Chitosan Functionalized with 2-Methylpyridine Cross-Linker Cellulose to Adsorb Pb(II) from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Characterization Materials
2.3. Preparation of 2-Pyridyl Methyl Chitosan (Cs-Py)
2.4. Preparation of Cellulose-Succinic (Com-1)
2.5. Preparation of Cellulose-Succinic-CDI (Com-2)
2.6. Preparation of Cellulose-Succinic-Chitosan (Com-3) and Cellulose-Succinic-Chitosan-Pyridine (Com-4)
2.7. Adsorption Kinetics
2.8. Adsorption Isotherms
3. Results
4. Characterization
4.1. Fourier Transform Infrared (FTIR) Analysis
4.2. Surface Analysis by XPS
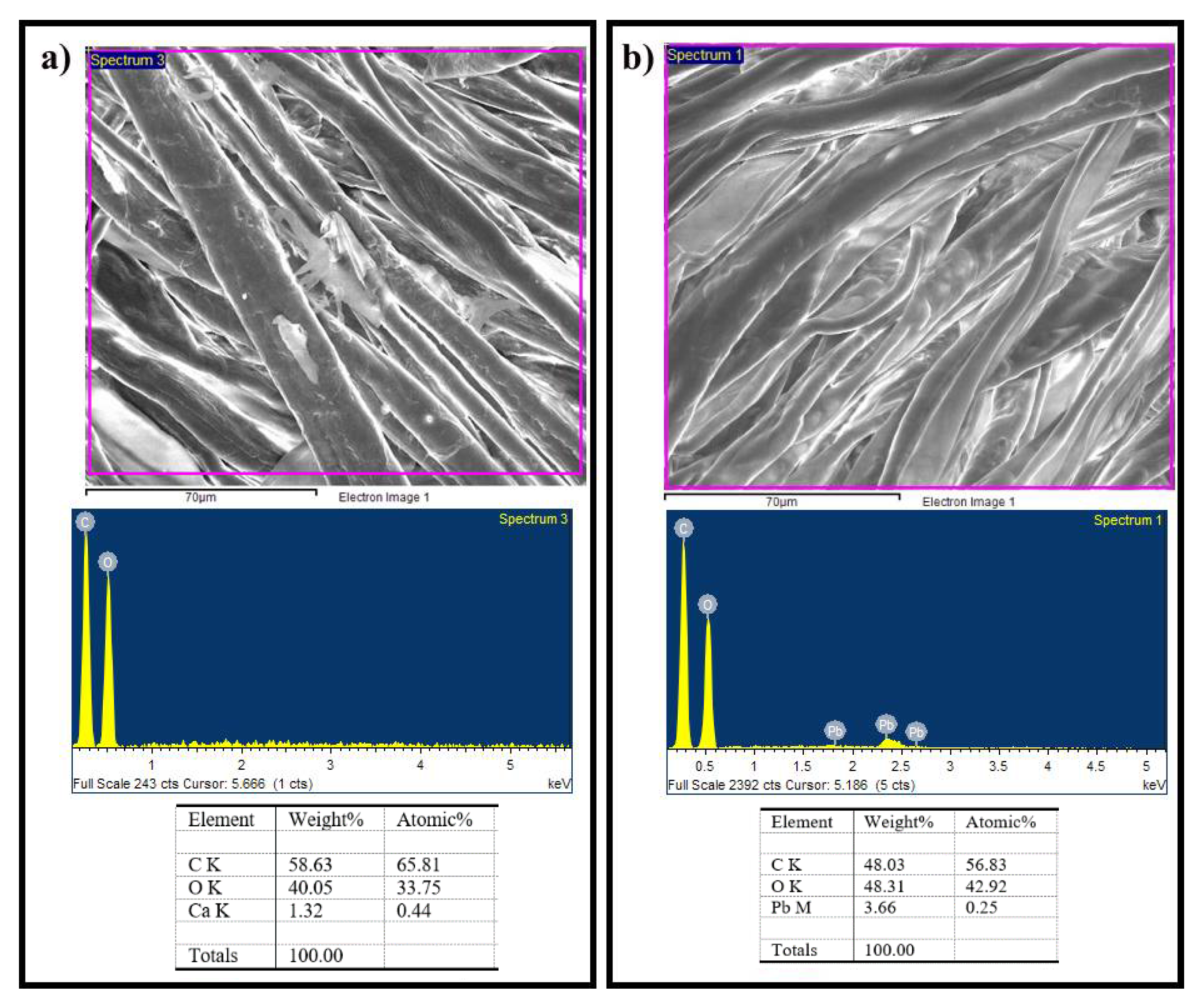
4.3. Surface Analysis by SEM and EDS
4.4. Kinetic Adsorption of Pb(II) by Functionalized Cellulose
4.5. Isothermal Adsorption of Pb(II) by Functionalized Cellulose
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- EU. Directive 2002/959EC of the European Parliament and of the council of 27 January 2003 on the Restriction of the use of Certain Hazardous Substances in Electrical and electronic Equipment. Off. J. Eur. Union 2003, L 37, 19–23. [Google Scholar]
- Vries, W.D.; Römkens, P.F.A.M.; Schütze, G. Critical soil concentrations of cadmium, lead, and mercury in view of health effects on humans and animals. Rev. Environ. Contam. Toxicol. 2007, 191, 91–130. [Google Scholar] [PubMed]
- Xalxo, R.; Keshavkant, S. Hydrolytic enzymes mediated lipid-DNA catabolism and altered gene expression of antioxidants under combined application of lead and simulated acid rain in Fenugreek (Trigonella foenum graecum L.). Ecotoxicology 2018, 27, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Xalxo, R.; Keshavkant, S. Melatonin, glutathione and thiourea attnuates lead and acid rain induced de leterious responses by regulating gene expression of antioxidants in Trigonella foenum graecum L. Chemosphere 2019, 221, 1–10. [Google Scholar] [CrossRef] [PubMed]
- ATSDR Agency for Toxic Substances and Disease Registry Toxicological Profile for Lead; U.S. Department of Health and Human Services: Washington, DC, USA, 2015.
- Gao, Y.; Trueman, B.F.; Stoddart, A.K.; Gagnon, G.A. Understanding the Impact of Extracellular Polymeric Substances on Lead Release in Drinking Water Systems. ACS Omega 2018, 3, 14824–14832. [Google Scholar] [CrossRef] [PubMed]
- Grupta, D.K.; Chatterjee, S.; Walther, C. Lead in Plants and the Environment, Impact of Lead Contamination on Agroecosystem and Human Health; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanada, A.; Eperon, G.E.; Pathank, S.K.; Johnston, M.H.; et al. Lead-free organic-inorganic tin halide perosvskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Tamayo y Ortiz, M.; Téllez-Rojo, M.M.; Hu, H.; Hernández-Ávila, M.; Wright, R.; Amarasiriwardena, C.; Lupoli, N.; Mercado-García, A.; Pantic, I.; Lamadrid-Figueroa, H. Lead in candy consumed and blood lead levels of children living in Mexico City. Environ. Res. 2016, 147, 497–502. