A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing
Abstract
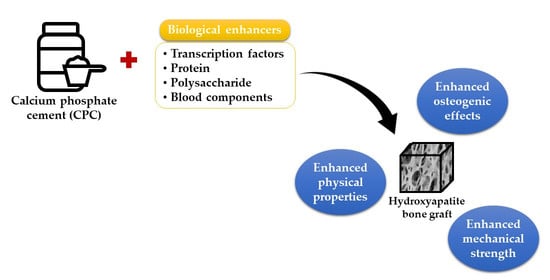
:1. Introduction
2. Literature Search
3. The Enhancement of CPC
3.1. Bone-Related Transcription Factors
3.2. Proteins
3.3. Polysaccharides
3.4. Blood Components
3.5. Combination of Biological Enhancers
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smrke, D.; Rožman, P.; Veselko, M.; Gubina, B. Treatment of Bone Defects—Allogenic Platelet Gel and Autologous Bone Technique; IntechOpen: London, UK, 2013. [Google Scholar]
- Wang, W.; Yeung, K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.; Allison, P.; Theologis, A.; Ashley, J.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2018, 37, 35–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pountos, I.; Giannoudis, P.V. Fracture Healing: Back to Basics and Latest Advances. In Fracture Reduction and Fixation Techniques; Springer: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar] [CrossRef]
- Yousefi, A.-M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; He, F.; Ye, J. Preparation, characterization and in vitro cell performance of anti-washout calcium phosphate cement modified by sodium polyacrylate. RSC Adv. 2017, 7, 32842–32849. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Shi, H.; Liang, Y.; Lu, T.; Lin, Z.; Ye, J. Improving osteogenesis of calcium phosphate bone cement by incorporating with manganese doped β-tricalcium phosphate. Mater. Sci. Eng. C 2019, 109, 110481. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Mason, D.E.; Kegelman, C.D.; Zhao, L.; Dawahare, J.H.; Kacena, M.A.; Boerckel, J.D. Effects of Bone Morphogenetic Protein-2 on Neovascularization During Large Bone Defect Regeneration. Tissue Eng. Part A 2019, 25, 1623–1634. [Google Scholar] [CrossRef]
- Poniatowski, L.A.; Wojdasiewicz, P.; Gasik, R.; Szukiewicz, D. Transforming Growth Factor Beta Family: Insight into the Role of Growth Factors in Regulation of Fracture Healing Biology and Potential Clinical Applications. Mediat. Inflamm. 2015, 2015, 137823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blom, E.J.; Klein-Nulend, J.; Klein, C.P.; Kurashina, K.; van Waas, M.A.; Burger, E.H. Transforming growth factorbeta1 incorporated during setting in calcium phosphate cement stimulates bone cell differentiation in vitro. J. Biomed. Mater. Res. 2000, 50, 67–74. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Yang, K.; Yuan, Y.; Liu, C. RhBMP-2-loaded calcium silicate/calcium phosphate cement scaffold with hierarchically porous structure for enhanced bone tissue regeneration. Biomaterials 2013, 34, 9381–9392. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, J.; Yin, M.; Wang, J.; Liu, C. Enhanced healing of rabbit segmental radius defects with surfacecoated calcium phosphate cement/bone morphogenetic protein-2 scaffolds. Mater. Sci. Eng. C 2014, 44, 326–335. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, W.; Jiang, Y.; Wu, X.; Xu, Z.; Yang, M.; Xie, J. Effects of strontiummodified calcium phosphate cement combined with bone morphogenetic protein-2 on osteoporotic bone defects healing in rats. J. Biomater. Appl. 2018, 33, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; He, Z.; Han, F.; Shi, C.; Zhou, P.; Ling, F.; Zhu, X.; Yang, H.; Li, B. Reinforcement of calcium phosphate cement using alkaline-treated silk fibroin. Int. J. Nanomed. 