Progress of 3D Bioprinting in Organ Manufacturing
Abstract
:1. Introduction
2. 3D Bioprinting Technologies
2.1. Inkjet-Based 3D Printing
2.2. Fused Deposition Modeling (FDM)
2.3. Extrusion-Based 3D Printing
2.4. Stereolithography (SLA)
2.5. Aerosol Jet Printing
3. Natural Polymers for Organ 3D Printing
3.1. Gelatin
3.2. Alginate
3.3. Fibrin
3.4. Chitosan
3.5. Agarose
4. Synthetic Polymers for Organ 3D Printing
4.1. PLA
4.2. PLGA
4.3. PU
4.4. PCL
4.5. Pluronic Acid (or Poloxamer)
5. Nanometer Material for Organ 3D Printing
6. Typical 3D Bioprinting Technologies for Bioartificial Organ Manufacturing
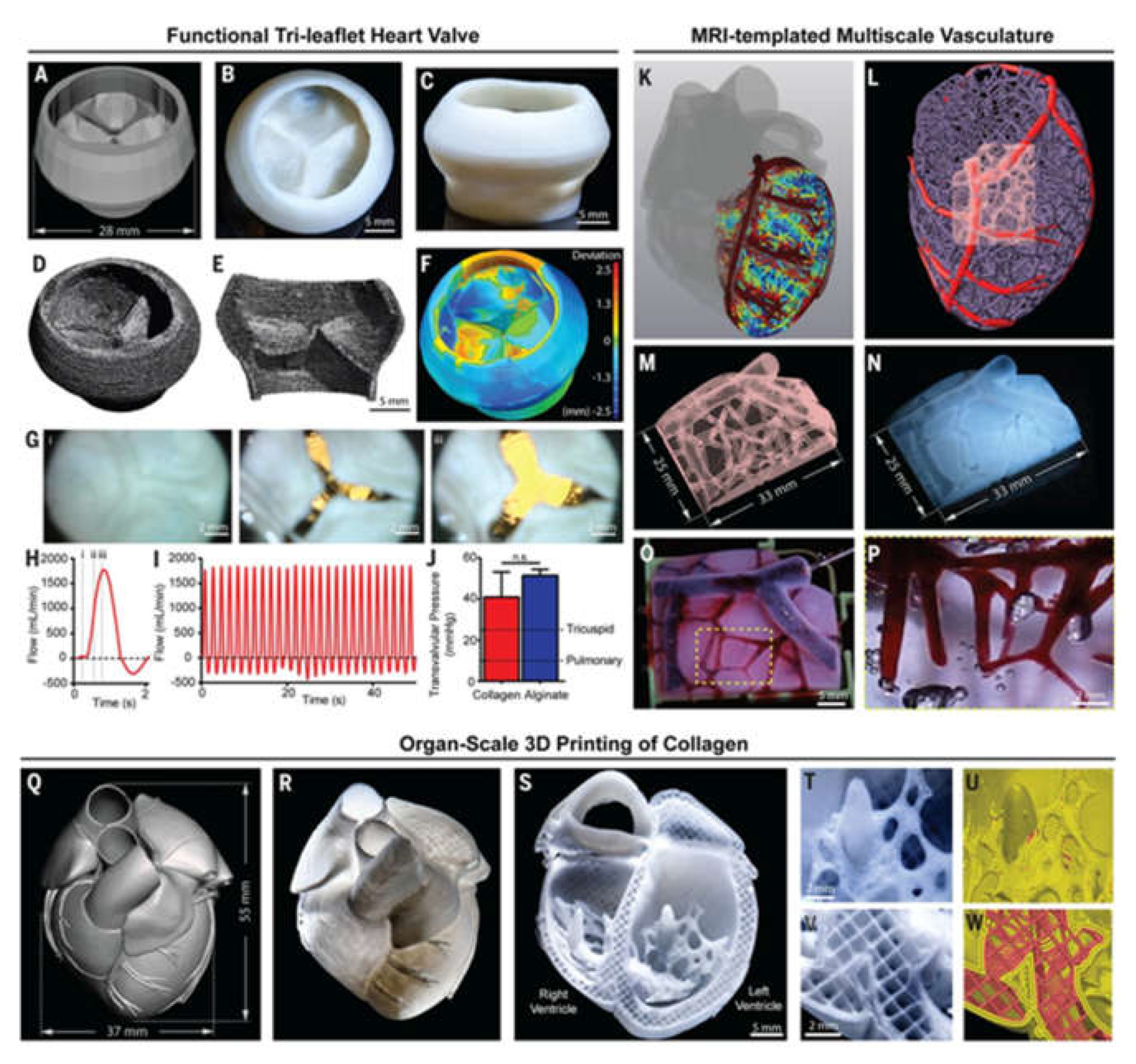
6.1. Heart 3D Bioprinting
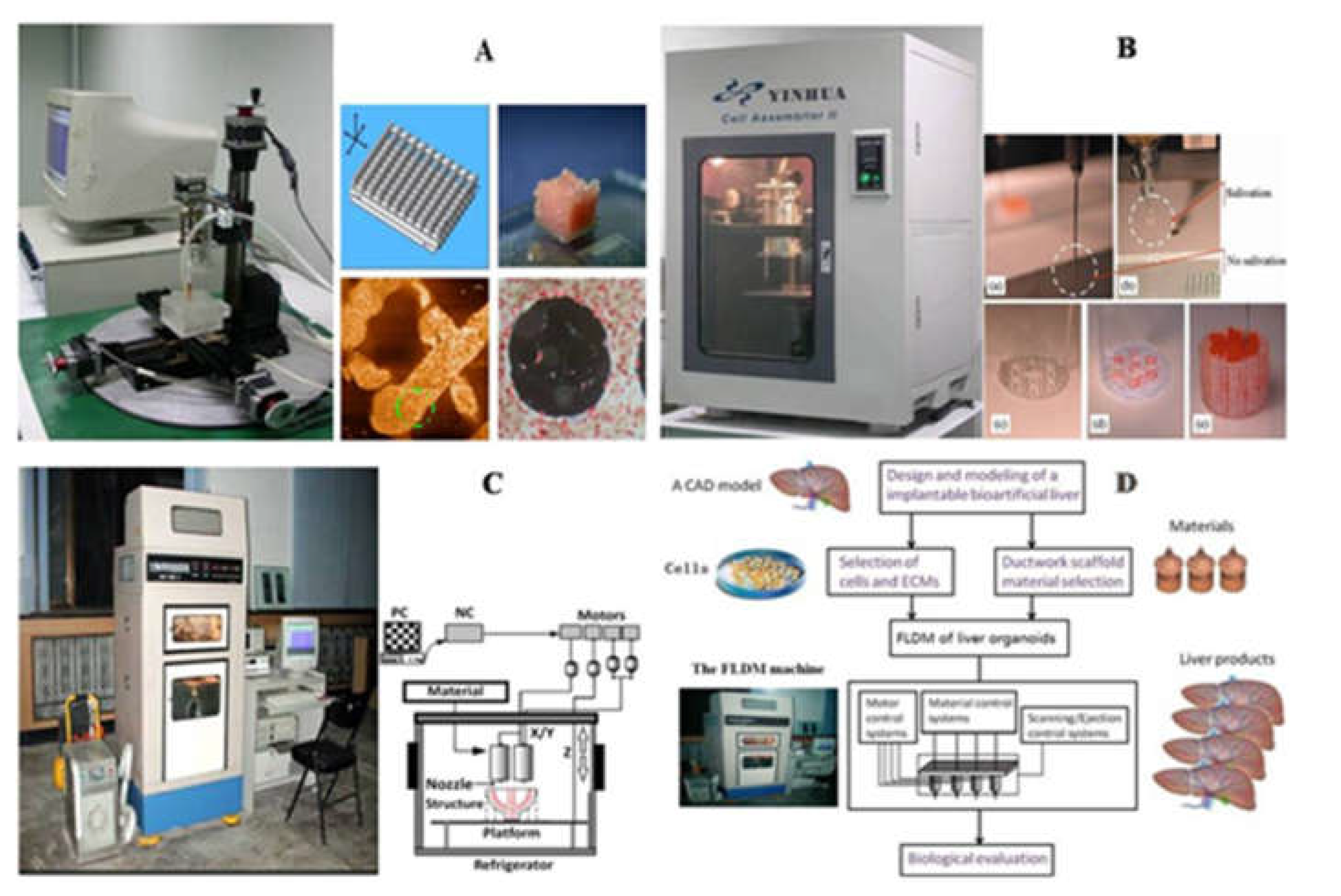
6.2. Liver 3D Bioprinting
6.3. Neural 3D Bioprinting
6.4. Skin 3D Bioprinting
7. Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full name |
| 3D | Three-dimensional |
| RP | rapid prototyping |
| AM | additive manufacturing |
| SFM | solid free-form surface manufacturing |
| CAD | computer-aided design |
| FDM | fused deposition modeling |
| PEGDA | PEG-diacrylate |
| GAG | glycosaminoglycans |
| PMC | polymer matrix composites |
| PCC | polymer ceramic composites |
| FRC | fiber reinforced composites |
| ABS | acrylonitrile butadiene styrene |
| PLA | polylactic acid |
| ASA | acrylonitrile styrene acrylate |
| PC | polycarbonate |
| PEEK | polyether ether ketone |
| ULTEM | polyether imide |
| HA | hyaluronic acid |
| AHA | aldehyde hyaluronic acid |
| CMC | N-carboxymethyl chitosan |
| GEL | gelatin |
| ALG | alginate |
| SLA | Stereolithography |
| GelMA | gelatin-methacryloyl |
| HAMA | methacrylated hyaluronic acid |
| SMC | smooth muscle cells |
| VIC | valve interstitial cells |
| TPP | tripolyphosphate |
| ZnO | zinc oxide |
| NPs | nanoparticles |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| PEG | Polyethylene glycol |
| NHS | N-hydroxysuccinimide |
| PBS | phosphate buffered saline |
| ACGO | agarose/chitosan/graphene oxide |
| PGA | glycolic acid |
| PLGA | polylactic-co-glycolic acid |
| PU | polyurethane |
| LA | lactic acid |
| GA | glycolic acid |
| FDA | Food and Drug Administration |
| BMSCs | bone marrow mesenchymal stem cells |
| UV | ultraviolet |
| DLDM | dual-nozzle low-temperature deposition manufacturing |
| WBPU | waterborne polyurethane |
| PPO | propylene oxide |
| 2DNM | two-dimensional nano materials |
| MOF | Metal Organic Frameworks |
| PG | polymer gel |
| iPSC | induced pluripotent cell |
| CMs | cardiomyocytes |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| SEC | sinusoidal endothelial cells |
| KC | Kupffer cells |
| HSC | hepatic stellate cells |
| ASCs | adipose-derived stem cells |
| NSC | neural stem cells |
| PEDOT | poly(3,4-ethylenedioxythiophene) |
| Cs | chitosan |
| GF | growth factor |
| ECM | extracellular matrix |
| FB | fibroblasts |
| EC | endothelial cells |
References
- Mallis, P.; Oikonomidis, C.; Dimou, Z.; Stavropoulos-Giokas, C.; Michalopoulos, E.; Katsimpoulas, M. Optimizing decellularization strategies for the efficient production of whole rat kidney scaffolds. Tissue Eng. Regen. Med. 2021, 18, 623–640. [Google Scholar] [CrossRef]
- Zhang, L.G.; Khademhosseini, A.; Webster, T.J. Tissue and Organ Regeneration: Advances in Micro and Nanotechnology; Pan Stanford Publishing: Stanford, CA, USA, 2014. [Google Scholar]
- Wang, X.; Yan, Y.; Zhang, R. Gelatin-based hydrogels for controlled cell assembly. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Ed.; Springer: New York, NY, USA, 2010; pp. 269–284. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Chinzei, R.; Tanak, Y.; Shimizu-Saito, K.; Hara, Y.; Kakinuma, S.; Watanabe, M.; Teramoto, K.; Arii, S.; Takase, K.; Sato, C.; et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology 2002, 36, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D bioprinting technologies for hard tissue and organ engineering. Materials 2016, 9, 802. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhu, K.; Li, J.; Wang, C.-S.; Wang, S.-B.; Chen, A.-Z. Fabrication of arbitrary 3D components in cardiac surgery: From macro-, micro- to nanoscale. Biofabrication 2017, 9, 032002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.S.; Heinrich, M.A.; De Ferrari, F.; Jang, H.L.; Bakht, S.M.; Alvarez, M.M.; Yang, J.; Li, Y.-C.; Trujillo-de Santiago, G.; et al. Rapid continuous multimaterial extrusion bioprinting. Adv. Mater. 2017, 29, 1604630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cristescu, R.; Chrisey, D.B.; Narayan, R.J. Solvent-based extrusion 3D printing for the fabrication of tissue engineering scaffolds. Int. J. Bioprinting 2020, 6, 211. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- You, F.; Eames, B.F.; Chen, X. Application of extrusion-based hydrogel bioprinting for cartilage tissue engineering. Int. J. Mol. Sci. 2017, 18, 1597. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Poloheimo, K.S.; Partanen, J.; Yliperttula, M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In Advances in Biomaterials Science and Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 437–463. [Google Scholar]