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of lead frombattery recycling plant wastewater. Ind. Eng. Chem. Res. 2002, 41, 1579–1582. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Khan, M.H.; Khandker, S.; Sarwar, A.F.M.; Yasmin, N.; Faruquee, M.H.; Yasmin, R. Blood lead leavels and health problems of lead acid battery workers in banglades. Sci. Word J. 2014, 2014, 974104. [Google Scholar]
- Ibarra, J.A.; Lerma, M.S.; Figueroa, D.M.; Franco, M.M.; Moreno, F.A.P. Evaluacion de la potencialidad de una chabasita natural mexicana en la remocion de plomo en agua. Rev. Int. Contam. Ambient 2013, 29, 201–210. [Google Scholar]
- Gwiazda, R.H.; Smith, D.R. Lead isotopes as a supplementary tool in the routine evaluation of household lead hazards. Environ. Health Perspect. 2000, 108, 1091–1097. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, X.; Yang, L.; Bai, X.; Ye, Z.; Zhou, L.; Wang, L. Selective removal for Pb2+ in aqueous environment by using novel macroreticular PVA beads. J. Hazard Mater. 2010, 181, 898–907. [Google Scholar] [CrossRef]
- Almutairi, F.M.; Williams, P.M.; Lovitt, R.W. Effect of membrane surface charge on filtration of heavy metal ions in the presence and absence of polyethylenimine. Desalin. Water Treat. 2012, 42, 131–137. [Google Scholar] [CrossRef]
- Peng, B.; Wan, J.; Li, X.; Zhang, Z.; Du, X.; Lei, Z. Separation and Recovery of Cadmium from Acidic Leach Liquors of Spent Ni-Cd Batteries using Molten Paraffin Wax Solvent Extraction. Sep. Sci. Technol. 2012, 47, 1255–1261. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removeal of heavy metal ions from wastewater: A review. J. Environ. Manag. 2011, 85, 833–846. [Google Scholar]
- Niu, Y.; Li, K.; Ying, D.; Wang, Y.; Jia, J. Novel recyclable adsorbent for the removal of copper (II) and lead (II) from aqueous solution. Bioresour. Technol. 2017, 229, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.; Brouillette, F.; Chabot, B. Phosphorylated cellulose/electrospun chitosan nanofibers media for removal of heavy metals from aqueous solutions. J. Appl. Polym. Sci. 2020, 138, 50021. [Google Scholar] [CrossRef]
- Luo, J.; Yu, D.; Hristovski, K.D.; Fu, K.; Shen, Y.; Westerhoff, P.; Crittenden, J.C. Critical Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Mechanism Identification and Engineering Design. Environ. Sci. Technol. 2021, 55, 4287–4304. [Google Scholar] [CrossRef] [PubMed]
