Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications
Abstract
:1. Introduction
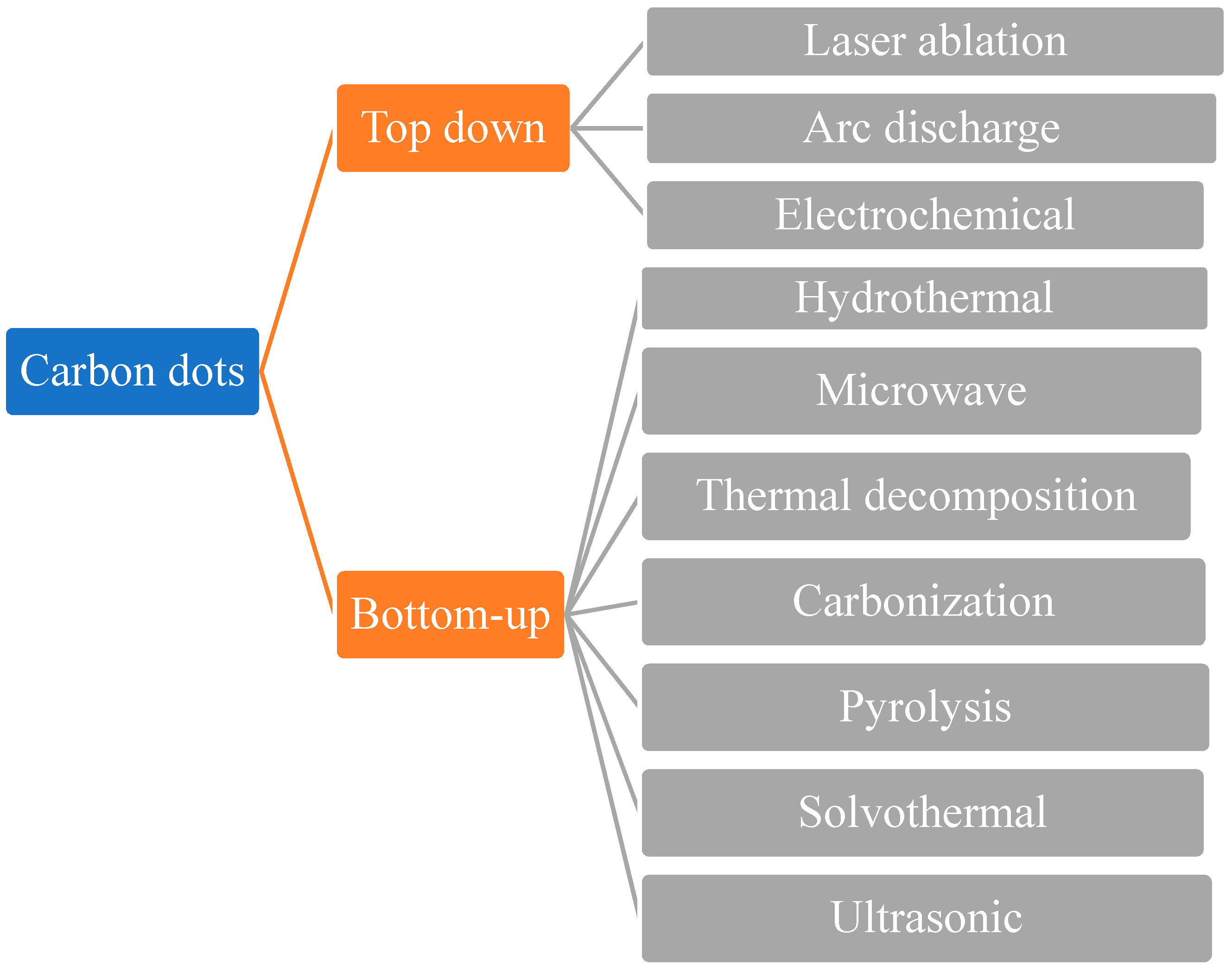
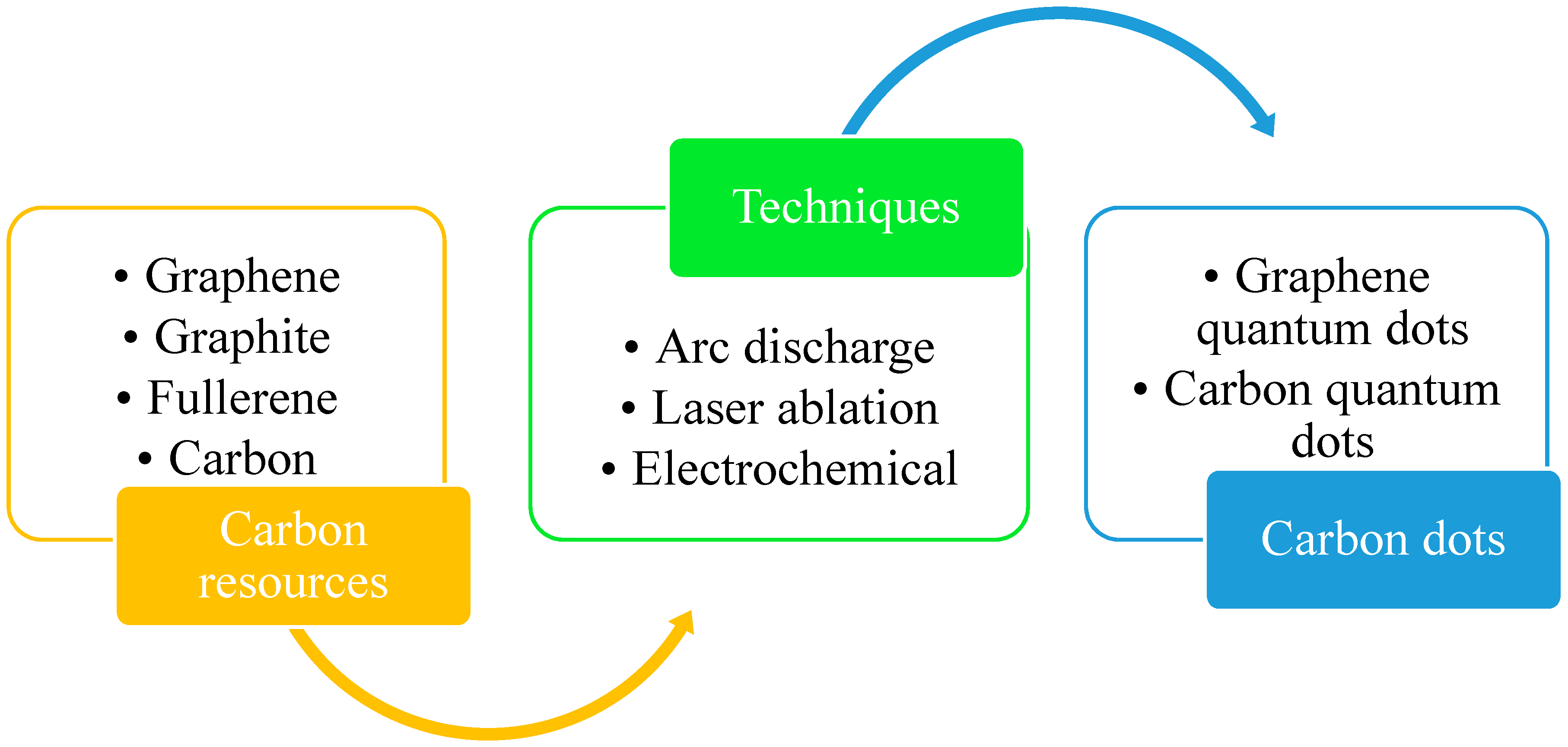
2. Synthesis of Carbon Dots
2.1. Laser Ablation (LA)
2.2. Arc Discharge (AcD)
2.3. Electrochemical Approach
2.4. Microwave-Assisted Synthesis
2.5. Thermal Decomposition
2.6. Carbonization Synthesis
2.7. Pyrolysis Synthesis Method
2.8. Solvothermal Method
2.9. Ultrasonic Treatment
3. Properties of CQDs
3.1. Structural Properties of C-Dots
3.2. Absorbance of C-Dots
4. Mechanisms Involved in the Photoluminescence Phenomenon
4.1. The Core Emission
4.2. The Surface States
4.3. The Molecular State
4.4. Factors Affecting the Properties of C-Dots
5. Emerging Applications of Carbon Dots
5.1. Bioimaging
5.2. Sensing
5.3. Drug Delivery
5.4. Catalysis
5.5. Optronics
5.6. Finger Print Recovery
5.7. Antibacterial Activity of CDs
5.8. Applications of CDs in Chemical Warfare
6. Surface Functionalization of CDs
7. Hetero Atomic Doping
- (i)
- AgNPs can be readily absorbed into E. coli’s surface and damage outer membrane permeability and fluidity [189].
