How Microgels Can Improve the Impact of Organ-on-Chip and Microfluidic Devices for 3D Culture: Compartmentalization, Single Cell Encapsulation and Control on Cell Fate
Abstract
:1. Introduction
2. Microencapsulation and Cellular Confinement
3. Applications of Microgels in OOC
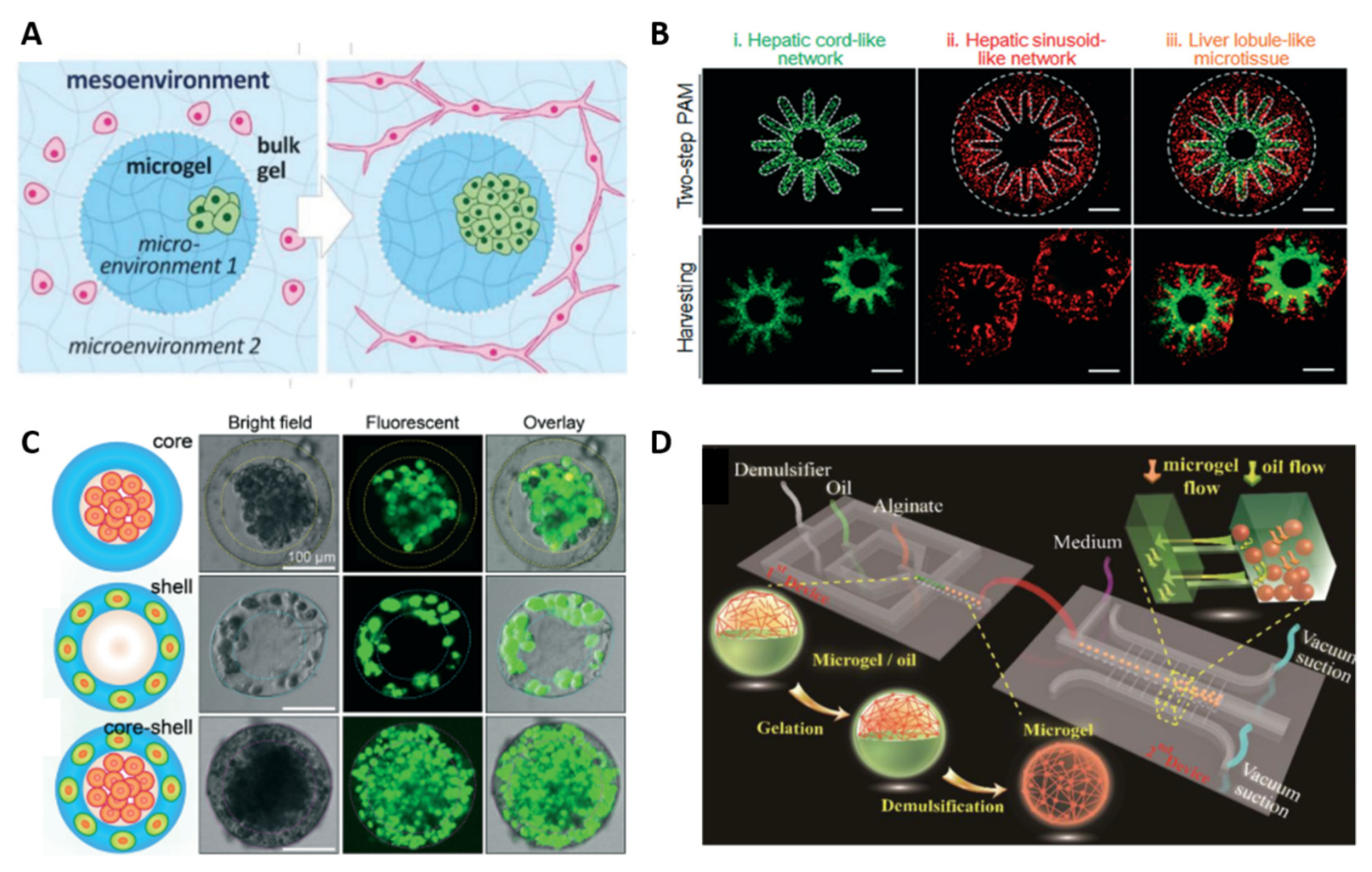
3.1. Compartmentalization
3.2. Single Cell Culture
3.3. Control on Proliferation, Polarity and Cell Fate
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verhulsel, M.; Vignes, M.; Descroix, S.; Malaquin, L.; Vignjevic, D.M.; Viovy, J.L. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials 2014, 35, 1816–1832. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, Y.; Cui, K.; Guo, Y.; Zhang, X.; Qin, J. Advances in Hydrogels in Organoids and Organs-on-a-Chip. Adv. Mater. 2019, 31, e1902042. [Google Scholar] [CrossRef]
- Ran, R.; Sun, Q.; Baby, T.; Wibowo, D.; Middelberg, A.P.J.; Zhao, C.-X. Multiphase microfluidic synthesis of micro- and nanostructures for pharmaceutical applications. Chem. Eng. Sci. 2017, 169, 78–96. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Yu, Y.; Hu, Y.; He, X.; Berk Usta, O.; Yarmush, M.L. Generation and manipulation of hydrogel microcapsules by droplet-based microfluidics for mammalian cell culture. Lab Chip 2017, 17, 1913–1932. [Google Scholar] [CrossRef] [PubMed]
- Castiaux, A.D.; Spence, D.M.; Martin, R.S. Review of 3D cell culture with analysis in microfluidic systems. Anal. Methods 2019, 11, 4220–4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Shim, K.Y.; Kim, B.; Sung, J.H. Hydrogel-based three-dimensional cell culture for organ-on-a-chip applications. Biotechnol. Prog. 2017, 33, 580–589. [Google Scholar] [CrossRef]
- Tenje, M.; Cantoni, F.; Porras Hernández, A.M.; Searle, S.S.; Johansson, S.; Barbe, L.; Antfolk, M.; Pohlit, H. A practical guide to microfabrication and patterning of hydrogels for biomimetic cell culture scaffolds. Organs-on-a-Chip 2020, 2, 100003. [Google Scholar] [CrossRef]
- Ooi, H.W.; Hafeez, S.; Van Blitterswijk, C.A.; Moroni, L.; Baker, M.B. Hydrogels that listen to cells: A review of cell-responsive strategies in biomaterial design for tissue regeneration. Mater. Horiz. 2017, 4, 1020–1040. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Luo, C. Gel integration for microfluidic applications. Lab Chip 2016, 16, 1757–1776. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chen, X.; Kang, Q.; Yan, X. Biomedical Application of Functional Materials in Organ-on-a-Chip. Front. Bioeng. Biotechnol. 2020, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Kemkemer, R.; Zenghao, Z.; Linxiao, Y.; Athanasopulu, K.; Frey, K.; Cui, Z.; Su, H.; Luo, L. Surface modification of Polydimethylsiloxane by hydrogels for microfluidic applications. Curr. Dir. Biomed. Eng. 2019, 5, 93–96. [Google Scholar] [CrossRef]