2018, 13, 7183–7193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Ye, X.; He, F.; Ye, J. Improving osteogenesis of calcium phosphate bone cement by incorporating with lysine: An in vitro study. Colloids Surf. B Biointerfaces 2019, 177, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, W.; Weir, M.D.; Xu, H.H. Biofunctionalized calcium phosphate cement to enhance the attachment and os-teodifferentiation of stem cells released from fast-degradable alginate-fibrin microbeads. Tissue Eng. Part A 2012, 18, 1583–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Zhou, H.; Weir, M.D.; Bao, C.; Xu, H.H. Umbilical cord stem cells released from alginate–fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater. 2012, 8, 2297–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, T.; Niu, J.; Pi, B.; Lu, Y.; Wang, J.; Zhang, W.; Li, B.; Yang, H.; Zhu, X. Osteogenesis enhancement of silk fibroin/α-TCP cement by N-acetyl cysteine through Wnt/β-catenin signaling pathway in vivo and vitro. J. Mech. Behav. Biomed. Mater. 2019, 101, 103451. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.; Xu, H.H. Collagen-Calcium Phosphate Cement Scaffolds Seeded with Umbilical Cord Stem Cells for Bone Tissue Engineering. Tissue Eng. Part A 2011, 17, 2943–2954. [Google Scholar] [CrossRef] [Green Version]
- Cuzmar, E.; Perez, R.A.; Manzanares, M.C.; Ginebra, M.P.; Franch, J. In Vivo Osteogenic Potential of Biomimetic Hydroxy-apatite/Collagen Microspheres: Comparison with Injectable Cement Pastes. PLoS ONE 2015, 10, e0131188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orshesh, Z.; Hesaraki, S.; Khanlarkhani, A. Blooming gelatin: An individual additive for enhancing nanoapatite precipitation, physical properties, and osteoblastic responses of nanostructured macroporous calcium phosphate bone cements. Int. J. Nanomed. 2017, 2017, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Bigi, A.; Torricelli, P.; Fini, M.; Bracci, B.; Panzavolta, S.; Sturba, L.; Giardino, R. A Biomimetic Gelatin-Calcium Phosphate Bone Cement. Int. J. Artif. Organs 2004, 27, 664–673. [Google Scholar] [CrossRef]
- Jang, J.H.; Shin, S.; Kim, H.J.; Jeong, J.; Jin, H.E.; Desai, M.S.; Lee, S.W.; Kim, S.Y. Improvement of physical properties of cal-cium phosphate cement by elastin-like polypeptide supplementation. Sci. Rep. 2018, 8, 5216. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Li, L.; Cui, X.; Tang, Z.; Wei, X.; Pan, H.; Li, B. Enhanced Proliferation and Differentiation Effects of a CGRP- and Sr-Enriched Calcium Phosphate Cement on Bone Mesenchymal Stem Cells. J. Appl. Biomater. Funct. Mater. 2016, 14, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Baranowski, A.; Ritz, U.; Götz, H.; Heinemann, S.; Mattyasovszky, S.; Rommens, P.M.; Hofmann, A. Effect of bone sialoprotein coated three-dimensional printed calcium phosphate scaffolds on primary human osteoblasts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2565–2575. [Google Scholar] [CrossRef]
- Klein, A.; Baranowski, A.; Ritz, U.; Mack, C.; Götz, H.; Langendorf, E.; Al-Nawas, B.; Drees, P.; Rommens, P.M.; Hofmann, A. Effect of bone sialoprotein coating on progression of bone formation in a femoral defect model in rats. Eur. J. Trauma Emerg. Surg. 2019, 46, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Bihuniak, J.D.; Insogna, K.L. The effects of dietary protein and amino acids on skeletal metabolism. Mol. Cell. Endocrinol. 2015, 410, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [Green Version]
- Zapp, C.; Minsky, B.B.; Boehm, H. Tuning RGD Motif and Hyaluronan Density to Study Integrin Binding. Front. Physiol. 2018, 9, 1022. [Google Scholar] [CrossRef]
- Mitroulis, I.; Alexaki, V.I.; Kourtzelis, I.; Ziogas, A.; Hajishengallis, G.; Chavakis, T. Leukocyte integrins: Role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol. Ther. 2014, 147, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaveri, T.; Lewis, J.S.; Dolgova, N.V.; Clare-Salzler, M.J.; Keselowsky, B.G. Integrindirected modulation of macrophage responses to biomaterials. Biomaterials 2014, 35, 3504–3515. [Google Scholar] [CrossRef] [Green Version]