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.F.; Zhang, W.M.; Khang, G.; Wang, X. In vitro vascularization of a combined system based on a 3D bioprinting technique. J. Tissue Eng. Regen. Med. 2016, 10, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.J.; Saksena, R.; Kolesky, D.B.; Vericella, J.J.; Kranz, S.J.; Muldowney, G.P.; Christensen, K.; Lewis, J.A. High-throughput printing via microvascular multinozzle arrays. Adv. Mater. 2013, 25, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Hubbell, K.; Schilling, A.F.; Dai, G.; Cui, X. Bioprinting cartilage tissue from mesenchymal stem cells and PEG hydrogel. Methods Mol. Biol. 2017, 1612, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Montero, M.; Odell, D.; Roundy, S.; Wright, P.K. Anisotropic material properties of fused deposition modeling ABS. Rapid Prototyp. J. 2002, 8, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.N.; Strong, R.; Gold, S.A. A review of melt extrusion additive manufacturing processes: Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-based 3D printing of polymer and associated composite: A review on mechanical properties, defects and treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, D.; Jin, Z. Preliminary investigation of poly-ether-ether-ketone based on fused deposition modeling for medical applications. Materials 2018, 11, 288. [Google Scholar] [CrossRef] [Green Version]
- Haleem, A.; Javaid, M. Polyether ether ketone (PEEK) and its 3D printed implants applications in medical field: An overview. Clin. Epidemiol. Glob. Health 2019, 7, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Lu, W.; Zhang, J.; Yang, C.; Wu, G. Preparation and performance evaluation of duotone 3D-printed polyetheretherketone as oral prosthetic materials: A proof-of-concept study. Polymers 2021, 13, 1949. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ye, X.; Wei, D.; Zhong, J.; Chen, Y.; Xu, G.; He, D. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair. ACS Appl. Mater. Interfaces 2014, 6, 14952–14963. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I. 3D Bioprinting; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, J.L.; Clark, E.A.; Murriky, A.; Smay, J.E. Three-dimensional printing of bone repair and replacement materials: Impact on craniofacial surgery. J. Craniofacial Surg. 2012, 23, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng. Part A 2008, 14, 41–48. [Google Scholar]
- Wang, X.; Ma, J.; Feng, Q.; Cui, F. Skeletal repair in of rabbits with calcium phosphate cements incorporated phosphorylated chitin reinforced. Biomaterials 2002, 23, 4591–4600. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Rutz, A.L.; Gargus, E.S.; Hyland, K.E.; Lewis, P.L.; Setty, A.; Burghardt, W.R.; Shah, R.N. Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties. Acta Biomater. 2019, 99, 121–132. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, L.; Du, X.; Fan, Z.; Wang, L.; Sun, W.; Cheng, Y.; Zhu, Y.; Chen, C. Reversible physical crosslinking strategy with optimal temperature for 3D bioprinting of human chondrocyte-laden gelatin methacryloyl bioink. J. Biomater. Appl. 2018, 33, 609–618. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X. Synthetic polymers for organ 3D printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 22, 110–115. [Google Scholar] [CrossRef]
- Magalhães, L.S.S.M.; Santos, F.E.P.; Elias, C.D.M.V.; Afewerki, S.; Sousa, G.; Furtado, A.S.A.; Marciano, F.R.; Lobo, A.O. Printing 3D hydrogel structures employing low-cost stereolithography technology. J. Funct. Biomater. 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Kim, K. Stereolithography 3D bioprinting. Methods Mol. Biol. 2020, 2140, 93–108. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D bioprinting method for fabrication of human corneal stroma equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, J.; Windmill, J.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of GelMA/PCL and dECM/PCL resins for 3D printing of acellular in vitro tissue scaffolds by stereolithography. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110958. [Google Scholar] [CrossRef]
- Serpelloni, M.; Cantù, E.; Borghetti, M.; Sardini, E. Printed smart devices on cellulose-based materials by means of aerosol-jet printing and photonic curing. Sensors 2020, 20, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhang, H.; Choi, J.P.; Moon, S.K.; Lee, B.; Koo, J. A post-treatment method to enhance the property of aerosol jet printed electric circuit on 3D printed substrate. Materials 2020, 13, 5602. [Google Scholar] [CrossRef]
- Efimov, A.; Arsenov, P.; Kornyushin, D.; Lizunova, A.; Volkov, I.; Ivanov, V. Aerosol jet printing of silver lines with a high aspect ratio on a heated silicon substrate. Materials 2020, 13, 730. [Google Scholar] [CrossRef] [Green Version]
- Basara, G.; Saeidi-Javash, M.; Ren, X.; Bahcecioglu, G.; Wyatt, B.C.; Anasori, B.; Zhang, Y.; Zorlutuna, P. Electrically conductive 3D printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater. 2020, 19. [Google Scholar] [CrossRef]
