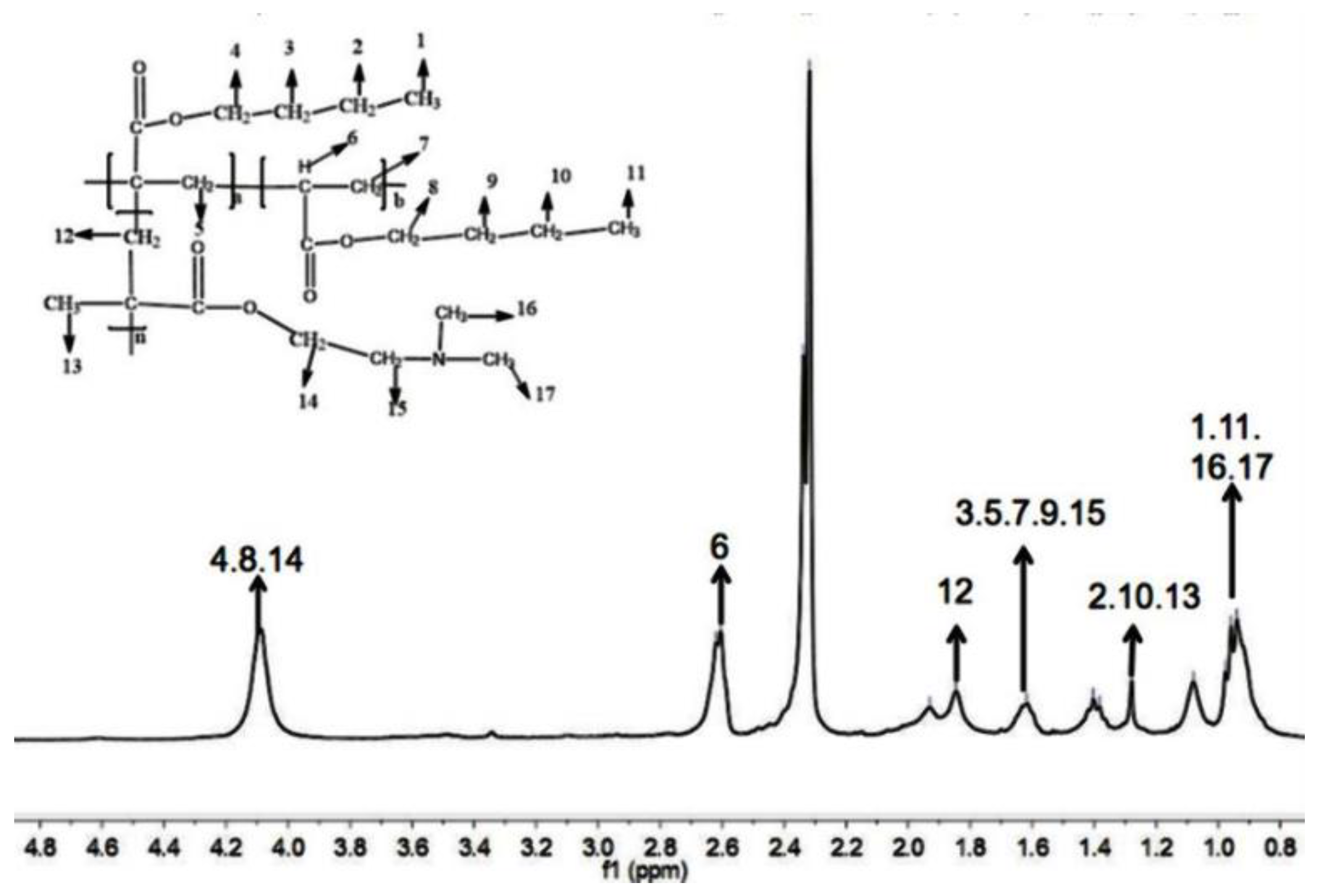
2.4.2. 1H NMR
2.4.3. GPC
2.5. Measurements
2.5.1. Transmittance
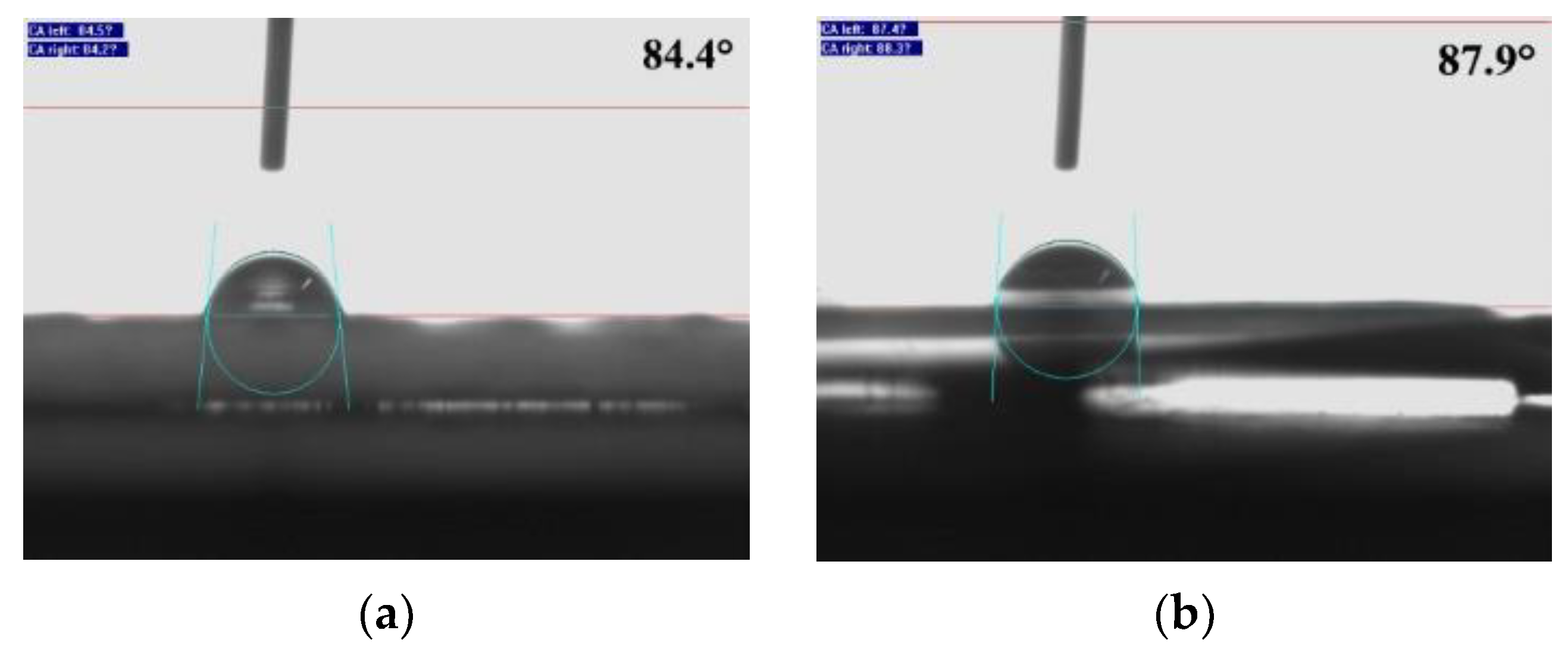
2.5.2. Contact Angle
2.5.3. Evaporation Rate
2.5.4. HLB Value
2.5.5. Water Absorption Rate
2.5.6. Dry Shrinkage
2.5.7. Mechanical Strength
2.5.8. Hydration Heat
2.5.9. XRD
3. Results and Discussion
3.1. Molecular Structure
3.1.1. IR Analysis
3.1.2. 1H NMR Analysis
3.1.3. Molecular Weight Analysis
3.2. Hydrophobicity Behavior
3.3. Hydrophobicity
3.4. Solution Properties of Polymer
3.4.1. HLB Value
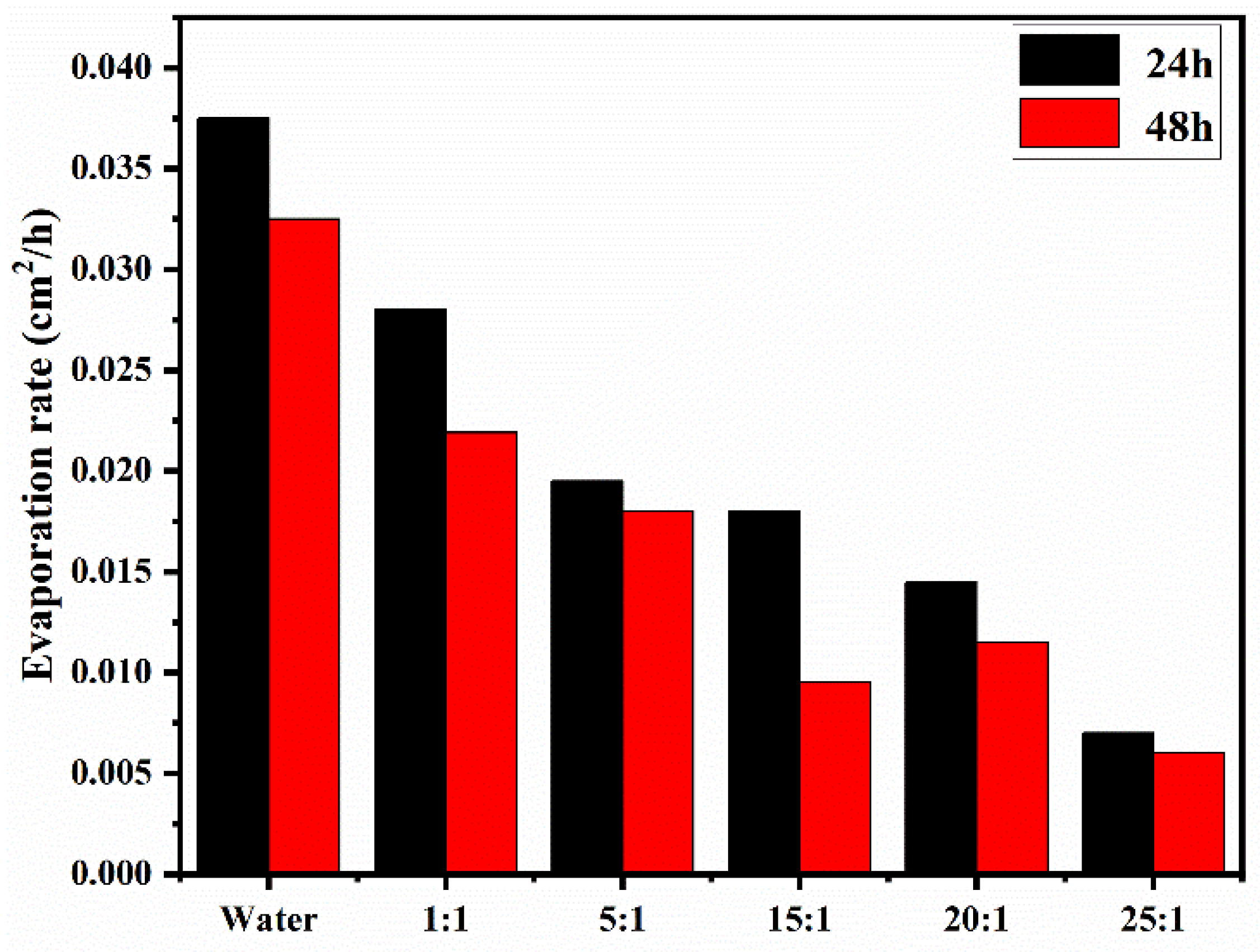
3.4.2. Evaporation Rate
3.5. Performance of Cement-Based Materials
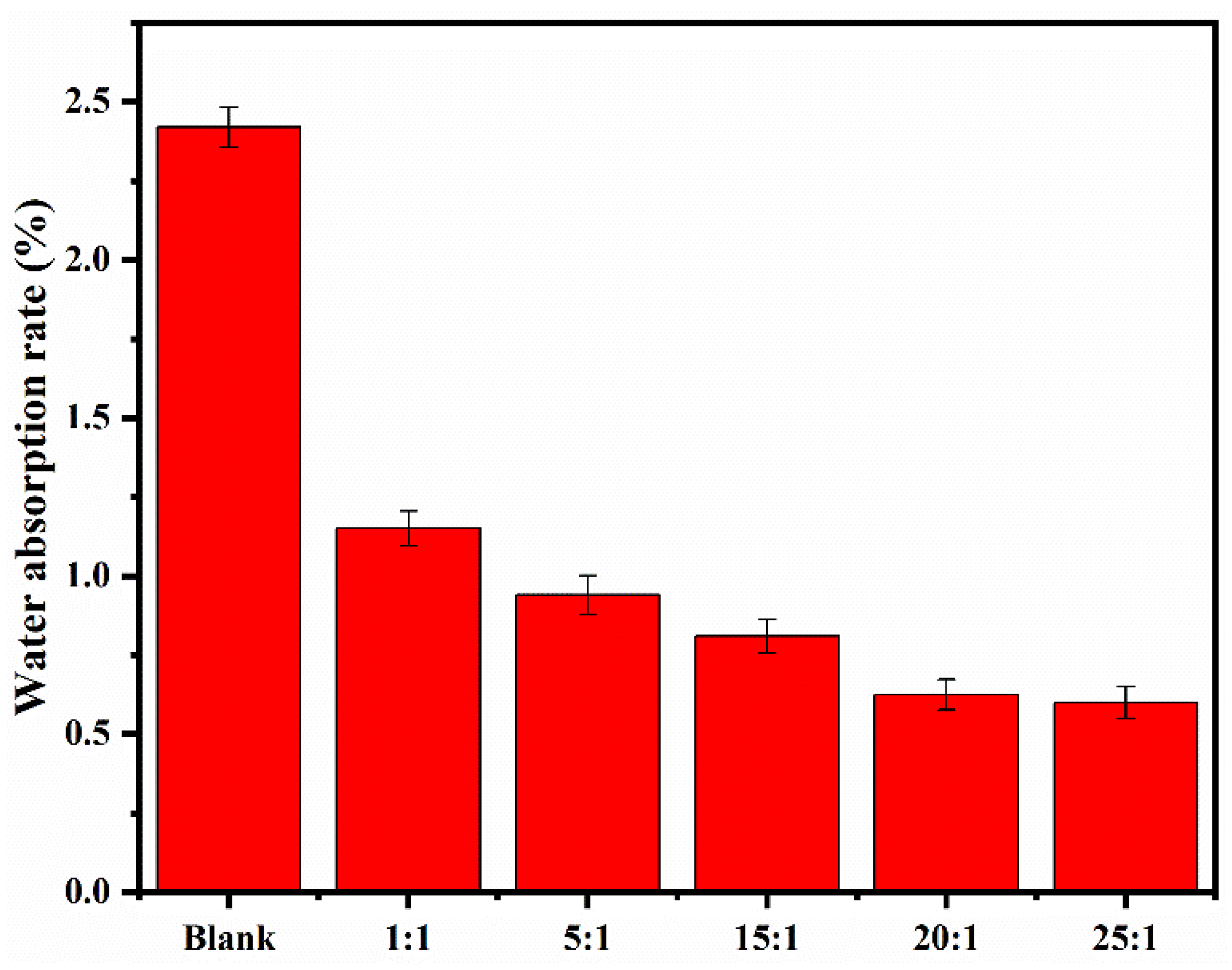
3.5.1. Water Absorption
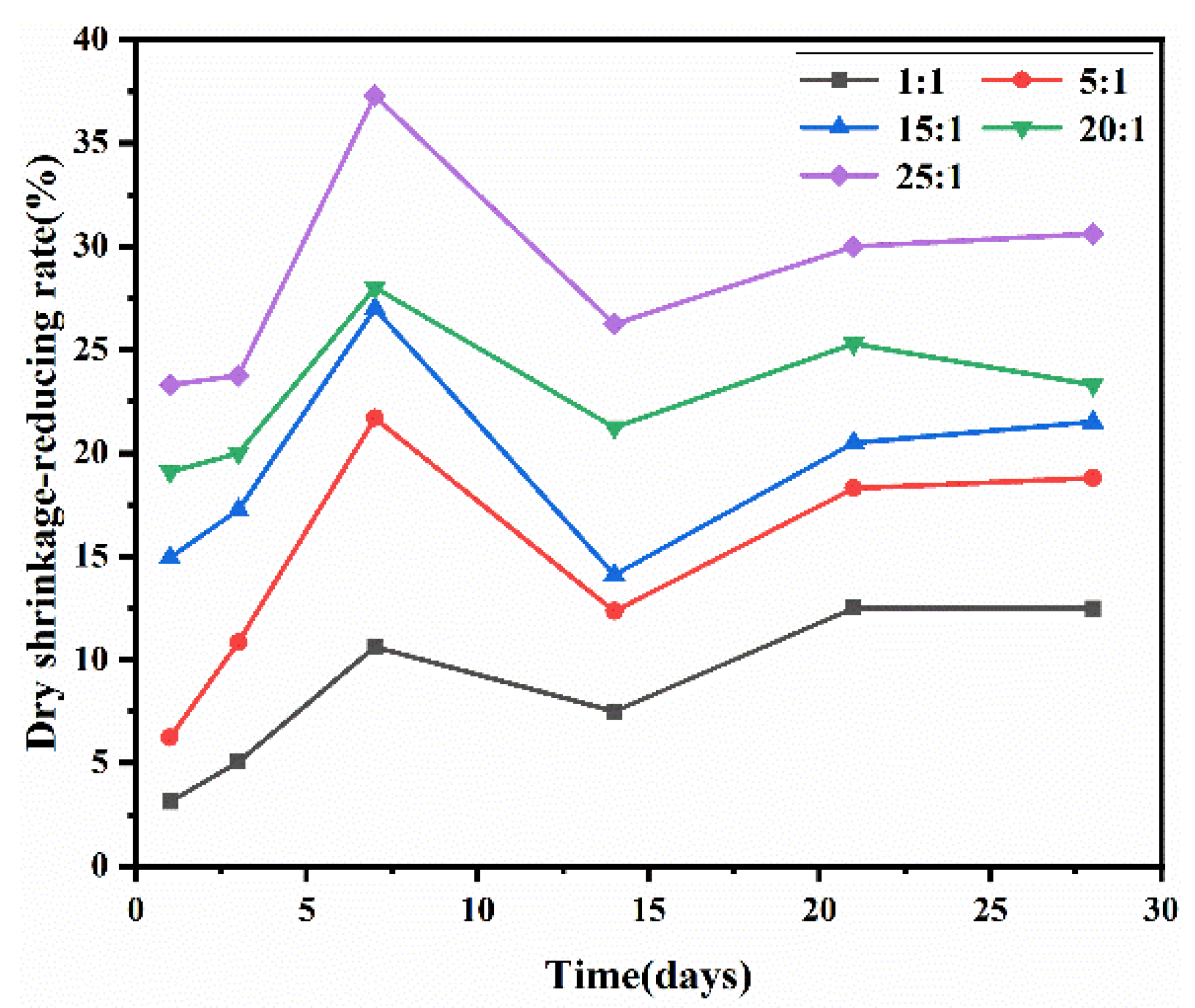
3.5.2. Dry Shrinkage
3.5.3. Mechanical Strength
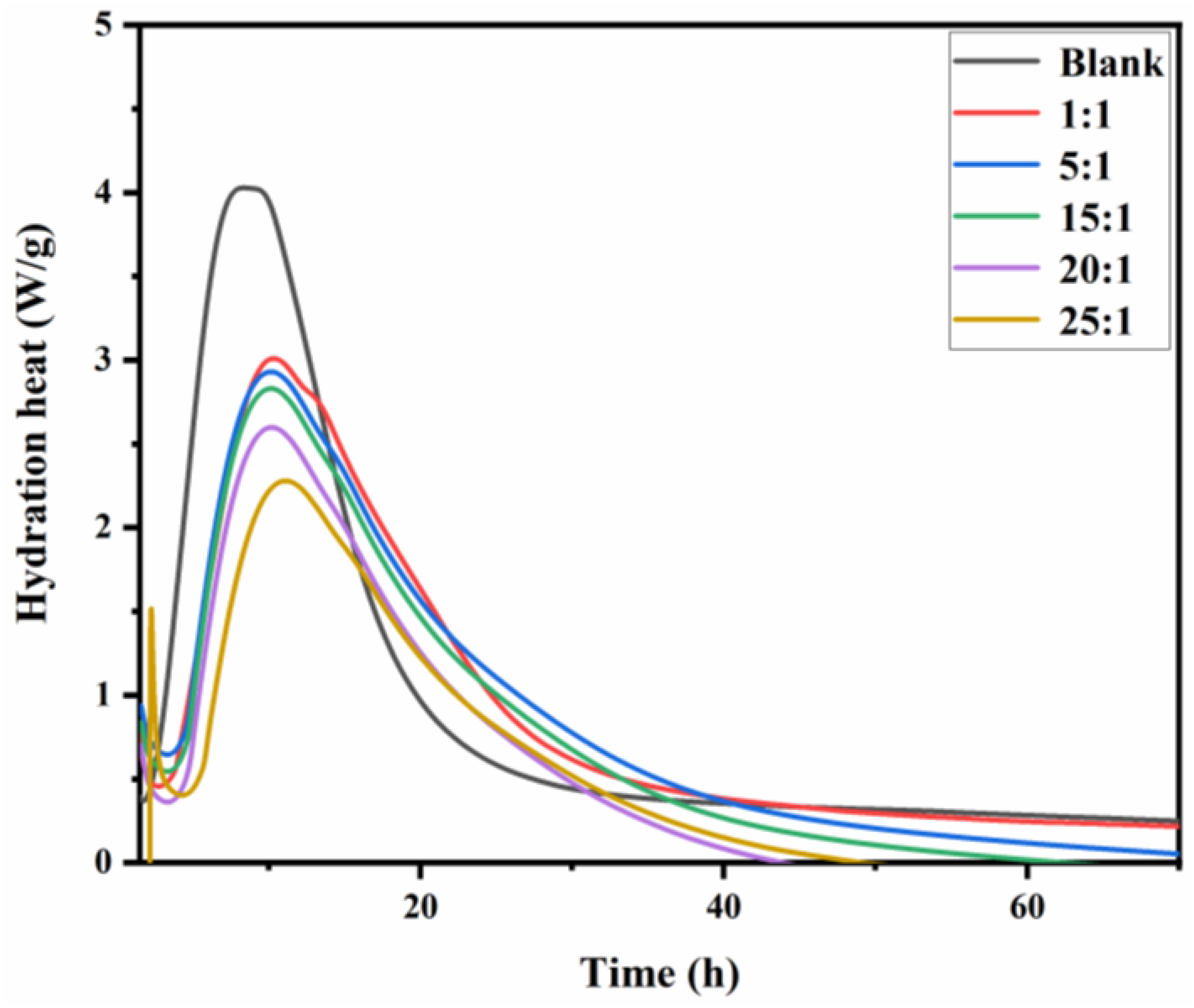
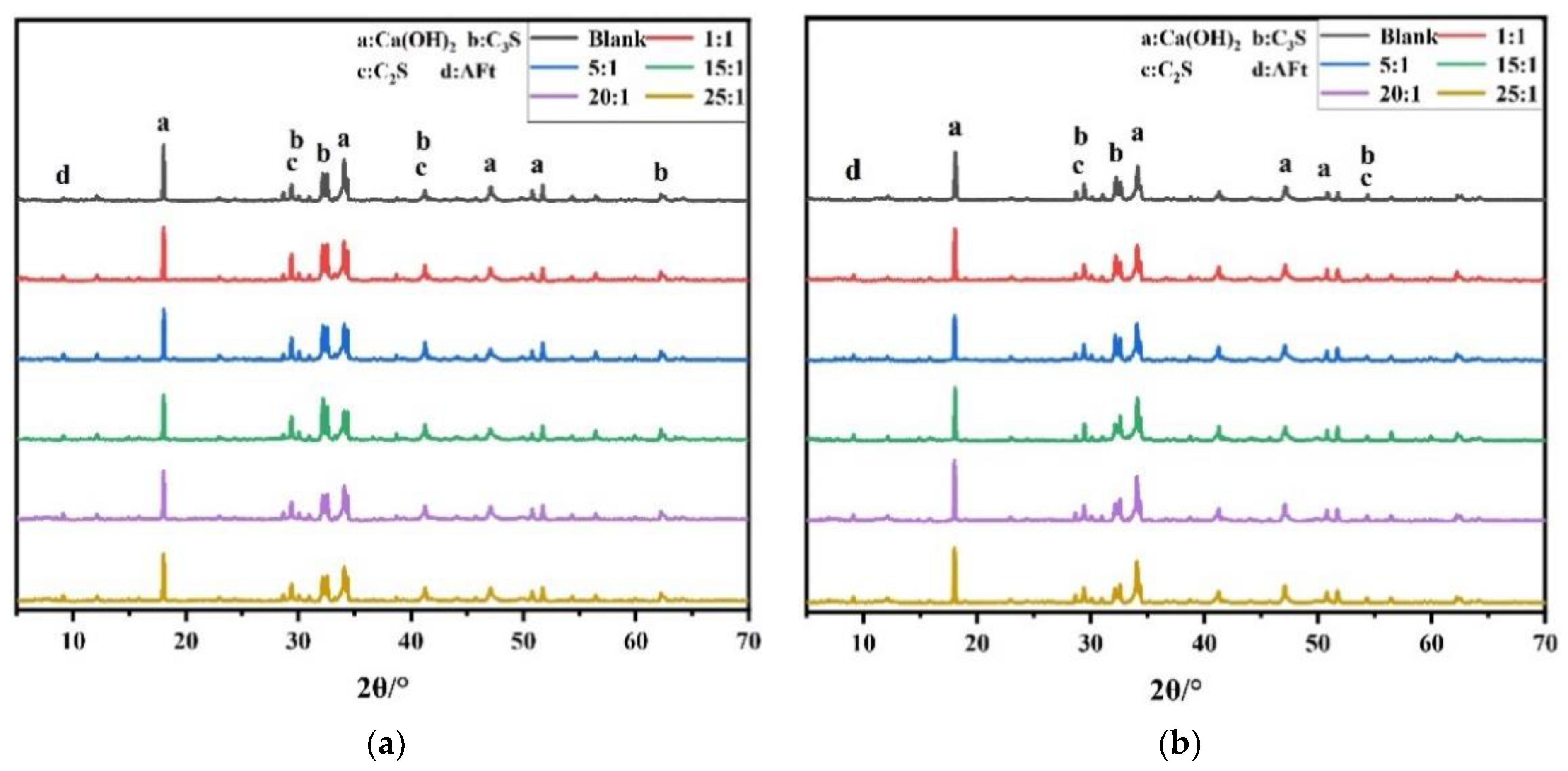
3.6. Hydration Behavior
3.7. Hydration Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, H.G.; Fan, J.C.; Zhao, Y.J.; Chen, H.Y.; Liu, H.X. Evaluation of concrete durability under complex environment. Concrete 2017, 12, 9–13. [Google Scholar]
- Shao, Z.M.; Zhang, C.; Zhong, X.L.; Sun, Y.S.; Gong, W.L.; Zheng, Q.; Peng, J. Development and applications of shrinkage reducing agents overseas. Concrete 2000, 10, 60–63. [Google Scholar]