- Saxena, S.; Lyon, L.A. Enabling method to design versatile biomaterial systems from colloidal building blocks. Mol. Syst. Des. Eng. 2016, 1, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Li, M.; Chen, Z.; Leong, K.W. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 2016, 16, 4482–4506. [Google Scholar] [CrossRef] [Green Version]
- Lienemann, P.S.; Rossow, T.; Mao, A.S.; Vallmajo-Martin, Q.; Ehrbar, M.; Mooney, D.J. Single cell-laden protease-sensitive microniches for long-term culture in 3D. Lab Chip 2017, 17, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Abdalla, A.M.E.; Xiao, L.; Yang, G. Biopolymer-based microcarriers for three-dimensional cell culture and engineered tissue formation. Int. J. Mol. Sci. 2020, 21, 1895. [Google Scholar] [CrossRef] [Green Version]
- Choe, G.; Park, J.; Park, H.; Lee, J.Y. Hydrogel biomaterials for stem cell microencapsulation. Polymers 2018, 10, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.G.A.; Ambhorkar, P.; Samanipour, R.; Yang, A.; Ghafoor, A.; Kim, K. Microfluidics-based fabrication of cell-laden microgels. Biomicrofluidics 2020, 14, 021501. [Google Scholar] [CrossRef]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Rossow, T.; Lienemann, P.S.; Mooney, D.J. Cell Microencapsulation by Droplet Microfluidic Templating. Macromol. Chem. Phys. 2017, 218, 1600380. [Google Scholar] [CrossRef]
- Alkayyali, T.; Cameron, T.; Haltli, B.; Kerr, R.G.; Ahmadi, A. Microfluidic and cross-linking methods for encapsulation of living cells and bacteria—A review. Anal. Chim. Acta 2019, 1053, 1–21. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Suea-Ngam, A.; Howes, P.D.; Srisa-Art, M.; Demello, A.J. Droplet microfluidics: From proof-of-concept to real-world utility? Chem. Commun. 2019, 55, 9895–9903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.-M.; Lee, J.-H.; Huh, Y.S.; Takayama, S. Alginate Microencapsulation for Three-Dimensional In vitro Cell Culture. ACS Biomater. Sci. Eng. 2021, 7, 2864–2879. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, Z.; Khan, M.; Mao, S.; Manibalan, K.; Li, N.; Lin, J.M.; Lin, L. Multifunctional Regulation of 3D Cell-Laden Microsphere Culture on an Integrated Microfluidic Device. Anal. Chem. 2019, 91, 12283–12289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, X.; Zeng, R.; Xu, F.; Li, X.J. Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip. Future Sci. OA 2017, 3, FSO187. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Yu, Y.; Cheng, Y.; Tian, C.; Zhao, G.; Zhao, Y. All-Aqueous-Phase Microfluidics for Cell Encapsulation. ACS Appl. Mater. Interfaces 2019, 11, 4826–4832. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Wang, H.; Wei, W.B.; Liu, H.; Jiang, L.; Qin, J.H. A Microfluidic Strategy for Controllable Generation of Water-in-Water Droplets as Biocompatible Microcarriers. Small 2018, 14, 1801095. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, J.J. Sub-100-micron calcium-alginate microspheres: Preparation by nitrogen flow focusing, dependence of spherical shape on gas streams and a drug carrier using acetaminophen as a model drug. Carbohydr. Polym. 2021, 269, 118262. [Google Scholar] [CrossRef]