- Malinin, N.; Pluskota, E.; Byzova, T.V. Integrin signaling in vascular function. Curr. Opin. Hematol. 2012, 19, 206–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, L.T.; Lakkakorpi, P.; Nakamura, I.; Rodan, G.A. Integrins and signaling in osteoclast function. Matrix Biol. 2000, 19, 97–105. [Google Scholar] [CrossRef]
- Hendesi, H.; Barbe, M.; Safadi, F.F.; Monroy, M.A.; Popoff, S.N. Integrin Mediated Adhesion of Osteoblasts to Connective Tissue Growth Factor (CTGF/CCN2) Induces Cytoskeleton Reorganization and Cell Differentiation. PLoS ONE 2015, 10, e0115325. [Google Scholar] [CrossRef] [Green Version]
- Green, J.L.; Heard, K.J.; Reynolds, K.M.; Albert, D. Oral and Intravenous Acetylcysteine for Treatment of Acetaminophen Toxicity: A Systematic Review and Meta-analysis. West. J. Emerg. Med. 2013, 14, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Guillerminet, F.; Beaupied, H.; Fabien-Soulé, V.; Tomé, D.; Benhamou, C.-L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: An in vitro and in vivo study. Bone 2010, 46, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar]
- Chen, K.-Y.; Yao, C.-H. Repair of bone defects with gelatin-based composites: A review. BioMedicine 2011, 1, 29–32. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Hnatuszko-Konka, K.; Gerszberg, A.; Kononowicz, A.K. Elastinlike polypeptides as a promising family of genetically-engineered protein based polymers. World J. Microbiol. Biotechnol. 2014, 30, 2141–2152. [Google Scholar] [CrossRef] [Green Version]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, J.; Xu, J.; Yao, H.; Li, X.; Tong, W.; Li, Y.; Dai, B.; He, X.; Chow, D.H.K.; Li, G.; et al. Calcitonin Gene-Related Peptide Enhances Distraction Osteogenesis by Increasing Angiogenesis. Tissue Eng. Part A 2021, 27, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Appelt, J.; Baranowsky, A.; Jahn, D.; Yorgan, T.; Köhli, P.; Otto, E.; Farahani, S.K.; Graef, F.; Fuchs, M.; Herrera, A.; et al. The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing. EBioMedicine 2020, 59, 102970. [Google Scholar] [CrossRef] [PubMed]
- Ganss, B.; Kim, R.H.; Sodek, J. Bone sialoprotein. Crit. Rev. Oral Biol. Med. 1999, 10, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.K.; Goldberg, H.A. Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 8562–8565. [Google Scholar] [CrossRef] [Green Version]
- Mintz, K.P.; Midura, R.J.; Fisher, L.W. Purification of bone sialoprotein from the medium of the rat osteoblast-like cell line UMR 106-01 BSP. J. Tissue Cult. Methods 1994, 16, 205–209. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lei, Z.; Peng, M.; Zhong, M.; Wan, Y.; Luo, H. Enhancement of mechanical and biological properties of calcium phosphate bone cement by incorporating bacterial cellulose. Mater. Technol. 2019, 34, 800–806. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nezafati, N. In vitro biocompatibility of chitosan/hyaluronic acid-containing calcium phosphate bone cements. Bioprocess Biosyst. Eng. 2014, 37, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Xu, H.H. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate—Chitosan composite scaffold. Biomaterials 2009, 30, 2675–2682. [Google Scholar] [CrossRef] [Green Version]
- Lian, Q.; Li, D.-C.; He, J.-K.; Wang, Z. Mechanical properties and in-vivo performance of calcium phosphate cement—Chitosan fibre composite. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 347–353. [Google Scholar] [CrossRef]
- Cui, X.; Huang, C.; Chen, Z.; Zhang, M.; Liu, C.; Su, K.; Wang, J.; Li, L.; Wang, R.; Li, B.; et al. Hyaluronic acid facilitates bone repair effects of calcium phosphate cement by accelerating osteogenic expression. Bioact. Mater. 2021, 6, 3801–3811. [Google Scholar] [CrossRef] [PubMed]