- Cantù, E.; Tonello, S.; Abate, G.; Uberti, D.; Sardini, E.; Serpelloni, M. Aerosol jet printed 3D electrochemical sensors for protein detection. Sensors 2018, 18, 3719. [Google Scholar] [CrossRef] [Green Version]
- Wang, X. Advanced polymers for three-dimensional (3D) organ bioprinting. Micromachines 2019, 10, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The application of hydrogels based on natural polymers for tissue engineering. Curr. Med. Chem. 2020, 27, 2658–2680. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural polymer-based hydrogels with enhanced mechanical performances: Preparation, structure, and property. Adv. Healthc. Mater. 2019, 17, e1900670. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C. 3D bioprinting of adipose-derived stem cells for organ manufacturing. In Enabling Cutting Edge Technology for Regenerative Medicine; Khang, G., Ed.; Springer, SBM Singapore Pte Ltd.: Singapore, 2018; pp. 3–14. [Google Scholar]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef]
- Riopel, M.; Trinder, M.; Wang, R. Fibrin, a scaffold material for islet transplantation and pancreatic endocrine tissue engineering. Tissue Eng. Part B Rev. 2015, 21, 34–44. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, P.; Ludeña, D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell Res. 2014, 322, 1–11. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Beneficial effects of hyaluronic acid. Adv. Food Nutr. Res. 2014, 72, 137–176. [Google Scholar] [CrossRef]
- Cortés, H.; Caballero-Florán, I.H.; Mendoza-Muñoz, N.; Córdova-Villanueva, E.N.; Escutia-Guadarrama, L.; Figueroa-González, G.; Reyes-Hernández, O.D.; Carmen, M.G.-D.; Varela-Cardoso, M.; Magaña, J.J.; et al. Hyaluronic acid in wound dressings. Cell. Mol. Biol. 2020, 66, 191. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014, 802, 5–29. [Google Scholar] [CrossRef]
- Ferreira-Duarte, A.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Ribeiro, J.C.V.; Vieira, R.S.; Melo, I.M.; Araújo, V.M.A.; Lima, V. Versatility of chitosan-based biomaterials and their use as scaffolds for tissue regeneration. Sci. World J. 2017, 2017, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maaloum, M.; Pernodet, N.; Tinland, B. Agarose gel structure using atomic force microscopy: Gel concentration and ionic strength effects. Electrophoresis 1998, 19, 1606–1610. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular interactions in gelatin/chitosan composite films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater. 2018, 69, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Yan, Y.; Zheng, W.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Rapid prototyping three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. J. Bioact. Compat. Polym. 2007, 22, 363–377. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Y.; Wang, X.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Three-dimensional gelatin and gelatin/hyaluronan hydrogel structures for traumatic brain injury. J. Bioact. Compat. Polym. 2007, 22, 19–29. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. A Polyurethane-gelatin hybrid construct for manufacturing implantable bioartificial livers. J. Bioact. Compat. Polym. 2008, 23, 409–422. [Google Scholar] [CrossRef]
- He, K.; Wang, X. Rapid prototyping of tubular polyurethane and cell/hydrogel constructs. J. Bioact. Compat. Polym. 2011, 26, 363–374. [Google Scholar] [CrossRef]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Hakam, M.S.; Imani, R.; Abolfathi, N.; Fakhrzadeh, H.; Sharifi, A.M. Evaluation of fibrin-gelatin hydrogel as biopaper for application in skin bioprinting: An in-vitro study. Bio-Med. Mater. Eng. 2017, 27, 669–682. [Google Scholar] [CrossRef]
- Zheng, F.; Derby, B.; Wong, J. Fabrication of microvascular constructs using high resolution electrohydrodynamic inkjet printing. Biofabrication 2020. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yi, H.-G.; Kim, S.-W.; Cho, D.-W. 3D cell printed tissue analogues: A new platform for theranostics. Theranostics 2017, 7, 3118–3137. [Google Scholar] [CrossRef] [Green Version]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2012, 101, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J. Drug Deliv. Sci. Technol. 2019, 55, 101379. [Google Scholar] [CrossRef]
- Elkaseer, A.; Schneider, S.; Scholz, S.G. Experiment-based process modeling and optimization for high-quality and resource-efficient FFF 3D printing. Appl. Sci. 2020, 10, 2899. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yu, Y.; Ozbolat, I.T. Direct bioprinting of vessel-like tubular microfluidic channels. J. Nanotechnol. Eng. Med. 2013, 4, 020902–0210017. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez-Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Cleetus, C.M.; Primo, F.A.; Fregoso, G.; Raveendran, N.L.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate hydrogels with embedded ZnO nanoparticles for wound healing therapy. Int. J. Nanomed. 2020, 15, 5097–5111. [Google Scholar] [CrossRef]