- Tian, Y.; Lipke, E.A. Microfluidic Production of Cell-Laden Microspheroidal Hydrogels with Different Geometric Shapes. ACS Biomater. Sci. Eng. 2020, 6, 6435–6444. [Google Scholar] [CrossRef] [PubMed]
- Kupikowska-Stobba, B.; Lewińska, D. Polymer microcapsules and microbeads as cell carriers for: In vivo biomedical applications. Biomater. Sci. 2020, 8, 1536–1574. [Google Scholar] [CrossRef]
- Weaver, J.D.; Headen, D.M.; Coronel, M.M.; Hunckler, M.D.; Shirwan, H.; García, A.J. Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site. Am. J. Transplant. 2019, 19, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Qu, X.; Tan, Y.; Xiao, J.; Le, Y.; Li, Y.; Liu, X.; Li, B.; Liao, X. Synthesis of photocrosslinkable hydrogels for engineering three-dimensional vascular-like constructs by surface tension-driven assembly. Mater. Sci. Eng. C 2020, 116, 111143. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, Y.; Zhao, C.; Shou, X.; Piao, Y.; Zhao, X.; Zhao, Y.; Wang, S. NK-Cell-Encapsulated Porous Microspheres via Micro fl uidic Electrospray for Tumor Immunotherapy. ACS Appl. Mater. Interfaces 2019, 11, 33716–33724. [Google Scholar] [CrossRef]
- Nie, M.; Chen, G.; Zhao, C.; Gan, J.; Alip, M.; Zhao, Y.; Sun, L. Bioactive Materials Bio-inspired adhesive porous particles with human MSCs encapsulation for systemic lupus erythematosus treatment. Bioact. Mater. 2021, 6, 84–90. [Google Scholar] [CrossRef]
- Lagus, T.P.; Edd, J.F. High-throughput co-encapsulation of self-ordered cell trains: Cell pair interactions in microdroplets. RSC Adv. 2013, 3, 20512–20522. [Google Scholar] [CrossRef] [Green Version]
- Pajoumshariati, S.R.; Azizi, M.; Wesner, D.; Miller, P.G.; Shuler, M.L.; Abbaspourrad, A. Microfluidic-Based Cell-Embedded Microgels Using Nonfluorinated Oil as a Model for the Gastrointestinal Niche. ACS Appl. Mater. Interfaces 2018, 10, 9235–9246. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, C.J. Hydrogel droplet microfluidics for high-throughput single molecule/cell analysis. Acc. Chem. Res. 2017, 50, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Sievers, J.; Stoppa, A.; Träber, N.; Zimmermann, R.; Welzel, P.B.; Werner, C. Cell-Instructive Multiphasic Gel-in-Gel Materials. Adv. Funct. Mater. 2020, 30, 1908857. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Neto, M.D.; Oliveira, M.B.; Mano, J.F. Microparticles in Contact with Cells: From Carriers to Multifunctional Tissue Modulators. Trends Biotechnol. 2019, 37, 1011–1028. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Utech, S.; Chen, D.; Prodanovic, R.; Lin, J.M.; Weitz, D.A. Controlled assembly of heterotypic cells in a core-shell scaffold: Organ in a droplet. Lab Chip 2016, 16, 1346–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husman, D.; Welzel, P.B.; Vogler, S.; Bray, L.J.; Träber, N.; Friedrichs, J.; Körber, V.; Tsurkan, M.V.; Freudenberg, U.; Thiele, J.; et al. Multiphasic microgel-in-gel materials