- Sprio, S.; Dapporto, M.; Montesi, M.; Panseri, S.; Lattanzi, W.; Pola, E.; Logroscino, G.; Tampieri, A. Novel Osteointegrative Sr-Substituted Apatitic Cements Enriched with Alginate. Materials 2016, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Weir, M.D.; Xu, H.H. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applica-tions. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, G.C.A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms; IntechOpen: London, UK, 2017; Volume 10. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Wang, X.; Qin, L.; Zhai, M.; Yuan, J.; Chen, J.; Li, D. Effect of hyaluronic acid in bone formation and its applications in dentistry. J. Biomed. Mater. Res. A 2016, 104, 1560–1569. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, Q.; Wang, C.; Shi, F.; Cao, H.; Yu, Y.; Zhang, M.; Liu, X. Hyaluronic acid/Hyaluronidase as biomarkers for bladder cancer: A diagnostic meta-analysis. Neoplasma 2017, 64, 901–908. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound Healing Promotion by Hyaluronic Acid: Effect of Molecular Weight on Gene Expression and In Vivo Wound Closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for os-teoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Seo, J.Y.; Park, J.Y.; Ji, Y.B.; Kim, K.; Choi, H.S.; Choi, S.; Kim, J.H.; Min, B.H.; Kim, M.S. An injectable, clickcrosslinked, cytomodulin-modified hyaluronic acid hydrogel for cartilage tissue engineering. NPG Asia Mater. 2019, 11, 30. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellier, C.; Lefevre, F.X.; Fayon, F.; Montouillout, V.; Despas, C.; Le Ferrec, M.; Boukhechba, F.; Walcarius, A.; Janvier, P.; Dutilleul, M.; et al. A straightforward approach to enhance the textural, mechanical and biological properties of injectable calcium phosphate apatitic cements (CPCs): CPC/blood composites, a comprehensive study. Acta Biomater. 2017, 62, 328–339. [Google Scholar] [CrossRef]
- Hasan, L.; Taz, M.; Lee, B.-T. Effects of platelet-rich plasma on biological activity and bone regeneration of brushite-based calcium phosphate cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 2316–2326. [Google Scholar] [CrossRef]
- Cho, A.-R.; Kim, H.-K.; Kwon, J.-Y.; Kim, T.-K.; Choi, Y.-M.; Kim, K.-H. The incorporation of platelet-rich plasma into calcium phosphate cement enhances bone regeneration in osteoporosis. Pain Physician 2014, 17, E737–E745. [Google Scholar]
- Ko, C.-L.; Chen, W.-C.; Chen, J.-C.; Wang, Y.H.; Shih, C.-J.; Tyan, Y.-C.; Hung, C.-C.; Wang, J.-C. Properties of osteoconductive biomaterials: Calcium phosphate cement with different ratios of platelet-rich plasma as identifiers. Mater. Sci. Eng. C 2013, 33, 3537–3544. [Google Scholar] [CrossRef]
- Lei, W.; Dong, J.; Cui, G.; Bi, L.; Li, J. The mechanical and biological stud ies of calcium phosphate cement-fibrin glue for bone reconstruction of rabbit femoral defects. Int. J. Nanomed. 2013, 8, 1317–1324. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Panda, A.; Kumar, S.; Kumar, A.; Bansal, R.; Bhartiya, S. Fibrin glue in ophthalmology. Indian J. Ophthalmol. 2009, 57, 371–379. [Google Scholar] [CrossRef]
- Nan, A. Miscellaneous Drugs, Materials, Medical Devices and Techniques. In Side Effects of Drugs Annual; Ray, S.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 523–532. [Google Scholar]
- Joch, C. The safety of fibrin sealants. Cardiovasc. Surg. 2003, 11, 23–28. [Google Scholar] [CrossRef]
- Seifert, J.; Klause, N.; Stobbe, J.; Egbers, H.J. Antibodies formed against fibrin glue components and their circulatory relevance. J. Invest. Surg. 1994, 7, 167–171. [Google Scholar] [CrossRef]