- Aranci, K.; Uzun, M.; Su, S.; Cesur, S.; Ulag, S.; Amin, A.; Guncu, M.; Aksu, B.; Kolayli, S.; Ustundag, C.; et al. 3D propolis-sodium alginate scaffolds: Influence on structural parameters, release mechanisms, cell cytotoxicity and antibacterial activity. Molecules 2020, 25, 5082. [Google Scholar] [CrossRef]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Hybrid hydrogels based on keratin and alginate for tissue engineering. J. Mater. Chem. B 2014, 2, 5441–5451. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping of polyurethane for the creation of vascular systems. J. Bioact. Compat. Polym. 2008, 23, 103–114. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Nakamura, M.; Henmi, C.; Yamaguchi, K.; Mochizuki, S.; Nakagawa, H.; Takiura, K. Development of a three-dimensional bioprinter: Construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng. 2009, 131, 035001. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, M.; Huang, Y.; Ogale, A.; Fu, J.; Markwald, R.R. Study of droplet formation process during drop-on-demand inkjetting of living cell-laden bioink. Langmuir 2014, 30, 9130–9138. [Google Scholar] [CrossRef]
- Thein-Han, W.; Xu, H.H. Prevascularization of a Gas-Foaming Macroporous Calcium Phosphate Cement Scaffold Via Coculture of Human Umbilical Vein Endothelial Cells and Osteoblasts. Tissue Eng. Part A 2013, 19, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B.; Lopez, J.; Faucon, M.; Pippenger, B.; Bareille, R.; Rémy, M.; Bellance, S.; et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010, 6, 2494–2500. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Q.; Qu, X.; Liu, J.; Chen, S. 3D printing of biomimetic microstructures for cancer cell migration. Biomed. Microdevices 2014, 16, 127–132. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Xiong, Z.; Liu, H.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Direct construction of a three-dimensional structure with cells and hydrogel. J. Bioact. Compat. Polym. 2005, 20, 259–269. [Google Scholar] [CrossRef]
- Zhao, X.; Du, S.; Chai, L.; Xu, Y.; Liu, L.; Zhou, X.; Wang, J.; Zhang, W.; Liu, C.-H.; Wang, X. Anti-cancer drug screening based on an adipose-derived stem cell/hepatocyte 3D printing technique. J. Stem Cell Res. Ther. 2015, 5, 273. [Google Scholar]
- Ducret, M.; Montembault, A.; Josse, J.; Pasdeloup, M.; Celle, A.; Benchrih, R.; Mallein-Gerin, F.; Alliot-Licht, B.; David, L.; Farges, J.-C. Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. Dent. Mater. 2019, 35, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. 3D printing of tissue/organ analogues for regenerative medicine. In Handbook of Intelligent Scaffolds for Regenerative Medicine, 2nd ed.; Pan Stanford Publishing: Palo Alto, CA, USA, 2016; pp. 557–570. [Google Scholar]
- Wang, X.; Yan, Y.; Lin, F.; Xiong, Z.; Wu, R.; Zhang, R.; Lu, Q. Preparation and characterization of a collagen/chitosan/heparin matrix for an implantable bioartificial liver. J. Biomater. Sci. Polym. Ed. 2005, 16, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Kohane, D.S.; Langer, R. Polymeric biomaterials in tissue engineering. Pediatr. Res. 2008, 63, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivashankari, P.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, J.; Kashii, M.; Hirao, M.; Oka, K.; Sugamoto, K.; Yoshikawa, H.; Akashi, M. Quick-forming hydroxyapatite/agarose gel composites induce bone regeneration. J. Biomed. Mater. Res. Part A 2007, 83, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Tseng, C.-S.; Dai, L.-G.; Hsu, S.-H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016, 83, 156–168. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.; Elisseeff, J.H.; Dorafshar, A.; Grayson, W.L. Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mott, E.J.; Busso, M.; Luo, X.; Dolder, C.; Wang, M.O.; Fisher, J.P.; Dean, D. Digital micromirror device (DMD)-based 3D printing of poly(propylene fumarate) scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Rizvi, G.M.; Bellehumeur, C.T.; Gu, P. Effect of processing conditions on the bonding quality of FDM polymer filaments. Rapid Prototyp. J. 2008, 15, 72–80. [Google Scholar] [CrossRef]
- Kruth, J.; Mercelis, P.; Van Vaerenbergh, J.; van Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2013, 11, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Ifkovits, J.L.; Burdick, J.A. Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007, 13, 2369–2385. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Aw, J.E.; Zhang, X.; Centellas, P.J.; Dean, L.M.; Lloyd, E.M.; Robertson, I.D.; Liu, Y.; Yourdkhani, M.; Moore, J.S.; et al. Rapid synchronized fabrication of vascularized thermosets and composites. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Ortiz-Hernandez, M.; Engel, E.; Planell, J.A.; Navarro, M. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 55–62. [Google Scholar] [CrossRef]