- Riaan, C.; William, P.B. Typical plastic shrinkage cracking behaviour of concrete. Mag. Concr. Res. 2013, 65, 486–493. [Google Scholar]
- Sadegh, G.; Mateusz, W.; Luis, B.; Pietro, L. Susceptibility of portland cement and blended cement concretes to plastic shrinkage cracking. Cem. Concr. Compos. 2018, 85, 44–55. [Google Scholar]
- Zhang, P.; Li, Q.F.; Chen, Y.Z.; Shi, Y.; Ling, Y.F. Durability of steel fiber-reinforced concrete containing SiO2 nano-particles. Materials 2019, 12, 2184. [Google Scholar] [CrossRef] [Green Version]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Durability evaluation of geopolymer and conventional concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhang, S.M.; Wei, G.; Wei, X.J.; Jin, L.Q.; Xu, K.X. Water transport in unsaturated cracked concrete under pressure. Adv. Civ. Eng. 2019, 8, 4504891. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Wang, D.M.; Wang, Y.R.; Zhang, L.; Gu, Y. Study on durability against dry-wet cycles and chloride ion erosion of concrete revetment materials at the water-level-fluctuations zone in yellow river delta wetlands. Wetlands 2020, 40, 2713–2727. [Google Scholar] [CrossRef]