- Cunha, M.R.d.; Menezes, F.A.; Santos, G.R.d.; Pinto, C.A.L.; Barraviera, B.; Martins, V.d.C.A.; Plepis, A.M.d.G.; Ferreira, R.S. Hydroxyapatite and a new fibrin sealant derived from snake venom as scaffold to treatment of cranial defects in rats. Mater. Res. 2015, 18, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Makkar, P.; Paul, K.; Lee, B. Incorporation of BMP-2 loaded collagen conjugated BCP granules in calcium phosphate cement based injectable bone substitutes for improved bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 713–724. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Liu, X.; Ge, B. Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: A pilot study. Clin Orthop Relat Res. 2010, 468, 1978–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Chen, L.; Yang, H.L.; Luo, Z.P.; Tang, T.S. Evaluation of an injectable silk fibroin enhanced calcium phosphate cement loaded with human recombinant bone morphogenetic protein-2 in ovine lumbar interbody fusion. J. Biomed. Mater. Res. A 2011, 97, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, H.; Weir, M.D.; Tang, M.; Bao, C.; Xu, H.H. Human Embryonic Stem Cell-Derived Mesenchymal Stem Cell Seeding on Calcium Phosphate Cement-Chitosan-RGD Scaffold for Bone Repair. Tissue Eng. Part A 2013, 19, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-Y.; Zhou, Z.-B.; He, Z.-W.; Ren, W.-P.; Yu, X.-W.; Huang, Y. Reinforcement of a new calcium phosphate cement with RGD-chitosan-fiber. J. Biomed. Mater. Res. Part A 2013, 102, 68–75. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, B.; Padalhin, A.R.; Lee, B.T. Incorporation of chitosan-alginate complex into injectable calcium phosphate ce-ment system as a bone graft material. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Wang, J.; Xie, Q.; Li, F.; Dong, L.; Xu, T. Injectable calcium phosphate–alginate–chitosan microencapsulated MC3T3-E1 cell paste for bone tissue engineering in vivo. Mater. Sci. Eng. C 2013, 33, 4633–4639. [Google Scholar] [CrossRef] [PubMed]
- Ekeuku, S.; Pang, K.-L.; Chin, K.-Y. The Skeletal Effects of Tanshinones: A Review. Molecules 2021, 26, 2319. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, M.A.; Chin, K.-Y.; Ramli, E.S.M. A Review of Potential Beneficial Effects of Honey on Bone Health. Evid. Based Complement. Altern. Med. 2019, 2019, 8543618. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Berberine and musculoskeletal disorders: The therapeutic potential and underlying molecular mechanisms. Phytomedicine 2019, 73, 152892. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Osteoprotective Effects of Kaempferol: The Evidence From In Vivo and In Vitro Studies. Drug Des. Dev. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Mohamad, N.-V.; Ibrahim, N.; Chin, K.-Y.; Shuid, A.N.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef] [Green Version]
- Jolly, J.J.; Mohd Fozi, N.F.; Chin, K.Y.; Wong, S.K.; Chua, K.H.; Alias, E.; Adnan, N.S.; Ima-Nirwana, S. Skeletal microenvi-ronment system utilising bovine bone scaffold co-cultured with human osteoblasts and osteoclast-like cells. Exp. Ther Med. 2021, 22, 680. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Criteria for an Ideal Bone Cement |
|---|---|
| Self-setting ability | The bone cement should harden in situ, forming solid hydroxyapatite. |
| Injectability | The bone cement could be pushed out through a syringe without separation of the liquid and powder that composed it. |
| Mouldability | The bone cement could be moulded according to the shape of a bone cavity |
| Biocompatibility | The bone cement should not cause any local or systemic adverse response. |
| Osteoconductivity | The bone cement should encourage host bone cells, capillaries, and tissue to move into it to facilitate bone repair. |
| Resorbability | The bone cement should be resorbable by the body. |
| Feasibility in controlled drug delivery | The bone cement could be designed to deliver a drug at a predetermined rate. |
| Mechanical strength | The bone cement could withstand application of force without failure and deformation. |