- Najaflou, S.; Rad, M.F.; Baghdadi, M.; Nabi Bidhendi, G.R. Removal of Pb(II) from contaminated waters using cellulose sulfate/chitosan aerogel: Equilibrium, kinetics, and thermodynamic studies. J. Environ. Manag. 2021, 286, 112167. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.T.; Okabe, H.; Hidaka, Y.; Hara, K. Removal of metal ions from aqueous solutions using carboxymethyl cellulose/sodium stryene sulfonate gels prepared by radiation grafting. Carbohydr. Polym. 2017, 157, 335–343. [Google Scholar]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and valorization of cellulose, ling and linocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Blessy, J.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose Nanocomposites: Fabrication and Biomedical Applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar]
- Isaad, J.; El Achari, A. Colorimetric sensing of cyaanide anions in aqueous media bases on functional surface modification of natural cellulose materials. Tetrahedron 2011, 67, 4939–4947. [Google Scholar] [CrossRef]
- Lv, X.; Liu, Y.; Zhang, J.; Zhao, M.; Zhu, K. Study on the adsorption behavior of glutaric acid modified Pb(II) imprinted chitosan-based composite membrane to Pb(II) in aqueous solution. Mater. Lett. 2019, 251, 172–175. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, R.; Sun, C.; Ji, C.; Chen, H.; Wang, C.; Niu, Y. Adsorption for metal ions of chitosan coated cotton fiber. J. Appl. Polym. Sci. 2008, 110, 2321–2327. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, J. Cellulose/chitosan composites prepared in ethylene diamine/potassium thiocyanate for adsorption if heavy metal ions. Cellulose 2017, 24, 2545–2557. [Google Scholar] [CrossRef]
- Alonso-Segura, D.; Hernández-García, L.; Menchaca-Arredondo, J.; Sánchez, M.; Chamorro-Garza, B.; Garza-Hernández, R. The Development and Characterization of a Cotton–Chitosan Composite for Lead Removal from Water. Polymers 2021, 13, 2066. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.; Song, Y.; Lee, H.J.; Lee, H. Synthesis and structural characterisation of Palladium(II) complex whit N,N′,N-Tridentate N′-substituted N,N-Di(2-picolyl)amines and their aplication to Methyl Methacrylate Polymerisation. Aust. J. Chem. 2014, 67, 953–961. [Google Scholar] [CrossRef]
- Suess, D.L.M.; Peters, J.C. Ligand design for site-selective installation of Pd and Pt centers to generate homo-and heteropolymetallic motifs. Chem. Commun. 2010, 46, 6554–6556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nugent, J.W.; Reibenspies, J.H.; Hancock, R.D. Controlling the fluorescence response of PET sensors via the metal-ion Contacting ability of the fluorophore:coumarin, Weaker pi contacter. Inorg. Chem 2015, 20, 9976–9988. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial-a review on recent modifications and applications. Int. J. Biol. Macromol. 2019, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, D.; Wei, Q.; Wei, D.; Yan, T.; Yan, L.; Hu, L.; Du, B. Rapid removal of Pb(II) from aqueous solution using branched polyethylenimine enhanced magnetic carboxymethyl chitosan optimized with response surface methodology. Sci. Rep. 2017, 7, 10264. [Google Scholar] [CrossRef]