- Bao, H.; Xu, G.; Wang, Q.; Peng, Y.Z.; Liu, J.Y. Study on the deterioration mechanism of cement-based materials in acid water containing aggressive carbon dioxide. Constr. Build. Mater. 2020, 243, 118233. [Google Scholar] [CrossRef]
- Qian, R.S.; Liu, C.; Liu, G.J.; Liu, Z.Y.; Pang, B.; She, W.; Zhang, Y.S. Effects of various inlet-gas mediums on apparent permeability of concrete under steady-state flow: Comparison between carbon-dioxide and oxygen. Cem. Concr. Compos. 2021, 119, 103995. [Google Scholar] [CrossRef]
- Marcos, L.; Encarnación, M.; María, M.; Juan, A.M. Use of zinc stearate to produce highly-hydrophobic adobe materials with extended durability to water and acid-rain. Constr. Build. Mater. 2017, 139, 114–122. [Google Scholar]
- Paweł, N.; Jerzy, H.; Andrzej, Ć. Study on properties of self-compacting concrete modified with nanoparticles. Arch. Civ. Mech. Eng. 2018, 18, 877–886. [Google Scholar]
- Husni, H.; Nazari, M.R.; Yee, H.M.; Rohim, R.; Yusuff, A.; Ariff, M.A.M.; Ahmad, N.N.R.; Leo, C.P.; Junaidi, M.U.M. Superhydrophobic rice husk ash coating on concrete. Constr. Build. Mater. 2017, 144, 385–391. [Google Scholar] [CrossRef]