- (ii)
- Smaller-sized AgNPs (7.3 ± 1.0 and 6.1 ± 0.8 nm) that permeate the E. coli membrane to associate with the respiratory chain and DNA.
- (iii)
- Ag+ release can trigger the death of E. coli.
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AP | 2-aminopyridine |
| AA | Ascorbic acid |
| AcD | Arc discharge |
| AEAPMS | N-(β-aminoethyl)-γ-aminopropyl methyl dimethoxysilane |
| AFM | Atomic force microscopy |
| Ag | Silver element |
| AgNP | Silver nanoparticles |
| AMP | Ampicillin |
| Au | Gold |
| BBr3 | Boron tribromide |
| Ca | Calcium |
| Ca | Citric acid monohydrate |
| Cd | Cadmium |
| CD or C-dots | Carbon dots |
| CD-CH | Carbon dots-chitosan |
| Cm | Centimeters |
| CMCDs | Cow-milk-derived carbon dots |
| CNPs | Carbon nanoparticles |
| CNTs | Carbon nanotubes |
| CQD | Crystalline carbon core |
| Cu | Copper |
| Cys | Cysteine |
| DD | Drug discovery |
| DEG | Diethylene glycol |
| DMF | Dimethylformamide |
| DNA | Deoxyribonucleic acid |
| ECE | Electrochemical exfoliation |
| FCD | Fluorescent carbon dots |
| Fe3O4 | Iron oxide |
| FG | Functional groups |
| HAuCl4 | Hydrogen tetrachloroaurate(III) hydrate |
| Hg | Mercury |
| HPPT | 4-hydroxy-1H-pyrrolo[3,4-c]pyridine-1,3,6(2H,5H)-trione |
| HT | Hydrothermal |
| IPCA | Imidazo[1,2-a]pyridine-7-carboxylic acid |
| LA | Laser ablation |
| LED | Light-emitting diode |
| MIC | Minimum inhibitory concentration |
| MnO2 | Manganese dioxide |
| MWCNTs | Multi-walled carbon nanotubes |
| NaNH2 | Sodium amide |
| NaOH | Sodium hydroxide |
| Ni | Nickel |
| Nm | Nanometers |
| NPs | Nanoparticles |
| NR-CDs | Nitrogen-rich blue, fluorescent carbon dots |
| OPD | O-phenylenediamine |
| PAHs | Polycyclic aromatic hydrocarbons |
| PD | Polymer dots |
| PEG | Polyethylene glycol |
| pH | Potential of hydrogen |
| PL | Photoluminescence |
| PNSCDs | Pseudomonas aeruginosa |
| PPEIE | Poly (propionyl ethylene-imine-ethyleneimine) |
| PPEI-EI | Poly (propionylethyleneimine-co-ethyleneimine |
| Pt | Platinum |
| PVA | Polyvinyl alcohol |
| QCE | Quantum confinement effect |
| QD | Quantum dots |
| QY | Quantum yield |
| RB | Rose Bengal (RB) |
| ROS | Reactive oxygen species |
| Rpm | Rotations per minute |
| SC | Sodium citrate |
| Se | Selenium |
| Si | Silicon |
| SiCl4 | Silicon tetrachloride |
| SWCNTs | Single-wall carbon nanotubes |
| Tarp | Tryptophan |
| TEM | Transmission electron microscopy |
| TETA | Triethylenetetramine |
| TiO2 | Titanium dioxide |
| TTDDA | 4, 7, 10-trioxa-1,13-tridecanediamine |
| UA | Uric acid |
| UV-Vis | Ultraviolet visible light |
| XRD | X-ray diffraction |
| Zn | Zinc |
References
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Choudhary, N.; Khan, S.H.; Malik, P.; Inwati, G.K.; Suriyaprabha, R.; Ravi, R.K. Synthesis and Characterisation of Nano-Biosorbents and Their Applications for Waste Water Treatment. In Handbook of Research on Emerging Developments and Environmental Impacts of Ecological Chemistry; Gheorghe, D., Ashok, V., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 252–290. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, V.K.; Choudhary, N.; Khan, S.H.; Malik, P.; Inwati, G.K.; Suriyaprabha, R.; Ravi, R.K. Recovery of Natural Nanostructured Minerals: Ferrospheres, Plerospheres, Cenospheres, and Carbonaceous Particles from Fly Ash. In Handbook of Research on Emerging Developments and Environmental Impacts of Ecological Chemistry; Gheorghe, D., Ashok, V., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 450–470. [Google Scholar]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Hu, X.-L.; Shang, Y.; Yan, K.-C.; Sedgwick, A.C.; Gan, H.-Q.; Chen, G.-R.; He, X.-P.; James, T.D.; Chen, D. Low-dimensional nanomaterials for antibacterial applications. J. Mater. Chem. B 2021, 9, 3640–3661. [Google Scholar] [CrossRef] [PubMed]
- Gougoulias, C.; Clark, J.; Shaw, L. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.; Tavker, N.; Choudhary, N.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M.; Hamid, A.A. Recent Advances in Methods for the Recovery of Carbon Nanominerals and Polyaromatic Hydrocarbons from Coal Fly Ash and their Emerging Applications. Crystals 2021, 11, 88. [Google Scholar] [CrossRef]
- Júnior, A.H.D.S.; Macuvele, D.L.P.; Riella, H.G.; Soares, C.; Padoin, N. Are carbon dots effective for ion sensing and antiviral applications? A state-of-the-art description from synthesis methods to cost evaluation. J. Mater. Res. Technol. 2021, 12, 688–716. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Kou, X.; Jiang, S.; Park, S.-J.; Meng, L.-Y. A review: Recent advances in preparations and applications of heteroatom-doped carbon quantum dots. Dalton Trans. 2020, 49, 6915–6938. [Google Scholar] [CrossRef] [PubMed]
- Doñate-Buendía, C.; Fernández-Alonso, M.; Lancis, J.; Mínguez-Vega, G. Pulsed laser ablation in liquids for the production of gold nanoparticles and carbon quantum dots: From plasmonic to fluorescence and cell labelling. J. Phys. Conf. Ser. 2020, 1537, 012013. [Google Scholar] [CrossRef]
- Elugoke, S.; Adekunle, A.; Fayemi, O.; Mamba, B.; Sherif, E.-S.; Ebenso, E. Carbon-Based Quantum Dots for Electrochemical Detection of Monoamine Neurotransmitters—Review. Biosensors 2020, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef]
- Başoğlu, A.; Ocak, Ü.; Gümrükçüoğlu, A. Synthesis of Microwave-Assisted Fluorescence Carbon Quantum Dots Using Roasted–Chickpeas and its Applications for Sensitive and Selective Detection of Fe3+ Ions. J. Fluoresc. 2020, 30, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 9. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon Dots as Potent Antimicrobial Agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Gnanamoorthy, G.; Ahmed, E.M.; Yadav, V.K.; Narayanan, V. New modification of (Platinum aminophosphate) nanoparticles surface: Superior photocatalytic properties and antimicrobial applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100148. [Google Scholar] [CrossRef]
- Dong, Y.; Chang, Y.; Gao, H.; Anchustegui, V.A.L.; Yu, Q.; Wang, H.; Liu, J.-H.; Wang, S. Characteristic synergistic cytotoxic effects toward cells in graphene oxide dressing with cadmium and copper ions. Toxicol. Res. 2019, 8, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Ruzycka-Ayoush, M.; Kowalik, P.; Kowalczyk, A.; Bujak, P.; Nowicka, A.M.; Wojewodzka, M. Quantum dots as targeted doxorubicin drug delivery nanosystems in human lung cancer cells. Cancer Nanotechnol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Feng, L.; Li, B.; Li, Z.; Chen, R.; Qin, C.; Gao, Y.; Xiao, L.; Jia, S. Suppressing the photobleaching and photoluminescence intermittency of single near-infrared CdSeTe/ZnS quantum dots with p-phenylenediamine. Opt. Express 2018, 26, 11889–11902. [Google Scholar] [CrossRef] [PubMed]
- Butera, E.; Zammataro, A.; Pappalardo, A.; Sfrazzetto, G.T. Supramolecular Sensing of Chemical Warfare Agents. ChemPlusChem 2021, 86, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Hazra, N.; Hazra, S.; Banerjee, A. Carbon dot mediated trihybrid formation by reduction of GO and in situ gold nanocluster fabrication: Photo-switching behaviour and degradation of chemical warfare agent stimulants. J. Mater. Chem. C 2020, 8, 15735–15741. [Google Scholar] [CrossRef]