| Brittleness | The bone cement should not be fractured easily when subjected to stress. |
| Anti-washout | The bone cement should be resistant to segregation under washing action. |
| Osteogenic property | The bone cement should encourage new bone formation by osteoblasts. |
| Enhancer | Characteristics of Enhanced CPC | Type of Study | Type of Cell, Sample, and Animal Model | Outcomes Observed in Animals | Reference |
|---|---|---|---|---|---|
| rhTGF-β1 | - | In vitro | Pre-osteoblastic and osteoblastic cells obtained from collagenase-treated fragments of adult rat long bones | Cell differentiation: ↑, ALP: ↑ | [12] |
| rhBMP-2 | - | In vitro | Myoblastic precursor cells | ALP: ↑, COL1: ↑, OCN: ↑, Runx-2: ↑ | [13] |
| In vivo | Bone defect at femur of female New Zealand rabbits | BV/TV: ↑, residue of material: ↓, newly formed bone area: ↑ | |||
| rhBMP-2 | Maximum compressive strength: ↑ | In vivo | Critical defect at the middle of the radius of male New Zealand rabbits | BMC: ↑, BMD: ↑, bone formation: ↑, bone regeneration: ↑, maximum load: ↑ | [14] |
| BMP-2 | - | In vivo | Bone defect at femoral metaphysis of ovariectomised rats | BV/TV: ↑, Tb.N: ↑, Tb.Th: ↑, Tb.Sp: ↓, newly formed bone: ↑, percentage of remaining biomaterial: ↓, ultimate load: ↑ | [15] |
| Enhancer | Characteristics of Enhanced CPC | Type of Study | Type of Cell, Sample, and Animal Model | Outcomes Observed in Animals | Reference |
|---|---|---|---|---|---|
| Alkaline-treated silk fibroin | Compressive strength: ↑, setting time: ↓, anti-washout: ↑, injectability: ↑ | Ex vivo | Sheep vertebra | Compressive strength: ↑, stiffness: ↑ | [17] |
| In vitro | MC3T3-E1 cells | No cytotoxicity, good cell morphology | |||
| Lysine | Compressive strength: ↑, apparent porosity: ↑ | In vitro | Bone mesenchymal stem cells | ALP: ↑, Runx-2: ↑, COL1: ↑, OCN: ↑ | [18] |
| Arginine-glycine-aspartate | Flexural strength: ↔, elastic modulus: ↔, work of fracture: ↔ | In vitro | Human umbilical cord mesenchymal stem cells | Viable cells: ↑, ALP: ↑, COL1: ↑, OCN: ↑, Runx-2: ↑, mineralisation: ↑ | [19] |
| Arginine-glycine-aspartate | Setting time: ↔, flexural strength: ↔, elastic modulus: ↔ | In vitro | Human umbilical cord mesenchymal stem cells | Cell density: ↑, ALP: ↑, OCN: ↑, COL1: ↑, mineralisation: ↑ | [20] |
| N-acetyl cysteine loaded silk fibroin | - | Ex vivo | Sheep vertebra | Compressive strength: ↑, stiffness: ↑ | [21] |
| In vivo | Bone defect at distal femoral metaphysis of male Sprague-Dawley rats | Maximum force: ↑, BV/TV: ↑, remaining material in bone: ↓ | |||
| In vitro | Rat bone marrow mesenchymal stromal cells | ALP: ↑, mineralisation: ↑, Runx-2: ↑, OSX: ↑, OCN: ↑, β-catenin: ↑ | |||
| Collagen | Flexural strength: ↑ | In vitro | Human umbilical cord mesenchymal stem cells | Mineral nodules: ↑, extracellular matrix formation: ↑, cell number: ↑, ALP: ↑, OCN: ↑, COL1: ↑, Runx-2: ↑, mineralisation: ↑ | [22] |
| Collagen microsphere | - | In vivo | Bone defect at femoral condyles of female New Zealand rabbits | New bone formation: ↑ | [23] |
| Gelatine | Initial and final setting time: ↓, compressive strength: ↑, elastic displacement: ↑ | In vitro | Human osteosarcoma (G-292) cells | Cell number: ↑, ALP: ↑ | [24] |
| Gelatine | Setting time: ↓, compressive strength: ↑ | In vitro | Human osteoblast-like (MG63) cells | Cell proliferation: ↑, ALP: ↑, type 1 pro-collagen: ↑, TGF-β1: ↑ | [25] |
| Elastin-like polypeptide | Micro-hardness: ↑, compressive strength: ↑, initial and final setting time: ↑, anti-washout, denser microstructure with fewer pores, crystallite formation: ↑ | In vitro | NIH3T3 cells | Viable cells, normal cell morphology, normal spreading pattern, normal cell distribution, no nuclear condensation in cells | [26] |