- Corcione, C.E.; Striani, R.; Capone, C.; Molfetta, M.; Vendetta, S.; Frigione, M. Preliminary study of the application of a novel hydrophobic photopolymerizable nano-structured coating on concrete substrates. Prog. Org. Coat. 2018, 121, 182–189. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, S.C.; Zhang, W.J.; Chen, X.; Hou, D.S.; Zhao, T.J.; Li, X.G. Preparation and mechanism of graphene oxide/isobutyltriethoxysilane composite emulsion and its effects on waterproof performance of concrete. Constr. Build. Mater. 2019, 208, 343–349. [Google Scholar] [CrossRef]
- Dai, J.; Akira, Y.; Wittmann, F.H.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Compos. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2008, 41, 59–71. [Google Scholar] [CrossRef]
- Santos, W.F.; Quattrone, M.; John, V.M.; Angulo, S.C. Roughness, wettability and water absorption of water repellent treated recycled aggregates. Constr. Build. Mater. 2017, 146, 502–513. [Google Scholar] [CrossRef]
- Theodosia, Z.; Eleni, R.; George, B. Performance evaluation of organic coatings against corrosion in reinforced cement mortars. Prog. Org. Coat. 2011, 72, 175–180. [Google Scholar]
- Li, G.; Yang, B.Y.; Guo, C.S.; Du, J.M.; Wu, X.S. Time dependence and service life prediction of chloride resistance of concrete coatings. Constr. Build. Mater. 2015, 83, 19–25. [Google Scholar] [CrossRef]