- Song, Y.; Chen, J.; Hu, D.; Liu, F.; Li, P.; Li, H.; Chen, S.; Tan, H.; Wang, L. Ratiometric fluorescent detection of biomakers for biological warfare agents with carbon dots chelated europium-based nanoscale coordination polymers. Sens. Actuators B Chem. 2015, 221, 586–592. [Google Scholar] [CrossRef]
- Tuccitto, N.; Fichera, L.; Ruffino, R.; Cantaro, V.; Sfuncia, G.; Nicotra, G.; Sfrazzetto, G.T.; Li-Destri, G.; Valenti, A.; Licciardello, A.; et al. Carbon Quantum Dots as Fluorescence Nanochemosensors for Selective Detection of Amino Acids. ACS Appl. Nano Mater. 2021, 4, 6250–6256. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, I.; Chaturvedi, P.; Chouksey, A.; Tandon, R.; Chaudhury, P. Study of simultaneous reversible and irreversible adsorption on single-walled carbon nanotube gas sensor. Mater. Chem. Phys. 2016, 177, 276–282. [Google Scholar] [CrossRef]
- Tuccitto, N.; Spitaleri, L.; Destri, G.L.; Pappalardo, A.; Gulino, A.; Sfrazzetto, G.T. Supramolecular Sensing of a Chemical Warfare Agents Simulant by Functionalized Carbon Nanoparticles. Molecules 2020, 25, 5731. [Google Scholar] [CrossRef]
- Tisdale, W.; Zhu, X.-Y. Artificial atoms on semiconductor surfaces. Proc. Natl. Acad. Sci. USA 2011, 108, 965–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.; Cao, X.; Zhu, L.; Zheng, M. Synthesis of Functional Nanomaterials for Electrochemical Energy Storage; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 13–29. [Google Scholar]
- Kingston, C.T.; Simard, B. Fabrication of Carbon Nanotubes. Anal. Lett. 2003, 36, 3119–3145. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Li, C.; Luo, P.; Tao, L.; Wei, Y.; Shi, G. Large scale preparation of graphene quantum dots from graphite with tunable fluorescence properties. Phys. Chem. Chem. Phys. 2013, 15, 9907–9913. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [Green Version]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum Dots in Bioanalysis: A Review of Applications across Various Platforms for Fluorescence Spectroscopy and Imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Ren, Y.; Guo, J.; Liu, Z.; Liu, L.; Yan, F. Imidazolium-type ionic liquid-based carbon quantum dot doped gels for information encryption. Nanoscale 2020, 12, 20965–20972. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, G.; Wang, S.; Mi, C.; Wei, S.; Tian, F.; Li, W.; Cao, H.; Cheng, Y. A review on diamond-like carbon films grown by pulsed laser deposition. Appl. Surf. Sci. 2021, 541, 148573. [Google Scholar] [CrossRef]
- Reyes, D.; Camacho, M.; Camacho, M.; Mayorga, M.; Weathers, D.; Salamo, G.; Wang, Z.; Neogi, A. Laser Ablated Carbon Nanodots for Light Emission. Nanoscale Res. Lett. 2016, 11, 424. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Central Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Calabro, R.; Yang, D.-S.; Kim, D.Y. Liquid-phase laser ablation synthesis of graphene quantum dots from carbon nano-onions: Comparison with chemical oxidation. J. Colloid Interface Sci. 2018, 527, 132–140. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Hoffman, J.; Morgiel, J.; Mościcki, T.; Stobiński, L.; Szymański, Z.; Małolepszy, A. Luminescent Carbon Dots Synthesized by the Laser Ablation of Graphite in Polyethylenimine and Ethylenediamine. Materials 2021, 14, 729. [Google Scholar] [CrossRef]
- Małolepszy, A.; Blonski, S.; Chrzanowska-Giżyńska, J.; Wojasiński, M.; Plocinski, T.; Stobinski, L.; Szymanski, Z. Fluorescent carbon and graphene oxide nanoparticles synthesized by the laser ablation in liquid. Appl. Phys. A 2018, 124, 282. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, H.; Duarte, A.J.; da Silva, J.C.E. Optical fiber sensor for Hg(II) based on carbon dots. Biosens. Bioelectron. 2010, 26, 1302–1306. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.; Zhao, N.; Yan, L.; Zhong, P.; Le, P.H. Double-pulse femtosecond laser ablation for synthesis of ultrasmall carbon nanodots. Mater. Res. Express 2020, 7, 015606. [Google Scholar] [CrossRef]
- Thongpool, V.; Asanithi, P.; Limsuwan, P. Synthesis of Carbon Particles using Laser Ablation in Ethanol. Procedia Eng. 2012, 32, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2015, 183, 519–542. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef] [PubMed]
- Varenikov, A.; Shapiro, E.; Gandelman, M. Decarboxylative Halogenation of Organic Compounds. Chem. Rev. 2021, 121, 412–484. [Google Scholar] [CrossRef]
- Bottini, M.; Tautz, L.; Huynh, H.; Monosov, E.; Bottini, N.; Dawson, M.I.; Bellucci, S.; Mustelin, T. Covalent decoration of multi-walled carbon nanotubes with silica nanoparticles. Chem. Commun. 2005, 2005, 758–760. [Google Scholar] [CrossRef]
- Michelsen, H.A.; Colket, M.B.; Bengtsson, P.-E.; D’Anna, A.; Desgroux, P.; Haynes, B.S.; Miller, J.H.; Nathan, G.J.; Pitsch, H.; Wang, H. A Review of Terminology Used to Describe Soot Formation and Evolution under Combustion and Pyrolytic Conditions. ACS Nano 2020, 14, 12470–12490. [Google Scholar] [CrossRef]
- Su, Y.; Xie, M.; Lu, X.; Wei, H.; Geng, H.; Yang, Z.; Zhang, Y. Facile synthesis and photoelectric properties of carbon dots with upconversion fluorescence using arc-synthesized carbon by-products. RSC Adv. 2013, 4, 4839–4842. [Google Scholar] [CrossRef]
- Biazar, N.; Poursalehi, R.; Delavari, H. Optical and structural properties of carbon dots/TiO2 nanostructures prepared via DC arc discharge in liquid. In Proceedings of the 6th International Biennial Conference on Ultrafine Grained and Nanostructured Materials: (UFGNSM2017), Kish Island, Iran, 12–13 November 2017. [Google Scholar]
- Dey, S.; Govindaraj, A.; Biswas, K.; Rao, C.N.R. Luminescence properties of boron and nitrogen doped graphene quantum dots prepared from arc-discharge-generated doped graphene samples. Chem. Phys. Lett. 2014, 595–596, 203–208. [Google Scholar] [CrossRef]
- Fang, S.; Lin, Y.; Hu, Y.H. Recent Advances in Green, Safe, and Fast Production of Graphene Oxide via Electrochemical Approaches. ACS Sustain. Chem. Eng. 2019, 7, 12671–12681. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Tan, H.; Zhang, T.; Duan, W.; Zhu, Y. Hydrothermal synthesis of N-doped carbon dots from an ethanolamine–ionic liquid gel to construct label-free multifunctional fluorescent probes for Hg2+, Cu2+ and S2O32−. Analyst 2019, 144, 3013–3022. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Zhang, D.; Ding, Y.; Zheng, X.; Xiang, Y.; Hua, J.; Zhang, Q.; Ji, X.; Li, B.; Wei, Y. Applications of hydrothermal synthesis of Escherichia coli derived carbon dots in in vitro and in vivo imaging and p-nitrophenol detection. Analyst 2019, 145, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Shim, J.-J.; Lee, Y.R. Microwave assisted green synthesis of fluorescent N-doped carbon dots: Cytotoxicity and bio-imaging applications. J. Photochem. Photobiol. B Biol. 2016, 161, 154–161. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhang, P.; Gao, M.X.; Liu, C.F.; Wang, W.; Leng, F.; Huang, C.Z. One-pot hydrothermal synthesis of highly luminescent nitrogen-doped amphoteric carbon dots for bioimaging from Bombyx mori silk-natural proteins. J. Mater. Chem. B 2013, 1, 2868–2873. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, P.; Zhang, F.; Feng, X.; Wang, Y.; Yang, Y.; Liu, X. Fluorescent probes for “off–on” highly sensitive detection of Hg2+ and L-cysteine based on nitrogen-doped carbon dots. Talanta 2016, 152, 288–300. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef]