- Charpentier, T.V.J.; Neville, A.; Lanigan, J.L.; Barker, R.; Smith, M.J.; Richardson, R. Preparation of Magnetic Carboxymethylchitosan Nanoparticles forAdsorption of Heavy Metal Ions. ACS Omega 2016, 1, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Barsbay, M.; Kavakh, P.A.; Tilki, S.; Kavakh, C.; Güven, O. Porous cellulosic adsorbent for the removal of Cd(II), Pb(II) and Cu(II) ions from aqueous media. Radiat. Phys. Cem. 2018, 142, 70–76. [Google Scholar] [CrossRef]
- Hashem, A.; Fletcher, A.J.; Younis, H.; Mauof, H.; Abou-Okeil, A. Adsorption of Pb(II) ions from contaminated water by 1,23,4-butanetetracarvoxylic acid-modified microsrustalalline cellulose: Isotherms, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2020, 164, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Bai, R. Mechanismos of lead adsoprtion on chitosan/PVA hydrogel Beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Gou, S. Mechanismos of lead biosorption on cellulose/chitin beads. Water Res. 2005, 39, 3755–3762. [Google Scholar] [CrossRef]
- Ununabonah, E.I.; Adebowale, K.O.; Olu-Owolabi, B.I.; Yang, L.Z.; Kong, L.X. Adsorption of Pb(II) and Cd(II) from aqueous solutions onto sodium tetraborate-modified Kalinite clay: Equilibrium and thermodynamic studies. Hydrometallury 2008, 93, 1–9. [Google Scholar] [CrossRef]
- Person, R.G. Hard and soft acids and base. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Khiari, R.; Rol, F.; Brochier Salon, M.C.; Bras, J.; Belgacem, M.N. Efficiency of Cellulose Carbonates to Produce Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 8155–8167. [Google Scholar] [CrossRef]
- Fras, L.; Johansson, L.S.; Stenius, P.; Laine, J.; Stana-Kleinschek, K.; Ribitsch, V. Analysis of the oxidation of cellulose fibres by titration and XPS. Colloids Surf. A Physicochem. Eng. Asp. 2005, 260, 101–108. [Google Scholar] [CrossRef]
- Ly, B.; Thielemans, W.; Dufresne, A.; Chaussy, D.; Belgacem, M.N. Surface functionalization of cellulose fibres and their incorporation in renewable polymeric matrices. Compos. Sci. Technol. 2008, 68, 3193–3201. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Maachou, H.; Genet, M.J.; Aliouche, D.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS analysis of chitosan–hydroxyapatitebiomaterials: From elements to compounds. Surf. Interface Anal. 2013, 45, 1088–1097. [Google Scholar] [CrossRef]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Jurado-López, B.; Vieira, R.S.; Rabelo, R.B.; Beppu, M.M.; Casado, J.; Rodríguez-Castellón, E. Formation of complexes between functionalized chitosan membranes and copper: A study by angle resolved XPS. Mater. Chem. Phys. 2017, 185, 152–161. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef]