- Wang, S.-F.; Wu, Y.-C.; Cheng, Y.-C.; Hu, W.-W. The development of polylactic acid/multi-wall carbon nanotubes/polyethylene glycol scaffolds for bone tissue regeneration application. Polymers 2021, 13, 1740. [Google Scholar] [CrossRef]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 2018, 7, e1700939. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.H.; Armstrong, P.A.; Lee, L.J.; Duan, B.; Kang, K.H.; Butcher, J.T. Optimizing photo-encapsulation viability of heart valve cell types in 3d printable composite hydrogels. Ann. Biomed. Eng. 2017, 45, 360–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-contacting biomaterials: In vitro evaluation of the hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Narmon, A.S.; Dewaele, A.; Bruyninckx, K.; Sels, B.F.; Van Puyvelde, P.; Dusselier, M. Boosting PLA melt strength by controlling the chirality of co-monomer incorporation. Chem. Sci. 2021, 12, 5672–5681. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Sarkar, D.; Yang, J.-C.; Gupta, A.S.; Lopina, S.T. Synthesis and characterization of L-tyrosine based polyurethanes for biomaterial applications. J. Biomed. Mater. Res. Part A 2009, 90, 263–271. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 2008, 29, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Kweon, H.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.; Lee, H.S.; Oh, J.S.; Akaike, T.; Cho, C.S. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Liang, Q.; Yu, F.; Xu, J.; Zhao, Q.; Sun, B. Synthesis and characterization of novel amphiphilic copolymer stearic acid-coupled F127 nanoparticles for nano-technology based drug delivery system. Colloids Surf. B Biointerfaces 2011, 88, 741–748. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109960. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X. Vascularization and adipogenesis of a spindle hierarchical adipose-derived stem cell/collagen/alginate-PLGA construct for breast manufacturing. IJITEE 2015, 4, 1–8. [Google Scholar]
- Duan, P.; Pan, Z.; Cao, L.; Gao, J.; Yao, H.; Liu, X.; Guo, R.; Liang, X.; Dong, J.; Ding, J. Restoration of osteochondral defects by implanting bilayered poly(lactide-co-glycolide) porous scaffolds in rabbit joints for 12 and 24 weeks. J. Orthop. Translat. 2019, 19, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, K.; Wang, X. Rapid prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater. Sci. Eng. C 2013, 33, 3220–3229. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H. Incorporation of DMSO and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryo-preservation. Cryobiology 2010, 61, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, T.; Yan, Y.; Zhang, R. Peroneal nerve regeneration along a new polyurethane-collagen guide conduit. J. Bioact. Compat. Polym. 2009, 24, 109–127. [Google Scholar] [CrossRef]
- Cui, T.; Yan, Y.; Zhang, R.; Liu, L.; Xu, W.; Wang, X. Rapid prototyping of a double-layer polyurethane–collagen conduit for peripheral nerve regeneration. Tissue Eng. Part C Methods 2009, 15, 1–9. [Google Scholar] [CrossRef]
- Cui, T.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping a new polyurethane-collagen conduit and its Schwann cell compatibilities. J. Bioact. Compat. Polym. 2009, 24, 5–17. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, D.; Zheng, Y.; Zhao, L.; Xu, T.; Guo, Z.; Irfan-Hussain, M.; Zeng, J.; Lou, L.; Sun, Y.; et al. A novel waterborne pol-yurethane with biodegradability and high flexibility for 3D printing. Biofabrication 2020, 12, 035015. [Google Scholar] [CrossRef]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.J.; Favia, P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.-X.; Hou, Y.; Bartolo, P.; Chiang, W.-H. Investigations of graphene and nitrogen-doped graphene enhanced polycaprolactone 3D scaffolds for bone tissue engineering. Nanomaterials 2021, 11, 929. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of thermoresponsive hydrogels for next generation tissue engineering: A review. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2013, 25, 3124–3130. [Google Scholar] [CrossRef]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef]
- Dung, T.H.; Huong, L.T.; Yoo, H. Morphological feature of pluronic F127 and its application in burn treatment. J. Nanosci. Nanotechnol. 2018, 18, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Liu, Y.; Hu, Y.; Ding, Y.; Lin, S.; Cao, W.; Wang, Q.; Wu, J.; Muhammad, F.; Zhao, X.; et al. Monitoring of Heparin Activity in Live Rats Using Metal–Organic Framework Nanosheets as Peroxidase Mimics. Anal. Chem. 2017, 89, 11552–11559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly stable Zr (IV)-based metal-organic frameworks for the detection and removal of antibiotics and organic explosives in water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Du, P.; Zhang, L.; Lei, J. Metal-organic framework (MOF) hybrid as a tandem catalyst for enhanced therapy against hypoxic tumor cells. Angew. Chem. Int. Ed. 2019, 58, 7808–7812. [Google Scholar] [CrossRef]