- Baluta, S.; Lesiak, A.; Cabaj, J. Simple and Cost-Effective Electrochemical Method for Norepinephrine Determination Based on Carbon Dots and Tyrosinase. Sensors 2020, 20, 4567. [Google Scholar] [CrossRef]
- Shinde, D.B.; Pillai, V.K. Electrochemical Preparation of Luminescent Graphene Quantum Dots from Multiwalled Carbon Nanotubes. Chem. A Eur. J. 2012, 18, 12522–12528. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, S.; Zhang, H.; Chen, J.; He, Y.; Li, F.; Weng, W.; Ni, J.; Bao, X.; Lin, Y. Carbon dots and chitosan composite film based biosensor for the sensitive and selective determination of dopamine. Analyst 2013, 138, 5417–5423. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a ferrocene derivative functional Au NPs/carbon dots nanocomposite and graphene. Anal. Chim. Acta 2016, 903, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, Y.; Tao, M.; Yan, X.; Liu, Y.; Zhou, X. An electrochemical and fluorescence dual-signal assay based on Fe3O4@MnO2 and N-doped carbon dots for determination of hydrogen peroxide. Microchim. Acta 2020, 187, 187. [Google Scholar] [CrossRef]
- Ng, H.M.; Lim, G.; Leo, C. Comparison between hydrothermal and microwave-assisted synthesis of carbon dots from biowaste and chemical for heavy metal detection: A review. Microchem. J. 2021, 165, 106116. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Stefanakis, D.; Philippidis, A.; Sygellou, L.; Filippidis, G.; Ghanotakis, D.; Anglos, D. Synthesis of fluorescent carbon dots by a microwave heating process: Structural characterization and cell imaging applications. J. Nanoparticle Res. 2014, 16, 2646. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Tian, F.; Li, W.; Li, F.; Liu, W. One-step synthesis of surface passivated carbon nanodots by microwave assisted pyrolysis for enhanced multicolor photoluminescence and bioimaging. J. Mater. Chem. 2011, 21, 13163–13167. [Google Scholar] [CrossRef]
- Kiran, S.; Misra, R.D.K. Mechanism of intracellular detection of glucose through nonenzymatic and boronic acid functionalized carbon dots. J. Biomed. Mater. Res. Part A 2015, 103, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Liu, M.L.; Bin Chen, B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Deng, W.; Chen, J.; Wang, Y.; Zhou, J.; Du, P.; Xu, W.; Wang, Q.; Wang, Q.; et al. Photoluminescent Cationic Carbon Dots as efficient Non-Viral Delivery of Plasmid SOX9 and Chondrogenesis of Fibroblasts. Sci. Rep. 2018, 8, 7057. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Wang, H.; Li, S.; Deng, Y.; Chen, X.; Ye, L.; Gu, W. Microwave-Assisted Polyol Synthesis of Gadolinium-Doped Green Luminescent Carbon Dots as a Bimodal Nanoprobe. Langmuir 2014, 30, 10933–10939. [Google Scholar] [CrossRef]
- Sarkar, T.; Bohidar, H.; Solanki, P.R. Carbon dots-modified chitosan based electrochemical biosensing platform for detection of vitamin D. Int. J. Biol. Macromol. 2018, 109, 687–697. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.-Q.; Zou, S.-Y.; Yang, Q.-J.; Huang, H.; Feng, J.-J.; Wang, A.-J. Microwave-assisted synthesis of N,P-doped carbon dots for fluorescent cell imaging. Microchim. Acta 2015, 183, 821–826. [Google Scholar] [CrossRef]
- Tabaraki, R.; Sadeghinejad, N. Microwave assisted synthesis of doped carbon dots and their application as green and simple turn off–on fluorescent sensor for mercury (II) and iodide in environmental samples. Ecotoxicol. Environ. Saf. 2018, 153, 101–106. [Google Scholar] [CrossRef]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzzá, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-Pot Microwave-Assisted Synthesis of Carbon Dots and in vivo and in vitro Antimicrobial Photodynamic Applications. Front. Microbiol. 2021, 12, 662149. [Google Scholar] [CrossRef]
- López, C.; Zougagh, M.; Algarra, M.; Rodriguez-Castellon, E.; Campos, B.; da Silva, J.E.; Jiménez, J.J.; Ríos, Á. Microwave-assisted synthesis of carbon dots and its potential as analysis of four heterocyclic aromatic amines. Talanta 2015, 132, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Pajewska-Szmyt, M.; Buszewski, B.; Gadzała-Kopciuch, R. Sulphur and nitrogen doped carbon dots synthesis by microwave assisted method as quantitative analytical nano-tool for mercury ion sensing. Mater. Chem. Phys. 2020, 242, 122484. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Cheng, C.; Yang, Y. Green and microwave-assisted synthesis of carbon dots and application for visual detection of cobalt(II) ions and pH sensing. Microchem. J. 2019, 147, 183–190. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Wang, N.; Koh, S.; Jeong, B.G.; Lee, D.; Kim, W.D.; Park, K.; Nam, M.K.; Lee, K.; Kim, Y.; Lee, B.-H.; et al. Highly luminescent silica-coated CdS/CdSe/CdS nanoparticles with strong chemical robustness and excellent thermal stability. Nanotechnology 2017, 28, 185603. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xie, Z.; Wallace, G.; Wang, X. Co-deposition of carbon dots and reduced graphene oxide nanosheets on carbon-fiber microelectrode surface for selective detection of dopamine. Appl. Surf. Sci. 2017, 412, 131–137. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Guan, W.; Huang, H.; Liu, Y. Carbon dots/g-C3N4/ZnO nanocomposite as efficient visible-light driven photocatalyst for tetracycline total degradation. Sep. Purif. Technol. 2017, 173, 295–303. [Google Scholar] [CrossRef]
- Armano, A.; Buscarino, G.; Messina, F.; Sciortino, A.; Cannas, M.; Gelardi, F.M.; Giannazzo, F.; Schilirò, E.; Agnello, S. Dynamic Modification of Fermi Energy in Single-Layer Graphene by Photoinduced Electron Transfer from Carbon Dots. Nanomaterials 2020, 10, 528. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Zhang, L.; Zhang, L.; Zhang, M.; Gong, A.; Tan, Y.; Miao, J.; Gong, Y.; Sun, M.; Ju, H.; et al. Engineered gadolinium-doped carbon dots for magnetic resonance imaging-guided radiotherapy of tumors. Biomaterials 2017, 121, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, Z.; Zhang, H.; Liu, C.-Y.; Zhang, Y.-G. Highly Luminescent Organosilane-Functionalized Carbon Dots. Adv. Funct. Mater. 2011, 21, 1027–1031. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.-G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 2015, 82, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.-Y.; Yang, Z.; Liu, Z.-G.; Wang, H.-X. Ionic liquid-assisted thermal decomposition synthesis of carbon dots and graphene-like carbon sheets for optoelectronic application. RSC Adv. 2016, 6, 61292–61300. [Google Scholar] [CrossRef]
- Hou, J.; Wang, W.; Zhou, T.; Wang, B.; Li, H.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, B.; Liu, Y.; Liu, Z.; Zhang, H.; Zhang, S.; Chang, J.; Lu, S. Green synthesis of nitrogen and sulfur co-doped carbon dots from Allium fistulosum for cell imaging. New J. Chem. 2019, 43, 718–723. [Google Scholar] [CrossRef]