- Abdel-Samad, H.; Watson, P. An XPS study of the adsorption of lead on goethitea-FeOOH. Appl. Surf. Sci. 1998, 136, 46–54. [Google Scholar] [CrossRef]
- Marchon, B.; Carrazza, J.; Heinemann, H.; Somorjai, G.A. TPD and XPS studies of O2, CO2, and H2O adsorption on clean polycrystalline graphite. Carbon 1998, 26, 507–514. [Google Scholar] [CrossRef]













| C 1s | N 1s | Pb 4f | O 1s | Si 2p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | C–C/C–H | C–OH/C–O | O–C–O | O=C–O/C=O | N–py | –NH2 | O=C–NH– | –NH3+ | Pb–O | Pb–NO3 | O–C | HO–C | –O–C=O | Si |
| Cellulose | 28.46 | 28.70 | 7.20 | 1.61 | 0.02 | 0.15 | 0.36 | 0.10 | 0.40 | 0.05 | 2.47 | 27.50 | 0.23 | 2.76 |
| Com-1 | 32.07 | 20.64 | 5.62 | 5.98 | 0.02 | 0.21 | 0.29 | 0.07 | 0.19 | 0.01 | 2.04 | 25.74 | 2.49 | 4.65 |
| Com-3 | 27.71 | 25.46 | 8.62 | 1.71 | 0.33 | 1.93 | 1.54 | 0.57 | 0.69 | 0.04 | 4.97 | 21.93 | 1.02 | 3.48 |
| Com-4 | 27.88 | 28.12 | 7.06 | 1.57 | 0.49 | 2.09 | 1.62 | 0.81 | 0.23 | 0.01 | 5.58 | 22.61 | 0.55 | 1.39 |
| Material | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | |
| Cellulose | 3.13 | 0.337 | 0.9349 | 5.27 | 0.291 | 0.99978 |
| Com-1 | 10.97 | 0.828 | 0.94566 | 22.5 | 0.177 | 0.99939 |
| Com-3 | 22.5 | 0.945 | 0.9942 | 22.6 | 0.147 | 0.99878 |
| Com-4 | 17.4 | 0.666 | 0.9949 | 23.8 | 0.097 | 0.99919 |
| Material | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | n | KF | R2 | |
| Cellulose | 6.62 | 0.115 | 0.9928 | 4.44 | 1.9474 | 0.9738 |
| Com-1 | 43.14 | 2.64 | 0.9999 | 4.56 | 20.504 | 0.5062 |
| Com-3 | 60.6 | 0.609 | 0.9994 | 3.69 | 24.928 | 0.6354 |
| Com-4 | 80.26 | 1.01 | 0.9997 | 5.9 | 20.506 | 0.5205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Montante, J.; Garza-Hernández, R.; Sánchez, M.; Moran-Palacio, E.; Niño-Medina, G.; Almada, M.; Hernández-García, L. Chitosan Functionalized with 2-Methylpyridine Cross-Linker Cellulose to Adsorb Pb(II) from Water. Polymers 2021, 13, 3166. https://doi.org/10.3390/polym13183166
Lozano-Montante J, Garza-Hernández R, Sánchez M, Moran-Palacio E, Niño-Medina G, Almada M, Hernández-García L. Chitosan Functionalized with 2-Methylpyridine Cross-Linker Cellulose to Adsorb Pb(II) from Water. Polymers. 2021; 13(18):3166. https://doi.org/10.3390/polym13183166
Chicago/Turabian StyleLozano-Montante, Jorge, Raquel Garza-Hernández, Mario Sánchez, Edgar Moran-Palacio, Guillermo Niño-Medina, Mario Almada, and Luis Hernández-García. 2021. "Chitosan Functionalized with 2-Methylpyridine Cross-Linker Cellulose to Adsorb Pb(II) from Water" Polymers 13, no. 18: 3166. https://doi.org/10.3390/polym13183166
APA StyleLozano-Montante, J., Garza-Hernández, R., Sánchez, M., Moran-Palacio, E., Niño-Medina, G., Almada, M., & Hernández-García, L. (2021). Chitosan Functionalized with 2-Methylpyridine Cross-Linker Cellulose to Adsorb Pb(II) from Water. Polymers, 13(18), 3166. https://doi.org/10.3390/polym13183166