- Ishiwata, T.; Furukawa, Y.; Sugikawa, K.; Kokado, K.; Sada, K. Transformation of metal-organic framework to polymer gel by cross-linking the organic ligands preorganized in metal-organic framework. J. Am. Chem. Soc. 2013, 135, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Tsotsalas, M.; Liu, J.; Tettmann, B.; Grosjean, S.; Shahnas, A.; Wang, Z.; Azucena, C.; Addicoat, M.; Heine, T.; Lahann, J.; et al. Fabrication of highly uniform gel coatings by the conversion of surface-anchored metal-organic frameworks. J. Am. Chem. Soc. 2014, 136, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Ianchis, R.; Ninciuleanu, C.M.; Gifu, I.C.; Alexandrescu, E.; Nistor, C.L.; Nitu, S.G.; Petcu, C. Hydrogel-clay nanocomposites as carriers for controlled release. Curr. Med. Chem. 2020, 27, 919–954. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, G.; Kang, M.; Lee, J.; Seo, J.; Do, J.; Hong, K.; Cha, J.; Shin, S.; Bae, H. Marine biomaterial-based bioinks for generating 3D printed tissue constructs. Mar. Drugs. 2018, 16, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaharwar, A.K.; Cross, L.M.; Peak, C.W.; Gold, K.; Carrow, J.K.; Brokesh, A.; Singh, K.A. 2D nanoclay for biomedical applications: Regenerative medicine, therapeutic delivery, and additive manufacturing. Adv. Mater. 2019, 31, 1900332. [Google Scholar] [CrossRef]
- Paul, A.; Manoharan, V.; Krafft, D.; Assmann, A.; Uquillas, J.A.; Shin, S.R.; Hasan, A.; Hussain, M.A.; Memic, A.; Gaharwar, A.K.; et al. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J. Mater. Chem. B 2016, 4, 3544–3554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [Green Version]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef]
- Gaharwar, A.; Dammu, S.A.; Canter, J.M.; Wu, C.-J.; Schmidt, G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Lueders, C.; Jastram, B.; Hetzer, R.; Schwandt, H. Rapid manufacturing techniques for the tissue engineering of human heart valves. Eur. J. Cardio Thora. Surg. 2014, 46, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Lee, S.J.; Cheng, H.-J.; Yoo, J.J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; AnilKumar, S.; Roman, B.; Tasnim, N.; Joddar, B. 3D Bioprinting of cardiac tissue and cardiac stem cell therapy. Transl. Res. 2019, 211, 64–83. [Google Scholar] [CrossRef]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; Zhuang, D.; Jiang, H.; Chen, X.; He, Y.; et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2020, 6, 1388–1401. [Google Scholar] [CrossRef]
- Betts, J.G. Anatomy & Physiology; Rice University: Houston, MA, USA, 2013. [Google Scholar]
- Michalopoulos, G.K. Liver regeneration after partial hepatectomy: Critical analysis of mechanistic dilemmas. Am. J. Pathol. 2010, 176, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.S.; Geerts, S.; Jindal, R.; Yarmush, M.L. Isolation and co-culture of rat parenchymal and non-parenchymal liver cells to evaluate cellular interactions and response. Sci. Rep. 2016, 6, 25329. [Google Scholar] [CrossRef] [PubMed]
- Senoo, H. Structure and function of hepatic stellate cells. Med. Electron. Microsc. 2004, 37, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, H.J.; Choi, D. Cell sources, liver support systems and liver tissue engineering: Alternatives to liver transplantation. Int. J. Stem Cells 2015, 8, 36–47. [Google Scholar] [CrossRef]
- Ikegami, T.; Maehara, Y. Transplantation: 3D printing of the liver in living donor liver transplantation. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 697–698. [Google Scholar] [CrossRef]
- Hong, N.; Yang, G.-H.; Lee, J.; Kim, G. 3D bioprinting and its in vivo applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [Green Version]
- Shoichet, M.S.; Tate, C.C.; Douglas-Baumann, M.; Laplaca, M.C. Strategies for regeneration and repair in the injured central nervous system. In Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment; Reichert, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S.-H. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Guan, S.; Li, W.; Ge, D.; Xu, J.; Sun, C.; Liu, T.; Ma, X. 3D culture of neural stem cells within conductive PEDOT layer-assembled chitosan/gelatin scaffolds for neural tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 890–901. [Google Scholar] [CrossRef]
- Lee, W.; Pinckney, J.; Lee, V.; Lee, J.-H.; Fischer, K.; Polio, S.; Park, J.-K.; Yoo, S.-S. Three-dimensional bioprinting of rat embryonic neural cells. NeuroReport 2009, 20, 798–803. [Google Scholar] [CrossRef]
- Tasoglu, S.; Demirci, U. Bioprinting for stem cell research. Trends Biotechnol. 2013, 31, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.-S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010, 223, 645–652. [Google Scholar] [CrossRef]
- Ishack, S.; Lipner, S.R. A review of 3-dimensional skin bioprinting techniques: Applications, approaches, and trends. Dermatol. Surg. 2020, 46, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Fauzi, M.B. Current insight of printability quality improvement strategies in natural-based bioinks for skin regeneration and wound healing. Polymers 2021, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Michael, S.; Sorg, H.; Peck, C.-T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the Dorsal Skin Fold Chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef] [Green Version]