- Dolai, S.; Bhunia, S.K.; Jelinek, R. Carbon-dot-aerogel sensor for aromatic volatile organic compounds. Sensors Actuators B Chem. 2017, 241, 607–613. [Google Scholar] [CrossRef]
- Lin, H.; Huang, J.; Ding, L. Preparation of Carbon Dots with High-Fluorescence Quantum Yield and Their Application in Dopamine Fluorescence Probe and Cellular Imaging. J. Nanomater. 2019, 2019, 5037243. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Su, S.; Qiu, J. Novel fluorescence resonance energy transfer optical sensors for vitamin B12 detection using thermally reduced carbon dots. New J. Chem. 2015, 39, 501–507. [Google Scholar] [CrossRef]
- Ma, C.; Yin, C.; Fan, Y.; Yang, X.; Zhou, X. Highly efficient synthesis of N-doped carbon dots with excellent stability through pyrolysis method. J. Mater. Sci. 2019, 54, 9372–9384. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Bakandritsos, A.; Kouloumpis, A.; Gournis, D.; Krysmann, M.; Giannelis, E.P.; Polakova, K.; Safarova, K.; Hola, K.; Zboril, R. Gd(iii)-doped carbon dots as a dual fluorescent-MRI probe. J. Mater. Chem. 2012, 22, 23327–23330. [Google Scholar] [CrossRef]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. J. Carbon Res. 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced in Vivo Cancer Therapeutic Efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Bin Yang, H.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Qian, Z.; Shan, X.; Chai, L.; Ma, J.; Chen, J.; Feng, H. Si-Doped Carbon Quantum Dots: A Facile and General Preparation Strategy, Bioimaging Application, and Multifunctional Sensor. ACS Appl. Mater. Interfaces 2014, 6, 6797–6805. [Google Scholar] [CrossRef]
- John, T.S.; Yadav, P.K.; Kumar, D.; Singh, S.K.; Hasan, S.H. Highly fluorescent carbon dots from wheat bran as a novel drug delivery system for bacterial inhibition. Luminescence 2020, 35, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, C.; Li, H.; Zhao, W. Synthesis of green-emitting carbon quantum dots with double carbon sources and their application as a fluorescent probe for selective detection of Cu2+ ions. RSC Adv. 2020, 10, 2536–2544. [Google Scholar] [CrossRef] [Green Version]
- Prasannan, A.; Imae, T. One-Pot Synthesis of Fluorescent Carbon Dots from Orange Waste Peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, Y. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper (II) ion detection. Sensors Actuators B Chem. 2014, 196, 647–652. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Chunduri, L.A.A.; Kurdekar, A.; Patnaik, S.; Dev, B.V.; Rattan, T.M.; Kamisetti, V. Carbon Quantum Dots from Coconut Husk: Evaluation for Antioxidant and Cytotoxic Activity. Mater. Focus 2016, 5, 55–61. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, S.; Wang, G.; Zhang, Y.; Zhao, H. Fluorescence Determination of Nitrite in Water Using Prawn-Shell Derived Nitrogen-Doped Carbon Nanodots as Fluorophores. ACS Sens. 2016, 1, 875–881. [Google Scholar] [CrossRef]
- Yuan, M.; Zhong, R.; Gao, H.; Li, W.; Yun, X.; Liu, J.; Zhao, X.; Zhao, G.; Zhang, F. One-step, green, and economic synthesis of water-soluble photoluminescent carbon dots by hydrothermal treatment of wheat straw, and their bio-applications in labeling, imaging, and sensing. Appl. Surf. Sci. 2015, 355, 1136–1144. [Google Scholar] [CrossRef]
- Shan, X.; Chai, L.; Ma, J.; Qian, Z.; Chen, J.; Feng, H. B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 2014, 139, 2322–2325. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Green, low-cost synthesis of photoluminescent carbon dots by hydrothermal treatment of willow bark and their application as an effective photocatalyst for fabricating Au nanoparticles–reduced graphene oxide nanocomposites for glucose detection. Catal. Sci. Technol. 2013, 3, 1027. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S.-J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.-T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent Carbon Quantum Dots-Synthesis, Functionalization and Sensing Application in FoodAnalysis. Nanomaterials 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cui, Y.; Liu, M.; Chen, J.; Wan, Q.; Wen, Y.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A one-step ultrasonic irradiation assisted strategy for the preparation of polymer-functionalized carbon quantum dots and their biological imaging. J. Colloid Interface Sci. 2018, 532, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, L. One-step sonochemical synthesis of versatile nitrogen-doped carbon quantum dots for sensitive detection of Fe2+ ions and temperature in vitro. Mater. Sci. Eng. C 2019, 101, 352–359. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Yu, H.; Kang, Z.; Lee, S.-T. Synthesis of fluorescent carbon nanoparticles directly from active carbon via a one-step ultrasonic treatment. Mater. Res. Bull. 2011, 46, 147–151. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Ngashangva, L.; Goswami, P. Carbon Dots: An Emerging Smart Material for Analytical Applications. Micromachines 2021, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Y.; Niu, N.; Chen, L. Synthesis of molecularly imprinted fluorescent probe based on biomass-derived carbon quantum dots for detection of mesotrione. Anal. Bioanal. Chem. 2019, 411, 5519–5530. [Google Scholar] [CrossRef]
- Demchenko, A.P. Excitons in Carbonic Nanostructures. C 2019, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.; Chen, P.-C.; Periasamy, A.P.; Chen, Y.-N.; Chang, H.-T. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Corpino, R.; Salis, M.; Mocci, F.; Thakkar, S.V.; Olla, C.; Ricci, P.C. On the Emission Properties of Carbon Dots: Reviewing Data and Discussing Models. C 2019, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Zhang, J.; Wang, H.; Kong, Y.; Xiao, Y.; Xu, W. Material and Optical Properties of Fluorescent Carbon Quantum Dots Fabricated from Lemon Juice via Hydrothermal Reaction. Nanoscale Res. Lett. 2018, 13, 175. [Google Scholar] [CrossRef]
- Wu, Z.L.; Liu, Z.X.; Yuan, Y.H. Carbon dots: Materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J. Mater. Chem. B 2017, 5, 3794–3809. [Google Scholar] [CrossRef] [PubMed]
- Slimane, A.B.; Najar, A.; Elafandy, R.; San-Román-Alerigi, D.P.; Anjum, D.; Ng, T.K.; Ooi, B.S. On the phenomenon of large photoluminescence red shift in GaN nanoparticles. Nanoscale Res. Lett. 2013, 8, 342. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.; Tang, L.; Teng, K.; Lau, S. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Fu, M.; Ehrat, F.; Wang, Y.; Milowska, K.; Reckmeier, C.J.; Rogach, A.L.; Stolarczyk, J.; Urban, A.; Feldmann, J. Carbon Dots: A Unique Fluorescent Cocktail of Polycyclic Aromatic Hydrocarbons. Nano Lett. 2015, 15, 6030–6035. [Google Scholar] [CrossRef]
- Ruprecht, V.; Wieser, S.; Marguet, D.; Schütz, G.J. Spot Variation Fluorescence Correlation Spectroscopy Allows for Superresolution Chronoscopy of Confinement Times in Membranes. Biophys. J. 2011, 100, 2839–2845. [Google Scholar] [CrossRef]