to recapitulate cellular mesoenvironments in vitro. Biomater. Sci. 2020, 8, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tian, C.; Zhao, L.; Wang, J. Pneumatic-aided micro-molding for flexible fabrication of homogeneous and heterogeneous cell-laden microgels. Lab Chip 2016, 16, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Shin, Y.; Lee, J.; Cha, C. Programmable multilayer printing of a mechanically-tunable 3D hydrogel co-culture system for high-throughput investigation of complex cellular behavior. Lab Chip 2021, 21, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, K.; Zhang, H.; Pang, B.; Choi, C.H.; Mao, A.S.; Liao, H.; Utech, S.; Mooney, D.J.; Wang, H.; et al. Microfluidic Templated Multicompartment Microgels for 3D Encapsulation and Pairing of Single Cells. Small 2018, 14, 1702955. [Google Scholar] [CrossRef]
- Zhong, M.; Wei, D.; Yang, Y.; Sun, J.; Chen, X.; Guo, L.; Wei, Q.; Wan, Y.; Fan, H.; Zhang, X. Vascularization in engineered tissue construct by assembly of cellular patterned micromodules and degradable microspheres. ACS Appl. Mater. Interfaces 2017, 9, 3524–3534. [Google Scholar] [CrossRef]
- Kamperman, T.; Karperien, M.; Le Gac, S.; Leijten, J. Single-Cell Microgels: Technology, Challenges, and Applications. Trends Biotechnol. 2018, 36, 850–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Neubauer, M.P.; Thiele, J.; Fery, A.; Huck, W.T.S. Artificial microniches for probing mesenchymal stem cell fate in 3D. Biomater. Sci. 2014, 2, 1661–1671. [Google Scholar] [CrossRef] [Green Version]
- Kamperman, T.; Henke, S.; Visser, C.W.; Karperien, M.; Leijten, J. Centering Single Cells in Microgels via Delayed Crosslinking Supports Long-Term 3D Culture by Preventing Cell Escape. Small 2017, 13, 1603711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubay, R.; Urban, J.N.; Darling, E.M. Single-Cell Microgels for Diagnostics and Therapeutics. Adv. Funct. Mater. 2021. [Google Scholar] [CrossRef]
- An, C.; Liu, W.; Zhang, Y.; Pang, B.; Liu, H.; Zhang, Y.; Zhang, H.; Zhang, L.; Liao, H.; Ren, C.; et al. Continuous microfluidic encapsulation of single mesenchymal stem cells using alginate microgels as injectable fillers for bone regeneration. Acta Biomater. 2020, 111, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Yang, Z.; Lyu, H.; Lau, K.Y.Y.; Shum, H.C. A Microfluidic System for One-Chip Harvesting of Single-Cell-Laden Hydrogels in Culture Medium. Adv. Biosyst. 2019, 3, e1900076. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.A.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T.; et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147. [Google Scholar] [CrossRef]