- Ye, X.M.; Zhu, C. Preparation and Properties of One-component Acrylate Waterproof Coatings. China Coat. 2018, 33, 56–59. [Google Scholar]
- Li, G.; Guo, C.H.; Gao, X.; Ji, Y.S.; Geng, O. Time dependence of carbonation resistance of concrete with organic film coatings. Constr. Build. Mater. 2016, 114, 269–275. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Shekarchi, M.; Hoseini, M. Time-dependent performance of concrete surface coatings in tidal zone of marine environment. Constr. Build. Mater. 2012, 30, 198–205. [Google Scholar] [CrossRef]
- Almusallam, A.A.; Khan, F.M.; Dulaijan, S.U.; Al-Amoudi, O.S.B. Effectiveness of surface coatings in improving concrete durability. Cem. Concr. Compos. 2003, 25, 473–481. [Google Scholar] [CrossRef]
- Liu, S.J.; Hu, Q.Q.; Zhao, F.Q.; Chu, X.M. Utilization of steel slag, iron tailings and fly ash as aggregates to prepare a polymer-modified waterproof mortar with a core-shell styrene–acrylic copolymer as the modifier. Constr. Build. Mater. 2014, 72, 15–22. [Google Scholar] [CrossRef]
- Feng, Z.J.; Wang, F.J.; Xie, T.; Qu, J.F.; Xue, M.S.; Li, W. Integral hydrophobic concrete without using silane. Constr. Build. Mater. 2019, 227, 116678. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Wang, Q.; Fan, X.R.; Yu, Y.Y.; Wang, P.; Zhang, Y.; Yuan, J.G.; Cavaco-Paulo, A. Preparation and rheological properties of starch-g-poly(butyl acrylate) catalyzed by horseradish peroxidase. Process Biochem. 2017, 59, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Morro, A.; Catalina, F.; Corrales, T.; Pablos, J.L.; Marin, I.; Abrusci, C. New blends of ethylene-butyl acrylate copolymers with thermoplastic starch. Characterization and bacterial biodegradation. Carbohyd. Polym. 2016, 149, 68–76. [Google Scholar] [CrossRef]
- Benjamin, C.C.; Craven, R.J.; Crone, W.C.; Lakes, R.S. Viscoelastic characterization of pH-sensitive 2-hydroxyethyl methacrylate (2-dimethylamino) ethyl methacrylate HEMA-DMAEMA hydrogels. Polym. Test. 2018, 72, 372–376. [Google Scholar] [CrossRef]
- Li, J.M.; Hu, C.S.; Shao, J.M.; Li, H.J.; Li, P.Y.; Li, X.C.; He, W.D. Fabricating ternary hydrogels of P(AM-co-DMAEMA)/PVA/β-CD based on multiple physical crosslinkage. Polymer 2017, 119, 152–159. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, D.S.; An, J.; Zhu, X.W.; Yin, R.; Luo, Q.Z.; Li, F.T. Microemulsion copolyerization of styrene and butyl acrylate initiated by BPO. Polym. Mater. Sci. Eng. 2003, 19, 100–103. [Google Scholar]