- Perez-Valle, A.; Del Amo, C.; Andia, I. Overview of current advances in extrusion bioprinting for skin applications. Int. J. Mol. Sci. 2020, 21, 6679. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.E.; Gou, M.; Xu, Y.; et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, X. Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Biotechnol. Bioeng. 2015, 112, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Spatial effects of stem cell engagement in 3D printing constructs. J. Stem. Cells Res. Rev. Rep. 2014, 1, 5–9. [Google Scholar]
- Wang, X. Overview on biocompatibilities of implantable biomaterials. In Advances in Biomaterials Science and Biomedical Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 111–155. [Google Scholar]
- Xu, Y.; Wang, X. Liver manufacturing approaches: The thresholds of cell manipulation with bio-friendly materials for multifunctional organ regeneration, In Organ Manufacturing; Wang, X., Ed.; Nova Science Publishers Inc: Hauppauge, NY, USA, 2015; pp. 201–225. [Google Scholar]
- Li, S.; Yan, Y.; Xiong, Z.; Weng, C.; Zhang, R.; Wang, X. Gradient hydrogel construct based on an improved cell assembling system. J. Bioact. Compat. Polym. 2009, 24, 84–99. [Google Scholar] [CrossRef]
- Xu, M.; Yan, Y.; Liu, H.; Yao, Y.; Wang, X. Control adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3-D structure established by cell-assembly technique. J. Bioact. Compat. Polym. 2009, 24, 31–47. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Yan, Y.; Yao, R.; Ge, Y. A cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials 2010, 31, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Bioartificial organ manufacturing technologies. Cell Transplant. 2018, 27, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J. Nanobiotechnol. 2021, 19, 194. [Google Scholar] [CrossRef]







| Printing Technology | Principle | Material | Advantages | Defects | Ref. |
|---|---|---|---|---|---|
| Inkjet 3D printing | Unsing acoustic, thermal or piezoelectric nozzle to jet biomaterials in drops | Polymer solutions or cell suspensions | Efficient control of “bioinks”, low cost and high throughput | Biomaterials need to be in a liquid state, and the viscosity needs to be precisely controlled | [17,18,19] |
| Fused deposition modeling | Thermoplastic material melted through one or more heated extrusion heads with small holes in a specific laying method | Thermoplastic polymers with a certain viscosity after heating, such as PCL, PLA, ABS, etc. | Low cost, a wide range of nonbiodegradable synthetic polymers with excellent mechanical properties can be printed | High printing temperature in which cells, growth factors and other bioactive agents cannot be incorporated | [20,21,22,23,24,25,26] |
| Extrusion based 3D printing | Polymeric solutions or hydrogels are drawn, extruded and deposited to form solid structures | A variety of natural polymers, such as alginate, collagen, and chitosan can be selected | High accuracy and speed, cells and other bioactive agents can be incorporated | Some of the extrusion setups may cause damage to cells | [27,28,29,30,31,32,33,34,35] |
| Stereolithography | Laser or projected light converts the liquid photosensitive material into a solid platform | Only photosensitive polymers can be used | High accuray | Most of the photosensitive resins are toxic to cells and the light in the printing process affect the survival rate of the cells | [36,37,38,39,40,41] |
| Aerosol jet printing | Ultrasonic or pneumatic atomization is formed by squeezing the “bioinks” around the airflow | Any substance that can be suspended in an aerosol | High resolution and flexibility, it can be printed on various substrates such as metals, semiconductors, and polymers | Denature DNA | [42,43,44,45,46] |
| Polymer | Chemistry | Characteristic | Disadvantage | Ref. |
|---|---|---|---|---|
| Gelatin | Partial degraded product of collagen | Excellent biocompatibility | Unstable solution at room temperature, fast degradation rate, and poor mechanical property | [51,52] |
| Alginate | A linear anionic polysaccharide copolymer | Rich source, low price, good hydrophilic property, easy to form penetrating networks | Few cells attachment sites and fast degradation rate | [53,54] |
| Fibrin | Polymerization product of fibrinogen | Excellent biocompatibility and biodegradability | Poor long-term stability and mechanical strength | [55,56,57] |
| Hyaluronic acid | A linear high molar mass natural polysaccharide | Non-allergic and non-inflammatory | Fast degradation rate and poor mechanical property | [58,59] |
| Collagen | A kind of protein composed of three intertwined polypeptide chains, which are connected to each other by hydrogen bonds and covalent bonds | In vivo immunogenicity, good cell compatibility | Poor mechanical properties | [60,61] |
| Silk fibroin | A natural protein from insects | Good biocompatibility and mechanical properties | Slow degradation rate | [62] |
| Chitosan | Obtained through deacetylation of chitin | Good biocompatibility, biodegradability, cell adhesion capability, low cost | Poor mechanical strength, unstable gel state | [63,64] |
| Agarose | Linear polysaccharide | Slow degradation rate, low cost, good mechanical properties after gelling | Poor cell compatibility | [65] |
| 3D Bioprinting Technique | “Bioink” Formulation | Crosslinking Method | Application | Ref. |