- Papaioannou, N.; Marinovic, A.; Yoshizawa, N.; Goode, A.E.; Fay, M.; Khlobystov, A.; Titirici, M.-M.; Sapelkin, A. Structure and solvents effects on the optical properties of sugar-derived carbon nanodots. Sci. Rep. 2018, 8, 6559. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, L.; Zhou, N.; Zhao, X.; Song, Y.; Maharjan, S.; Zhang, J.; Lu, L.; Wang, H.; Yang, B. The crosslink enhanced emission (CEE) in non-conjugated polymer dots: From the photoluminescence mechanism to the cellular uptake mechanism and internalization. Chem. Commun. 2014, 50, 13845–13848. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.; Tian, J.; Jia, L.; Yu, J.S. Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon 2014, 77, 775–782. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Wang, J.; Lu, Y. Facile synthesis of biocompatible N, S-doped carbon dots for cell imaging and ion detecting. RSC Adv. 2015, 5, 16368–16375. [Google Scholar] [CrossRef]
- Varisco, M.; Zufferey, D.; Ruggi, A.; Zhang, Y.; Erni, R.; Mamula, O. Synthesis of hydrophilic and hydrophobic carbon quantum dots from waste of wine fermentation. R. Soc. Open Sci. 2017, 4, 170900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shabasy, R.M.; Elsadek, M.F.; Ahmed, B.M.; Farahat, M.F.; Mosleh, K.M.; Taher, M.M. Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications. Processes 2021, 9, 388. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- do D’Angelis, E.S.; Barbosa, C. Carbon Dots (C-dots) from Cow Manure with Impressive Subcellular Selectivity Tuned by Simple Chemical Modification. Chem. A Eur. J. 2015, 21, 5055–5060. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low Dimens. Syst. Nanostruct. 2021, 126, 114417. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, Y.; Li, M.; Xing, M.; Wu, Q. Carbon quantum dots from carbonized walnut shells: Structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater. Sci. Eng. C 2017, 79, 473–480. [Google Scholar] [CrossRef]
- Xu-Cheng, F.; Xuan-Hua, L.; Jin, J.-Z.; Zhang, J.; Wei, G. Facile synthesis of bagasse waste derived carbon dots for trace mercury detection. Mater. Res. Express 2018, 5, 065044. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Facile Synthesis of Nitrogen-Doped Carbon Dots from Lignocellulosic Waste. Nanomaterials 2019, 9, 1500. [Google Scholar] [CrossRef] [Green Version]
- Xiao-Yan, W.; Xue-Yan, H.; Tian-Qi, W.; Xu-Cheng, F. Crown daisy leaf waste—Derived carbon dots: A simple and green fluorescent probe for copper ion. Surf. Interface Anal. 2019, 52, 148–155. [Google Scholar] [CrossRef]
- Godavarthi, S.; Kumar, K.M.; Vélez, E.V.; Hernandez-Eligio, A.; Mahendhiran, M.; Hernandez-Como, N.; Aleman, M.; Gomez, L.M. Nitrogen doped carbon dots derived from Sargassum fluitans as fluorophore for DNA detection. J. Photochem. Photobiol. B Biol. 2017, 172, 36–41. [Google Scholar] [CrossRef]
- Soni, H.; Pamidimukkala, P.S. Green synthesis of N, S co-doped carbon quantum dots from triflic acid treated palm shell waste and their application in nitrophenol sensing. Mater. Res. Bull. 2018, 108, 250–254. [Google Scholar] [CrossRef]
- Pankaj, A.; Tewari, K.; Singh, S.; Singh, S.P. Waste candle soot derived nitrogen doped carbon dots based fluorescent sensor probe: An efficient and inexpensive route to determine Hg(II) and Fe(III) from water. J. Environ. Chem. Eng. 2018, 6, 5561–5569. [Google Scholar] [CrossRef]
- Venkatesan, S.; Jhonsi, M.A.; Kathiravan, A.; Muthupandian, A. Fuel waste to fluorescent carbon dots and its multifarious applications. Sens. Actuators B Chem. 2019, 282, 972–983. [Google Scholar] [CrossRef]
- Chung, H.K.; Wongso, V.; Sambudi, N.S. Biowaste-derived carbon dots/hydroxyapatite nanocomposite as drug delivery vehicle for acetaminophen. J. Sol-Gel Sci. Technol. 2020, 93, 214–223. [Google Scholar] [CrossRef]
- Aruni Abdul Manaf, S.; Hegde, G.; Kumar Mandal, U.; Tin Wui, W.; Roy, P. Functionalized Carbon Nano-scale Drug Delivery Systems from Biowaste Sago Bark For Cancer Cell Imaging. Curr. Drug Deliv. 2017, 14, 1071–1077. [Google Scholar] [CrossRef]
- Kang, Z.; Liu, Y. Catalytic Applications of Carbon Dots. In Carbon Nanoparticles and Nanostructures; Yang, N., Jiang, X., Pang, D.-W., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 257–298. [Google Scholar]
- Yao, Y.-Y.; Gedda, G.; Girma, W.M.; Yen, C.-L.; Ling, Y.-C.; Chang, J.-Y. Magnetofluorescent Carbon Dots Derived from Crab Shell for Targeted Dual-Modality Bioimaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 13887–13899. [Google Scholar] [CrossRef]
- Qu, A.; Xie, H.; Xu, X.; Zhang, Y.; Wen, S.; Cui, Y. High quantum yield graphene quantum dots decorated TiO2 nanotubes for enhancing photocatalytic activity. Appl. Surf. Sci. 2016, 375, 230–241. [Google Scholar] [CrossRef]
- Hod, I.; González-Pedro, V.; Tachan, Z.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J.; Zaban, A. Dye versus Quantum Dots in Sensitized Solar Cells: Participation of Quantum Dot Absorber in the Recombination Process. J. Phys. Chem. Lett. 2011, 2, 3032–3035. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G.C. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef]
- Stepanidenko, E.; Ushakova, E.; Fedorov, A.; Rogach, A. Applications of Carbon Dots in Optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Cappai, A.; Melis, C.; Stagi, L.; Ricci, P.C.; Mocci, F.; Carbonaro, C.M. Insight into the Molecular Model in Carbon Dots through Experimental and Theoretical Analysis of Citrazinic Acid in Aqueous Solution. J. Phys. Chem. C 2021, 125, 4836–4845. [Google Scholar] [CrossRef]
- Surendran, P.; Lakshmanan, A.; Vinitha, G.; Ramalingam, G.; Rameshkumar, P. Facile preparation of high fluorescent carbon quantum dots from orange waste peels for nonlinear optical applications. Luminescence 2020, 35, 196–202. [Google Scholar] [CrossRef]
- Achilleos, D.S.; Kasap, H.; Reisner, E. Photocatalytic hydrogen generation coupled to pollutant utilisation using carbon dots produced from biomass. Green Chem. 2020, 22, 2831–2839. [Google Scholar] [CrossRef]
- Aggarwal, R.; Saini, D.; Singh, B.; Kaushik, J.; Garg, A.K.; Sonkar, S.K. Bitter apple peel derived photoactive carbon dots for the sunlight induced photocatalytic degradation of crystal violet dye. Sol. Energy 2020, 197, 326–331. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Free, M.L. Light emitting diodes based on carbon dots derived from food, beverage, and combustion wastes. Phys. Chem. Chem. Phys. 2015, 17, 27642–27652. [Google Scholar] [CrossRef]
- Shabashini, A.; Panja, S.K.; Nandi, G.C. Applications of Carbon Dots (CDs) in Latent Fingerprints Imaging. Chem. Asian J. 2021, 16, 1057–1072. [Google Scholar] [CrossRef]
- Verhagen, A.; Kelarakis, A. Carbon Dots for Forensic Applications: A Critical Review. Nanomaterials 2020, 10, 1535. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Sukmayani, W.; Khairunisa, S.Q.; Witaningrum, A.M.; Indriati, D.W.; Matondang, M.Q.Y.; Chang, J.-Y.; Kotaki, T.; Kameoka, M. Design of boronic acid-attributed carbon dots on inhibits HIV-1 entry. RSC Adv. 2016, 6, 92996–93002. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Nair, A.B.; Deb, P.K. Exploring the Potential of Carbon Dots to Combat COVID-19. Front. Mol. Biosci. 2020, 7, 616575. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.; Gedanken, A. Green Synthesis of Multifunctional Carbon Dots with Antibacterial Activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Yoo, J.; Lim, B.; Kwon, W.; Rhee, S.-W. Improving the functionality of carbon nanodots: Doping and surface functionalization. J. Mater. Chem. A 2016, 4, 11582–11603. [Google Scholar] [CrossRef]