| CGRP | Pore size distribution: ↔, compressive strength: ↔ | In vitro | Rat bone marrow mesenchymal stromal cells | Cell proliferation: ↑, ALP: ↑, BMP-2: ↑, osteonectin: ↑, Runx-2: ↑ | [27] |
| Bone sialoprotein | - | In vitro | Human primary osteoblasts | Cell number: ↑, ALP: ↔, OPN: ↔, OSX: ↑, Runx-2: ↔, osteonectin: ↑ | [28] |
| Bone sialoprotein | - | In vivo | Bone defect at femoral condyles of male Wistar rats | BV/TV: ↔, bone ingrowth: ↔ | [29] |
| Enhancer | Characteristics of Enhanced CPC | Type of Study | Type of Cell, Sample, and Animal Model | Outcomes Observed in Animals | Reference |
|---|---|---|---|---|---|
| Bacterial cellulose | Thermal stability: ↑, compressive strength: ↑ | In vitro | MC3T3-E1 cells | Cell growth and proliferation: ↑ | [50] |
| Chitosan | - | In vitro | Osteoblastic cells | ALP: ↑ | [51] |
| Chitosan | Flexural strength: ↑, elastic modulus: ↑ | In vitro | Rat bone marrow mesenchymal stem cells | ALP: ↑ | [52] |
| Chitosan | Compressive strength: ↑ | In vivo | Bone defect at radius of mature dogs | Amount of implant debris: ↓, new bone callus formation: ↑ | [53] |
| Hyaluronic acid | - | In vitro | Osteoblastic cells | ALP: ↑ | [51] |
| Hyaluronic acid | Compressive strength: ↑ | In vitro | Human bone marrow mesenchymal stromal cells | ALP: ↑, OPN: ↑, Runx-2: ↑ | [54] |
| In vivo | Bone defect at metaphyseal region of medial tibia in female Sprague-Dawley rats | BV/TV: ↑, Tb.Pf: ↑, BMD: ↑, bone and vessel formation: ↑, mineralisation: ↑, OCN: ↑, COL1: ↑, BMP-2: ↑ | |||
| Alginate | Injectability: ↑, cohesion: ↑, compressive strength: ↑, Young’s modulus: ↑, setting time: ↔ | In vitro | Human osteoblast-like cells | Viable cells: ↑ | [55] |
| Alginate hydrogel microbeads | Flexural strength: ↑, work of failure: ↑ | In vitro | Human umbilical cord mesenchymal stem cells | ALP: ↑, OCN: ↑, COL1: ↑, OSX: ↑ | [56] |
| Enhancer | Characteristics of Enhanced CPC | Type of Study | Type of Cell, Sample, and Animal Model | Outcomes Observed in Animals | Reference |
|---|---|---|---|---|---|
| Blood composite | Initial setting time: ↑, compressive strength: ↔, stiffness: ↓ | In vivo | Bone defect at distal femoral end of adult female New Zealand white rabbits | Degradation rate: ↑, new bone formation: ↑ | [68] |
| Platelet-rich plasma | No disintegration of paste consistency, setting time: ↓, compressive strength: ↔ | In vitro | MC3T3-E1 cells | No cytotoxic effect, cell proliferation: ↑, cell-to-cement interaction: ↑ | [69] |
| In vivo | Bone defect at femoral head of male New Zealand white rabbits | Residuary material: ↓, BV/TV: ↑ | |||
| Platelet-rich plasma | - | In vivo | Bone defect at distal 1/3 of the caudal vertebra body in ovariectomised female Sprague-Dawley rats | BV/TV: ↑, Tb.Th: ↑, Tb.N: ↑, Tb.Sp: ↓, BMD: ↑, new bone formation: ↑, osteogenesis grade: ↑ | [1] |
| Platelet-rich plasma | - | In vitro | Progenitor bone cells | ALP: ↑, diametral tensile strength: ↔ | [70] |
| In vivo | Bone defect at femur of rabbits | New trabecular bone formation: ↑, breakdown of bulk dense implants into pieces was observed. | |||
| Fibrin glue | - | In vivo | Bone defect at femoral condyles of male New Zealand white rabbits | Compressive strength: ↑, elastic modulus: ↑, new bone formation: ↑ | [71] |
| Enhancer | Characteristics of Enhanced CPC | Type of Study | Type of Cell, Sample, and Animal Model | Outcomes Observed in Animals | Reference |
|---|---|---|---|---|---|
| (A) Combination of BMP-2 and other enhancers | |||||
| BMP-2-loaded collagen | Setting time: ↓, compressive strength: ↑, disintegration or degree of cohesion: ↔ | In vitro | MC3T3-E1 cells | Cell viability: ↑, cell density: ↑ | [78] |
| In vivo | Bone defect at the parietal part of femur of New Zealand white rabbits | New bone tissue formation: ↑, degradation of material: ↑ | |||