- Siltanen, C.; Yaghoobi, M.; Haque, A.; You, J.; Lowen, J.; Soleimani, M.; Revzin, A. Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids. Acta Biomater. 2016, 34, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Du, Y.; Wang, L.; Kaji, H.; Bae, H.; Khademhosseini, A. Patterned Differentiation of Individual Embryoid Bodies in Spatially Organized 3D Hybrid Microgels. Adv. Mater. 2010, 22, 5276–5281. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Gao, H.; Wen, H.; Huang, H.; Li, Q.; Liang, M.; Liu, Y.; Dong, H.; Cao, X. Engineering the cellular mechanical microenvironment to regulate stem cell chondrogenesis: Insights from a microgel model. Acta Biomater. 2020, 113, 393–406. [Google Scholar] [CrossRef]
- Spiteri, C.; Caprettini, V.; Chiappini, C. Biomaterials-based approaches to model embryogenesis. Biomater. Sci. 2020, 8, 6992–7013. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Rao, V.V.; Golden, A.C.; Bell, D.J.; Grim, J.C.; Anseth, K.S. Mesenchymal stem cell-inspired microgel scaffolds to control macrophage polarization. Bioeng. Transl. Med. 2021, 6, e10217. [Google Scholar] [CrossRef]


| Ref. | Polymer | Synthesis | Application |
|---|---|---|---|
| [26] | Alginate | Chemical cross-linking within a multifunctional integrated microfluidic device | Study of cell−cell communications in a tumor-endothelial cell co-culture model |
| [29] | Alginate | (water/water) High-throughput droplet microfluidic system chemical cross-linking | Encapsulating rat pancreatic islets (β-TC6) for therapy |
| [38] | Alginate/gelatin | Droplet microfluidics using nonfluorinated oils and chemical cross-linking | Gastrointestinal niche exploiting crypt cells (as functional unit of the gastrointestinal tract) and Peyer’s patch cells (as functional unit of the immune system) to study intercellular interactions |
| [43] | Alginate | Chemical cross-linking of core-shell droplets within a flow focusing microfluidic device | “Organ in a droplet”: 3D liver model in a drop by controlled assembly of heterotypic cells in a 3D core–shell droplets |
| [44] | “Microgel-in-gel” based on poly(ethylene glycol)-heparin | Droplet microfluidics and crosslinking by Michael-type addition reaction | Modulation of micro- and mesoenvironmental parameters to reflect fundamental tissue properties or direct the maturation of 3D cell assemblies |
| [45] | Collagen, gelatin and agarose. | Pneumatic-aided micro-molding and physical gelation at 37 °C (collagen, gelatin) and 4 °C (agarose) | 3D liver microtissue composed of a radially orchestrated network of hepatic cords and sinusoids |
| [46] | Methacrylic-gelatin based microgels covered by a secondary hydrogel overlay. | Multilayer printing technique and crosslinking by ultraviolet irradiation | Study of mutual influence on proliferation and migration in a co-culture system |
| [47] | Alginate | Chemical cross-linking within a flow-focusing microfluidic device | Pairing of single cells using multi-compartmental microgels for the study of cell-cell interactions |
| [48] | Collagen and gelatin | Electrostatic droplet method (collagen) and double emulsion (gelatin), using chemical and physical cross-linking, respectively | A biomimetic construction of bone tissue was realized using functional modules that mimic the osteon-like structures |