- Tuccitto, N.; Riela, L.; Zammataro, A.; Spitaleri, L.; Li-Destri, G.; Sfuncia, G.; Nicotra, G.; Pappalardo, A.; Capizzi, G.; Sfrazzetto, G.T. Functionalized Carbon Nanoparticle-Based Sensors for Chemical Warfare Agents. ACS Appl. Nano Mater. 2020, 3, 8182–8191. [Google Scholar] [CrossRef]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Otis, G.; Bhattacharya, S.; Malka, O.; Kolusheva, S.; Bolel, P.; Porgador, A.; Jelinek, R. Selective Labeling and Growth Inhibition of Pseudomonas aeruginosa by Aminoguanidine Carbon Dots. ACS Infect. Dis. 2019, 5, 292–302. [Google Scholar] [CrossRef]
- Lin, F.; Bao, Y.-W.; Wu, F.-G. Carbon Dots for Sensing and Killing Microorganisms. C 2019, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection Methodologies for Pathogen and Toxins: A Review. Sensors 2017, 17, 1885. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Zhong, Z.; Gao, X.; Jia, L. Graphene Oxide Quantum Dots Assisted Construction of Fluorescent Aptasensor for Rapid Detection of Pseudomonas aeruginosa in Food Samples. J. Agric. Food Chem. 2018, 66, 10898–10905. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Bouckaert, J.; Dumitrascu, N.; Szunerits, S.; Boukherroub, R. Enhanced antibacterial activity of carbon dots functionalized with ampicillin combined with visible light triggered photodynamic effects. Colloids Surf. B Biointerfaces 2018, 170, 347–354. [Google Scholar] [CrossRef]
- Shi, B.; Su, Y.; Zhang, L.-L.; Huang, M.; Liu, R.; Zhao, S. Nitrogen and Phosphorus Co-Doped Carbon Nanodots as a Novel Fluorescent Probe for Highly Sensitive Detection of Fe3+ in Human Serum and Living Cells. ACS Appl. Mater. Interfaces 2016, 8, 10717–10725. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Safardoust-Hojaghan, H.; Salavati-Niasari, M.; Amiri, O.; Hassanpour, M. Preparation of highly luminescent nitrogen doped graphene quantum dots and their application as a probe for detection of Staphylococcus aureus and E. coli. J. Mol. Liq. 2017, 241, 1114–1119. [Google Scholar] [CrossRef]
- Jian, H.-J.; Wu, R.-S.; Lin, T.-Y.; Li, Y.-J.; Lin, H.-J.; Harroun, S.G.; Lai, J.-Y.; Huang, C.-C. Super-Cationic Carbon Quantum Dots Synthesized from Spermidine as an Eye Drop Formulation for Topical Treatment of Bacterial Keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef]
- Liu, J.; Lu, S.; Tang, Q.; Zhang, K.; Yu, W.; Sun, H.; Yang, B. One-step hydrothermal synthesis of photoluminescent carbon nanodots with selective antibacterial activity against Porphyromonas gingivalis. Nanoscale 2017, 9, 7135–7142. [Google Scholar] [CrossRef]
- Thakur, M.; Pandey, S.; Mewada, A.; Patil, V.; Khade, M.; Goshi, E.; Sharon, M. Antibiotic Conjugated Fluorescent Carbon Dots as a Theranostic Agent for Controlled Drug Release, Bioimaging, and Enhanced Antimicrobial Activity. J. Drug Deliv. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Verma, A.; Shivalkar, S.; Sk, P.; Samanta, S.K.; Sahoo, A.K. Nanocomposite of Ag nanoparticles and catalytic fluorescent carbon dots for synergistic bactericidal activity through enhanced reactive oxygen species generation. Nanotechnology 2020, 31, 405704. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Gao, H. Synthesis and drug detection performance of nitrogen-doped carbon dots. J. Lumin. 2014, 149, 159–162. [Google Scholar] [CrossRef]
- Han, S.; Zhang, H.; Zhang, J.; Xie, Y.; Liu, L.; Wang, H.-X.; Li, X.; Liu, W.; Tang, Y. Fabrication, gradient extraction and surface polarity-dependent photoluminescence of cow milk-derived carbon dots. RSC Adv. 2014, 4, 58084–58089. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]

| Source Material of Carbon | Size of C-Dots | Morphology | Applications | References |
|---|---|---|---|---|
| Graphite in polyethylenimine and ethylenediamine | A fairly spherical shape with diameters from 1 to 3 nm | Luminescent CD material for various applications | [42] | |
| Graphite target irradiation | 2–3 nm | Various potential associated applications | [43] | |
| C-dot NPs functionalized with PEG200 and N-acetyl-l-cysteine | Thin (about 750 nm), homogenous and smooth (roughness of 2.7 ± 0.7 Å) film | Sensor for Hg (II) ions | [44] | |
| Graphite powders | Ultrasmall size 1 nm | Sensing and catalytic applications | [45] | |
| Graphite | Flowerlike morphology of the particles with a broad size distribution ranging from 200–500 nm. | Photoluminescence-ability-related applications | [46] | |
| Mercapto-succinic acid | [47] | |||
| Carbon solid | Induced in acetone with laser pulses of 1064, 532 and 355 nm | Close-spherical amorphous CNDs with sizes between 5 and 20 nm | Light-emitting purposes | [48] |
| Scheme 52 | Size/Shape of C-Dots | Applications | References |
|---|---|---|---|
| Carbon byproducts | Optoelectronic applications | [52] | |
| TiO2 nanostructures coupled with carbon dots | Spherical shaped with an average size of 27 nm | Potential optical sensing applications | [53] |
| Boron-doped graphene quantum dots | Zero-dimensional graphene quantum dots (GQDs) | Potential luminescence and optical applications | [54] |
| CNTs and oxidized CNTs | [50] |
| Source Material of Carbon | Morphology of C-Dots | Applications | References |
|---|---|---|---|
|
Sodium citrate and urea (by electrochemical carbonization) | Varies from 1.0 to 3.5 nm and with an average size of 2.4 nm (water soluble, fluorescent) | Sensing application of Hg2+ determination in real samples | [62] |
| Orange juice + in ethanol and after dichloromethane | Small-sized power preparation | Detection of neurotransmitters, using nontoxic reagents | [63] |
| MWCNTs in propylene carbonate | Green luminescent, (GQDs) with a uniform size of 3, 5 and 8.2 (±0.3) nm | Biomarkers, nanoelectronic devices and chemosensors | [64] |
| A carbon dots (CDs) and chitosan (CS) composite film modified by glassy carbon electrode | A fine power for electrode coating for composite formation | Biosensing of dopamine | [65] |
| HAuCl4 into carbon nanodots solution | Composite containing Au/carbon dot NC, graphene and a ferrocene derivative | Sensing and detection of ascorbic acid, dopamine, uric acid and acetaminophen | [66] |
| Fe3O4@MnO2 and N-doped carbon dots (NCDs). | Powdered fluorescence quencher and electrochemical enhancer material | Determination of hydrogen peroxide | [67] |
| Source Material of Carbon | Size/Shape of C-Dots | Applications | References |
|---|---|---|---|
| Sucrose as C source, and diethylene glycol (DEG) as the reaction medium | Water dispersive and transparent with average diameter about 5 nm | Optical nanoprobing | [76] |
| Chitosan (1%) solution + acetic acid (1%) solution, addition of CDs to obtain carbon dots-chitosan (CD-CH) composite. | A thin film of CD-CH composite was prepared on ITO glass substrate | Sensing of vitamin D2 | [77] |