- Jia, R. Particle Formation of Vinvl Chloride Microsuspension Polymerization and Single Electron Transfer Polymerization in Microsuspension System; Zhejiang University: Zhengjiang, China, 2015. [Google Scholar]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. J. Am. Oil Chem. Soc. 1954, 5, 249–256. [Google Scholar]
- Lin, I.J.; Friend, J.P.; Zimmels, Y. The effect of structural modifications on the hydrophilic-lipophilic balance of ionic surfactants. J. Colloid Interface Sci. 1973, 45, 378–386. [Google Scholar] [CrossRef]
- JGJ/T 70-2009. Standard for Test Method of Performance on Building Mortar; ASTM: West Conshohocken, PA, USA, 2009.
- GB/T 2419-2005. Test Method for Fluidity of Cement Mortar; ASTM: West Conshohocken, PA, USA, 2005.
- JC/T 603-2004. Standard Test Method for Drying Shrinkage of Mortar; ASTM: West Conshohocken, PA, USA, 2004.
- Mao, Q.J.; Ma, J.F.; Wang, Z.M.; Lan, M.Z.; Cui, S.P. Shrinkage reduction of cement-based materials containing polycarboxylate superplasticizer. Mag. Concr. Res. 2021, 73, 217–227. [Google Scholar] [CrossRef]
- GB/T 17671-1999. Method of Testing Cements-Deteminition of Strength; ASTM: West Conshohocken, PA, USA, 1999.