|---|---|---|---|---|
| One nozzle extrusion-based 3D bioprinting | Hepatocytes in gelatin/chitosan hydrogel | 3% sodium tripolyphosphate (TPP) | Hepatic tissue manufacturing | [70] |
| Hepatocytes in gelatin hydrogel | 2.5% glutaraldehyde | Hepatic tissue manufacturing | [71] | |
| Hepatocytes in gelatin/fibrinogen hydrogel | Thrombin induced polymerization | Hepatic tissue manufacturing | [72] | |
| Gelatin/hyluronan | 2% glutaraldehyde | Brain tissue repair | [73] | |
| Two-nozzle low-temperature extrusion-based 3D printing | Polyurethane (PU)-gelatin/5% or 10% lysine hydrogel | 0.25% glutaraldehyde | Liver manufacturing | [74] |
| PU-adipose-derived stem cell (ADSC)/gelatin/alginate/fibrinogen/glycerol or dimethyl sulfoxide (DMSO) hydrogel | Double crosslinking with CaCl2 and thrombin solutions | Bioartificial liver manufacturing | [75] | |
| One-syringe extrusion-based 3D printing | Nanosilicate/GelMA | UV light (320–500 nm) for 60 s at an intensity of 6.9 mW/cm2 | Electrical conductive agent for bone tissue engineering | [76] |
| EnvisionTEC 3D-Bioplotter® | Polyethylene glycol (PEG)/gelatin-PEG/fibrinogen | Gelatin scaffolds were cross-linked with 15 mM EDC and 6 mM NHS, fibrinogen-containing samples were treated post-printing with 10 U/mL thrombin in 40 mM CaCl2 for ~30 min | Grid structures for cell seeding | [77] |
| Dual-syringe Fab@Home printing device | Gelatin ethanolamide methacrylate (GE-MA)-methacrylated hyaluronic acid (HA-MA) (GE-MA-HA-MA)/HepG2 C3A, NIH 3T3, or Int-407 cell | Ultraviolet (UV) light (365 nm, 180 mW/cm2) photocrosslinking | Tubular hydrogel structures for cell attachment | [78] |
| EHD inkjet printing system | GelMA solution | Illuminating with a UV light source | Microvascular constructs | [79] |
| 3D Bioprinting Technique | “Bioink” Formulation | Crosslinking Method | Bioprinter | Ref. |
|---|---|---|---|---|
| One/two nozzle extrusion-based 3D bioprinting | GelMA/alginate/PEGTA | Photo-crosslinking/ CaCl2 solution | Novogen MMX Bioprinter™ | [87] |
| Alginate/chitosan hydrogel | CaCl2 solution | EFD® Nordson printer | [88] | |
| Nanocellulose-alginate | CaCl2 solution | 3D discovery printer | [89] | |
| Zinc oxide (ZnO) nanoparticles (NPs)/alginate | CaCl2 solution | BioBot 1 | [90] | |
| Propolis/sodium alginate | CaCl2 solution | Ultimaker2+ | [91] | |
| Sodium alginate/keratin | CaCl2 solution | Ultimus V | [92] | |
| Inkjet-based 3D bioprinting | Collagen/sodium alginate | CaCl2 solution | HP DeskJet 550C | [93] |
| Alginate solution | CaCl2 solution as substrate | SEA-Jet™ | [94] | |
| Sodium alginate solution | CaCl2 solution | A platform-assisted 3D inkjet bioprinting system | [95] | |
| Alginate solution | CaCl2 solution after printing | MicroFab MJ-ABL piezoelectric inkjet printhead printer | [96] | |
| One/two-syringe extrusion-based 3D printing | Gelatin/glucose-alginate hydrogel | CaCl2 solution after printing | Fab@Home Model1-3 | [97] |
| Laser-based bioprinting | Sodium alginate/ Nano-HA | Laser | BioLP workstation | [98] |
| Sodium alginate solution | CaCl2 solution | Matrix-assisted pulsed-laser evaporation direct-write | [99] |
| Polymer | Chemistry | Characteristic | Ref. |
|---|---|---|---|
| PLA | A linear thermoplastic aliphatic polyester, mainly produced from starch raw materials through saccharification, fermentation and other chemical reactions | Good biocompatibility and biodegradability, can be completely degraded under certain conditions | [122,123,124,125] |
| PLGA | A synthetic copolymer of lactic acid (LA) and glycolic acid (GA), synthesized by the ring-opening copolymerization of cyclic dimer (1,4-dioxane-2,5-dione), glycolic acid and lactic acid | Good biocompatibility and controllable biodegradation rate | [126,127] |
| PU | A set of linear units (–NH–(C=O)–O–) connected by oligodiol (i.e., soft segment) and organic (i.e., hard segment) units through carbamate (i.e., carbamate) | Controllable degradation rate and mechanical properties, can be modified to have heat-sensitive properties | [128,129] |
| PCL | Catalysis with metal anion complex catalyst ε- Formation of caprolactone monomer by ring opening polymerization | Good biocompatibility and biodegradability | [130,131] |
| Pluronic Acid | Compound with a basic structure of poly (ethylene oxide) (PEO)-poly (propylene oxide) (PPO)-PEO | Easy to prepare, good cell affinity, and heat-sensitive | [132,133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3D Bioprinting in Organ Manufacturing. Polymers 2021, 13, 3178. https://doi.org/10.3390/polym13183178
Song D, Xu Y, Liu S, Wen L, Wang X. Progress of 3D Bioprinting in Organ Manufacturing. Polymers. 2021; 13(18):3178. https://doi.org/10.3390/polym13183178
Chicago/Turabian StyleSong, Dabin, Yukun Xu, Siyu Liu, Liang Wen, and Xiaohong Wang. 2021. "Progress of 3D Bioprinting in Organ Manufacturing" Polymers 13, no. 18: 3178. https://doi.org/10.3390/polym13183178
APA StyleSong, D., Xu, Y., Liu, S., Wen, L., & Wang, X. (2021). Progress of 3D Bioprinting in Organ Manufacturing. Polymers, 13(18), 3178. https://doi.org/10.3390/polym13183178