| N-phosphonomethyl aminodiacetic acid and ethylenediamine (thermolysis + microwave) | Uniform dispersion with the average size of 3.3 nm | Cellular imaging | [78] |
| Citric acid, urea and thiourea | ~10 nm | Detection of Hg2+ and I- in tap, river and mineral waters and fish samples | [79] |
| Cystine as source for C, N and S and glycerol as the reaction solvent | Fine-sized powder | Detecting Hg(II) in spiked tap and lake waters | [80] |
| From lactose by microwave-assisted hydrochloric acid | Average size 10 nm (fluorescent water-soluble carbon dots (CDs)) | Analysis of various heterocyclic aromatic amines | [81] |
| Citric acid was used and glutathione or thiourea as precursors of doping elements | Simple fine powder | Mercury in river water and wastewater | [82] |
| Kelp (algae) as main carbon source and ethylenediamine as nitrogen dopant | Below 10 nm | Detection of Co2+ based on fluorescence quenching | [83] |
| Source Material of Carbon | Synthesis Conditions | Size/Shape of C-Dots | Applications | References |
|---|---|---|---|---|
| Ascorbic acid (AA) and uric acid (UA) | Electrochemical method | Fine carbon fiber electrode | Highly sensitive and selective dopamine (DA) detection | [86] |
| Carbon dots (CDs)/g-C3N4/ZnO nanocomposite | Facile impregnation-thermal method | Composite by using impregnation-thermal method | Tetracycline photocatalysis in the water environment | [87] |
| Citric acid monohydrate, as carbon precursor solid-phase composite of CDs deposited on graphene | Carbonization+ microwave | CD–graphene-interaction-based nanomaterial 1–10 nm | Light conversation applications | [88] |
| Gadolinium-doped carbon dots (Gd-doped CDs) | ~18 nm with dispersibility in water | MRI-guided radiotherapy of tumors | [89] | |
| Citric acid | 240 °C for only 1 min | ~0.9 nm | [90] | |
| Citric acid | 200 °C for 30 min | 0.7–1 nm | [91] | |
| 1-butyl 3-methyl imidazolium bromide and L -cysteine | 240 °C | 1.0–3.5 nm | [92] | |
| Citric acid and dicyandiamide | Spherically shaped | [93] |
| Source Material of Carbon | Size of C-Dots | Applications | References |
|---|---|---|---|
| L-cysteine+ citric acid | Near-spherical particles with diameters in the range of 2–4 nm, crystalline | Fluorescent agent for cell imaging | [96] |
| Citric acid | 4.8–9 nm | [97] | |
| 6-O-(O-O-dilauroyl-tartaryl)- D -glucose | ~ 2.4 ± 0.5 nm | [95] |
| Source Material of Carbon | Size of C-Dots | References |
|---|---|---|
| L-aspartic acid and D-glucose | Diameter of CDs was 2.28 ± 0.42 nm | [102] |
| Citric acid via thermal pyrolysis method, capping agent: diethylenetriamine | CDs ranged from 5 to 8 nm | [101] |
| Tris base, gadopentetic acid and betaine hydrochloride | ~3.2 nm | [99] |
| Source Material of Carbon | Hydrothermal Conditions | Size of C-Dots | Applications | References |
|---|---|---|---|---|
| Wheat bran | 180 °C, 3 h | Drug delivery | [105] | |
| Tartaric acid and bran | Autoclave at 150 °C for 8 h in the oven | ~4.85 nm | G-CQDs were used as a fluorescent probe for detection of Cu2+ ions | [106] |
| Orange peels | 180 °C, 12 h | LEDs, photocatalysis | [107] | |
| Cereals and grains waste | 200 °C, 12 h | Imaging, sensing, labeling | ||
| Bamboo waste | 200 °C, 6 h | Bioimaging (in vivo) | [108] | |
| Lemon peels | 200 °C, 12 h | Sensing and photocatalytic | [109] | |
| Coconut husks | 200 °C, 3 h | pH sensing | [110] | |
| Sugarcane bagasse | 190 °C, 24 h | Drug delivery | [14] | |
| Prawn shells | 180 °C, 12 h | Nitrite detection | [14,111] | |
| Wheat straw | 250 °C, 10 h | - | [112] | |
| Carbon tetra chloride | ~3.3 nm | - | [103] | |
| SiCl4 and hydroquinone | SS autoclave at 200 °C for 2 h | 7 ± 2 nm | - | [104] |
| Hydroquinone and boron source BBr3 | ~16 nm | - | [113] | |
| Low-cost wastes of willow bark | Hydrothermal + carbonization | Glucose detection | [114] |
| Source Material of Carbon | Size of C-Dots | Applications | References |
|---|---|---|---|
| Activated carbon | 5–10 nm | - | [117] |
| Activated carbon | 5–10 nm | - | [121] |
| Source Material of Carbon | Methods of Synthesis | Applications | References |
|---|---|---|---|
| Onion waste | Hydrothermal | Multicolor imaging and Fe3+ detection | [141] |
| Lychee waste | Solvohydrothermal | Multicolor cell imaging and Fe3+ detection | [141] |
| Cow dung waste | Chemical oxidation | Live-cell imaging with subcellular selectivity | [142] |
| Banana peel waste | Hydrothermal | In vivo bioimaging | [143] |
| Walnut shells | Carbonization | Intracellular bioimaging | [144] |
| Wheat straw and cereals | Hydrothermal | Cell imaging and in vivo bioimaging | [112] |
| Source Material of Carbon | Morphology of C-Dots | Applications | References |
|---|---|---|---|
| Bagasse waste | Hydrothermal (HT) | Hg2+ detection | [145] |
| Lignocellulose waste | Copper ion | [146] | |
| Crown daisy leaf waste | Copper ion | [147] | |
| Sargassum fluitans | DNA detection | [148] | |
| Mango peels waste | Mesotrione detection | [124] | |
| Glucose source | Sensing | [123,140] | |
| Palm shell waste | Ultrasonic | Nitrophenol detection | [149] |
| Waste tea residue | Chemical oxidation | Tetracycline detection | [14] |
| Waste candle soot | Hg2+ and Fe3+ detection | [150] | |
| Kerosene fuel soot | Picric acid, Fe3+ and Cu2+ detection | [151] |
| Source Material of Carbon | Methods | Applications | References |
|---|---|---|---|
| Wheat bran | HT | DD | [105] |
| Sugarcane bagasse | Burning and HT | DD | [152] |
| Waste sago bark | Catalyst-free pyrolysis | Anticancer drug delivery | [153] |
| Bamboo leaves | Reflux | DD, tumor imaging | [154] |
| Crab shells | Microwave | DD | [155] |
| Source Material of Carbon | Methods | Applications | References |
|---|---|---|---|
| Waste frying oil | HT | Photocatalyst | [14] |
| Citrus fruits peel waste (lemon + orange) | HT | Photocatalyst and sensing | [161] |
| Lignocellulose waste | Pyrolysis | Photocatalyst attached to pollutant utilization | [162] |
| Bitter apple waste | Pyrolysis | Photocatalyst | [163] |
| Waste food | HT | Light-emitting diodes | [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications. Polymers 2021, 13, 3190. https://doi.org/10.3390/polym13183190
Khayal A, Dawane V, Amin MA, Tirth V, Yadav VK, Algahtani A, Khan SH, Islam S, Yadav KK, Jeon B-H. Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications. Polymers. 2021; 13(18):3190. https://doi.org/10.3390/polym13183190
Chicago/Turabian StyleKhayal, Areeba, Vinars Dawane, Mohammed A. Amin, Vineet Tirth, Virendra Kumar Yadav, Ali Algahtani, Samreen Heena Khan, Saiful Islam, Krishna Kumar Yadav, and Byong-Hun Jeon. 2021. "Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications" Polymers 13, no. 18: 3190. https://doi.org/10.3390/polym13183190
APA StyleKhayal, A., Dawane, V., Amin, M. A., Tirth, V., Yadav, V. K., Algahtani, A., Khan, S. H., Islam, S., Yadav, K. K., & Jeon, B.-H. (2021). Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications. Polymers, 13(18), 3190. https://doi.org/10.3390/polym13183190