| Ref. | Polymer | Synthesis Method | Application |
|---|---|---|---|
| [16] | TG-PEG hydrogel (polyethylene glycol precursors crosslinked by the transglutaminase FXIII) | Droplet microfluidics and enzymatic crosslinking | In vitro mimicking of stem cell niches (microniches) |
| [51] | Tyramine-conjugated polymers (dextran, hyaluronic acid) | Droplet microfluidics and enzymatic crosslinking | Preventing cell escape by cell centering to enable long-term culture and differentiation of stem cells |
| [53] | Alginate microgels | Droplet microfluidics and chemical crosslinking | Treatment of bone defects: osteogenesis and mineralization |
| [54] | Gelatin methacryloyl (GelMA) | Droplet microfluidics and gelation through ultraviolet irradiation | High-throughput analysis of single cells |
| Ref. | Polymer | Synthesis Method | Application |
|---|---|---|---|
| [59] | Natural or synthetic hydrogel | Bioprinting, photolithography, microcontact printing, microfluidics and chemical and photo-crosslinking | To model in vitro early stages of embryogenesis and gastrulation |
| [57] | GelMA/PEG | Combination of micromolding and photolithography techniques and photo-crosslinking | Polarization of individual embryoid bodies (EBs) with spatially patterned vasculogenic differentiation by encapsulating individual EBs inside microgels |
| [60] | PEG | Inverse suspension polymerization and chemical crosslinking | Macrophages can be encapsulated in microgel networks and polarized an inflammatory (M1) or anti-inflammatory (M2a) phenotypes |
| [58] | Gelatin/hyaluronic acid | Droplet microfluidics and crosslinking by Michael addition reaction | Mouse bone marrow mesenchymal stem cell (BMSC) proliferation, distribution and chondrogenesissyste |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argentiere, S.; Siciliano, P.A.; Blasi, L. How Microgels Can Improve the Impact of Organ-on-Chip and Microfluidic Devices for 3D Culture: Compartmentalization, Single Cell Encapsulation and Control on Cell Fate. Polymers 2021, 13, 3216. https://doi.org/10.3390/polym13193216
Argentiere S, Siciliano PA, Blasi L. How Microgels Can Improve the Impact of Organ-on-Chip and Microfluidic Devices for 3D Culture: Compartmentalization, Single Cell Encapsulation and Control on Cell Fate. Polymers. 2021; 13(19):3216. https://doi.org/10.3390/polym13193216
Chicago/Turabian StyleArgentiere, Simona, Pietro Aleardo Siciliano, and Laura Blasi. 2021. "How Microgels Can Improve the Impact of Organ-on-Chip and Microfluidic Devices for 3D Culture: Compartmentalization, Single Cell Encapsulation and Control on Cell Fate" Polymers 13, no. 19: 3216. https://doi.org/10.3390/polym13193216
APA StyleArgentiere, S., Siciliano, P. A., & Blasi, L. (2021). How Microgels Can Improve the Impact of Organ-on-Chip and Microfluidic Devices for 3D Culture: Compartmentalization, Single Cell Encapsulation and Control on Cell Fate. Polymers, 13(19), 3216. https://doi.org/10.3390/polym13193216