- Plank, J.; Yu, B. Preparation of hydrocalumite-based nanocomposites using polycarboxylate comb polymers possessing high grafting density as interlayer spacers. Appl. Clay Sci. 2010, 47, 378–383. [Google Scholar] [CrossRef]
- Robert, M.S.; Francis, X.W.; David, J.K. Spectrometric Identification of Organic Compounds; East China University of Science and Technology Press: Shanghai, China, 2017. [Google Scholar]
- Ralph, L.S. The Systematic Identification of Organic Compounds; Chemical Industry Press: Beijing, China, 2007. [Google Scholar]
- Symone, L.M.A.; LaShanda, T.J.K. Nucleation effects of high molecular weight polymer additives on low molecular weight gels. Polym. J. 2018, 50, 775–786. [Google Scholar]
- Lee, H.K.; Lee, Y.H.; Zhang, Q.; Phang, Y.I.; Tan, G.M.R.; Cui, Y.; Ling, X.Y. Superhydrophobic surface-enhanced raman scattering platform fabricated by assembly of ag nanocubes for trace molecular sensing. ACS Appl. Mater. Inter. 2013, 5, 11409–11418. [Google Scholar] [CrossRef]
- Zhang, L.F.; Qian, X.Q.; Yu, C.D.; Fang, M.H.; Qian, K.L.; Lai, J.Y. Influence of evaporation rate on pore size distribution, water loss, and early-age drying shrinkage of cement paste after the initial setting. Constr. Build. Mater. 2019, 226, 299–306. [Google Scholar] [CrossRef]
- Shirin-Abadi, A.R.; Darabi, A.; Jessop, P.G.; Cunningham, M.F. Tuning the aggregation and redispersion behavior of CO2-switchable latexes by a combination of DMAEMA and PDMAEMA-b-PMMA as stabilizing moieties. Polymer 2016, 106, 303–312. [Google Scholar] [CrossRef]
- Mei, Y.J.; Wang, P.M.; Liang, N.X. Mechanism of the effect of styrene butadiene latex on the water absorption and carbonization of cement mortar. J. Build. Mater. 2007, 3, 276–281. [Google Scholar]
- Zhao, H.X. Research on Factors and Characterization and Suppressing Measures of Efflorescence on Cement-Based Decorative Mortar; Chongqing University: Chongqing, China, 2015. [Google Scholar]
- Iman, M.; Khayat, K.H. Effect of shrinkage reducing admixture on early expansion and strength evolution of calcium sulfoaluminate blended cement. Cem. Concr. Compos. 2018, 92, 82–91. [Google Scholar]
- Zuo, W.Q.; Feng, P.; Zhong, P.H.; Tian, Q.; Gao, N.X.; Wang, Y.J.; Yu, C.; Miao, C.W. Effects of novel polymer-type shrinkage-reducing admixture on early age autogenous deformation of cement pastes. Cem. Concr. Res. 2017, 100, 413–422. [Google Scholar] [CrossRef]
- Yang, J.Y.; Liu, L.B.; Liao, Q.L.; Wu, J.M.; Li, J.J.; Zhang, L.H. Effect of superabsorbent polymers on the drying and autogenous shrinkage properties of self-leveling mortar. Constr. Build. Mater. 2019, 201, 401–407. [Google Scholar] [CrossRef]
- Chen, M.Z.; Zhou, M.K.; Wu, S.P.; He, Z. Effect of shrinkage-reducing admixture on hydration and shrinkage deformation of cement-based materials at early ages. Eng. J. Wuhan Univ. 2007, 1, 78–82. [Google Scholar]
- Uchikawa, H.; Hanehara, S.; Shirasaka, T.; Sawaki, D. Effect of admixture on hydration of cement, adsorptive behavior of admixture and fluidity and setting of fresh cement paste. Cem. Concr. Res. 1992, 22, 1115–1129. [Google Scholar] [CrossRef]
- Qiao, D. Influence and Mechanism of Shrinkage-Reducing Admixtures on Shrinkage Reduction of Cement-Based Materials; Chongqing University: Chongqing, China, 2010. [Google Scholar]
- Xu, Y.H. Investigation of Key Transform Technology on the Application of High Performance Cement; Wuhan University of Technology: Wuhan, China, 2006. [Google Scholar]
- Ren, H.T.; Lei Guo, L. Effect of addition of shrinkage-reducing admixture on properties of cement mortar. J. Arch. Civ. Eng. 2012, 3, 59–64. [Google Scholar]



| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2Oeq | f-CaO | C3S | C2S | C3A | C4AF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference cement | 22.93 | 4.29 | 2.89 | 66.23 | 1.92 | 0.35 | 0.70 | 0.64 | 58.78 | 21.38 | 6.49 | 8.77 |
| Sample | PBA-g-PDMAEMA-1:1 | PBA-g-PDMAEMA-5:1 | PBA-g-PDMAEMA-15:1 | PBA-g-PDMAEMA-20:1 | PBA-g-PDMAEMA-25:1 |
|---|---|---|---|---|---|
| Monomer ratio (DMAEMA:PBA) | 1:1 | 5:1 | 15:1 | 20:1 | 25:1 |
| Mw of copolymer (g/mol) | 14,122 | 35,474 | 88,854 | 116,525 | 142,234 |
| Mw of side chain (g/mol) | 157 | 785 | 2355 | 3140 | 3925 |
| Sample | 1:1 | 5:1 | 15:1 | 20:1 | 25:1 |
|---|---|---|---|---|---|
| HLB value | 11.0 | 6.5 | 4.8 | 4.5 | 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Song, X.; Wang, Z.; Xia, C.; Li, T.; Li, X.; Xu, Q.; Cui, S.; Qian, S. Polymer for Internal Hydrophobization of Cement-Based Materials: Design, Synthesis, and Properties. Polymers 2021, 13, 3069. https://doi.org/10.3390/polym13183069
Liu X, Song X, Wang Z, Xia C, Li T, Li X, Xu Q, Cui S, Qian S. Polymer for Internal Hydrophobization of Cement-Based Materials: Design, Synthesis, and Properties. Polymers. 2021; 13(18):3069. https://doi.org/10.3390/polym13183069
Chicago/Turabian StyleLiu, Xiao, Xiaofei Song, Ziming Wang, Chunlei Xia, Ting Li, Xiaoning Li, Qian Xu, Suping Cui, and Shanshan Qian. 2021. "Polymer for Internal Hydrophobization of Cement-Based Materials: Design, Synthesis, and Properties" Polymers 13, no. 18: 3069. https://doi.org/10.3390/polym13183069