| BMP-2 loaded gelatine microsphere | - | In vivo | Bone defect at lumbar vertebrae of female ovariectomised goats | Pushout value: ↑, bone mineralisation: ↑ | [79] |
| rhBMP-2 loaded silk fibroin | - | In vivo | Interbody defect at midpoint of disc space of mature sheep | Stiffness: ↑, BV/TV: ↑, ceramic residue volume: ↓ | [80] |
| (B) Combination of protein and polysaccharide | |||||
| Chitosan with arginine-glycine-aspartate motif | Setting time: ↔, flexural strength: ↑, elastic modulus: ↔, work of fracture: ↑ | In vitro | Human embryonic stem cell-derived mesenchymal stem cells | Percentage of live cells: ↑, cell density: ↑, OCN: ↑, COL1: ↑, mineralisation: ↑ | [81] |
| Chitosan with arginine-glycine-aspartate motif | Flexural strength: ↑ | In vitro | Mouse pluripotent C3H10T1/2(C3) cells | Cell number: ↑, cell proliferation: ↑, ALP: ↑, | [82] |
| In vivo | Bone defect at femoral condyles of New Zealand white rabbits | New bone volume: ↑ | |||
| (C) Combination of two different polysaccharides | |||||
| Chitosan-alginate complex | Initial and final setting time: ↓, no disintegration, compressive strength: ↑ | In vivo | Bone defect at femoral head of male New Zealand white rabbits | New bone formation: ↑, implant remaining: ↓ | [83] |
| Alginate-chitosan microencapsulated MC3T3-E1 cells | Setting time: ↔, compressive strength: ↓ | In vivo | BALB/c nude mice | Scaffold remaining: ↓, lamellar-bone-like mineral structure: ↑, newly formed collagen: ↑, mineralisation rate: ↑ | [84] |
| Bone-Related Transcription Factors | Proteins | Polysaccharides | Blood Components | Bone-Related Transcription Factors + Proteins | Proteins + Polysaccharide | Polysaccharide + Polysaccharide | |
|---|---|---|---|---|---|---|---|
| Physical properties | - | ↑ injectability ↑ anti-washout ↓ setting time (except for elastin-like polypeptide) Pore size: lysine increased, elastin-like polypeptide reduced but CGRP has no change in porosity | ↑ injectability ↑ thermal stability ↑ cohesion No change in setting time | Whole blood increased but PRP reduced setting time | ↓ setting time No change in disintegration or cohesion | No change in setting time | ↓ setting time No disintegration |
| Mechanical properties | ↑ compressive strength | ↑ compressive strength (except for CGRP) ↑ flexural strength ↑ elasticity ↑ work of fracture ↑ micro-hardness | ↑ compressive strength ↑ flexural strength ↑ elasticity ↑ work of fracture | No improvement in compressive strength ↓ stiffness | ↑ compressive strength | ↑ flexural strength No change in elasticity ↑ work of fracture | ↑ compressive strength |
| Biological properties | ↑ osteogenesis ↑ bone density, microstructure, and strength | No cytotoxicity ↑ osteogenesis (except bone sialoprotein) ↑ bone microstructure and strength | No cytotoxicity ↑ osteogenesis ↑ bone density and microstructure | No cytotoxicity ↑ osteogenesis ↑ bone density and microstructure | No cytotoxicity ↑ osteogenesis ↑ bone microstructure and strength | No cytotoxicity ↑ osteogenesis ↑ bone microstructure | ↑ bone formation and mineralisation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.K.; Wong, Y.H.; Chin, K.-Y.; Ima-Nirwana, S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers 2021, 13, 3075. https://doi.org/10.3390/polym13183075
Wong SK, Wong YH, Chin K-Y, Ima-Nirwana S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers. 2021; 13(18):3075. https://doi.org/10.3390/polym13183075
Chicago/Turabian StyleWong, Sok Kuan, Yew Hoong Wong, Kok-Yong Chin, and Soelaiman Ima-Nirwana. 2021. "A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing" Polymers 13, no. 18: 3075. https://doi.org/10.3390/polym13183075
APA StyleWong, S. K., Wong, Y. H., Chin, K.-Y., & Ima-Nirwana, S. (2021). A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers, 13(18), 3075. https://doi.org/10.3390/polym13183075