Fabrication of Eco-Friendly Polyelectrolyte Membranes Based on Sulfonate Grafted Sodium Alginate for Drug Delivery, Toxic Metal Ion Removal and Fuel Cell Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Polyelectrolyte Membranes (PEMs)
2.3. Swelling Studies
2.4. 5-Fluorouracil (5FU) Encapsulation and Release Studies
2.5. Sorption Studies of Cu(II) Ions
2.6. Ion Exchange Capacity, Oxidative Stability, Proton Conductivity and Methanol Permeability Studies
2.7. Characterization
3. Results and Discussion
3.1. Synthesis of Sulfonate Grafted Sodium Alginates
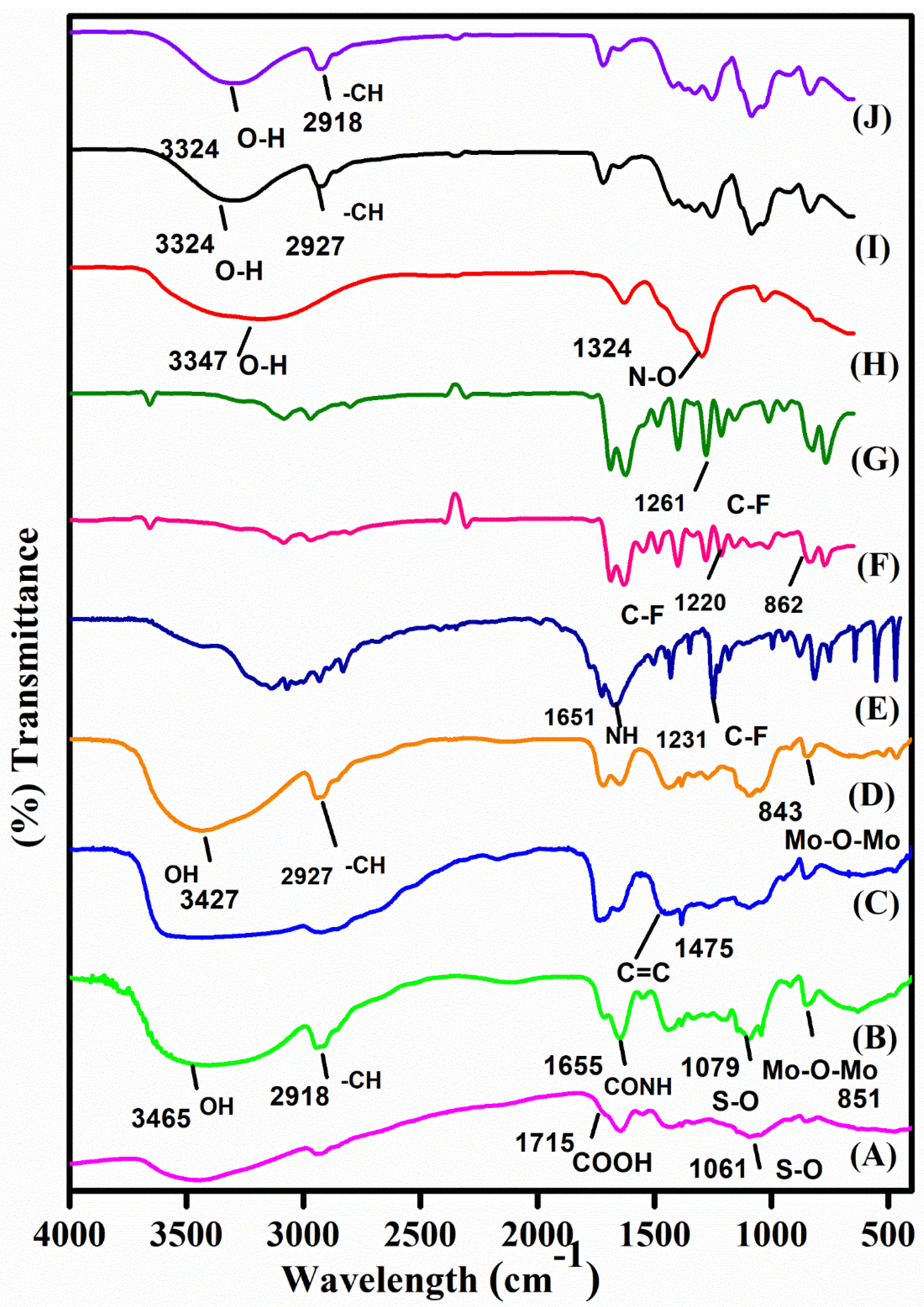
3.2. FTIR Studies
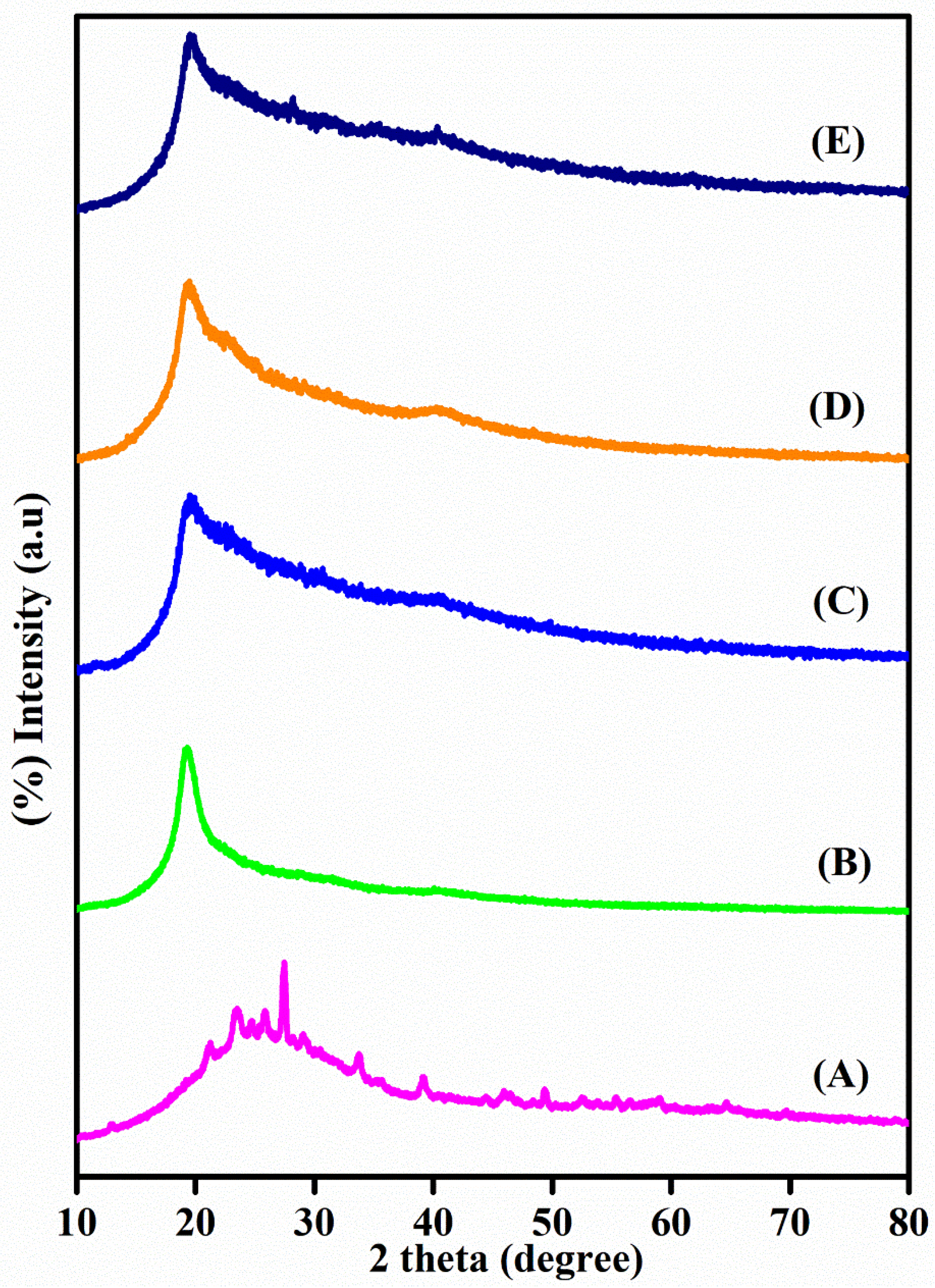
3.3. XRD Studies
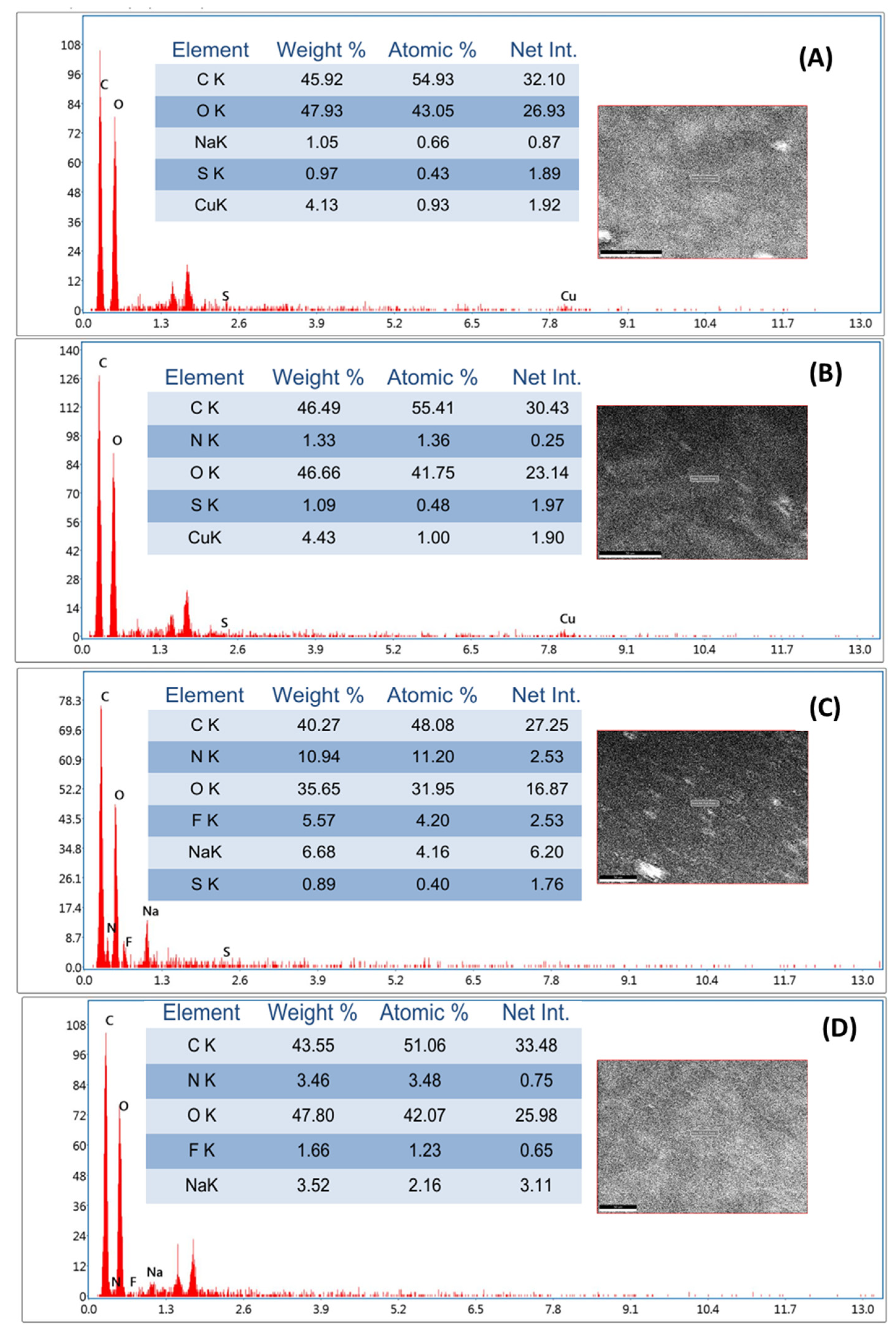
3.4. SEM and EDAX Studies
3.5. Equilibrium Swelling Studies
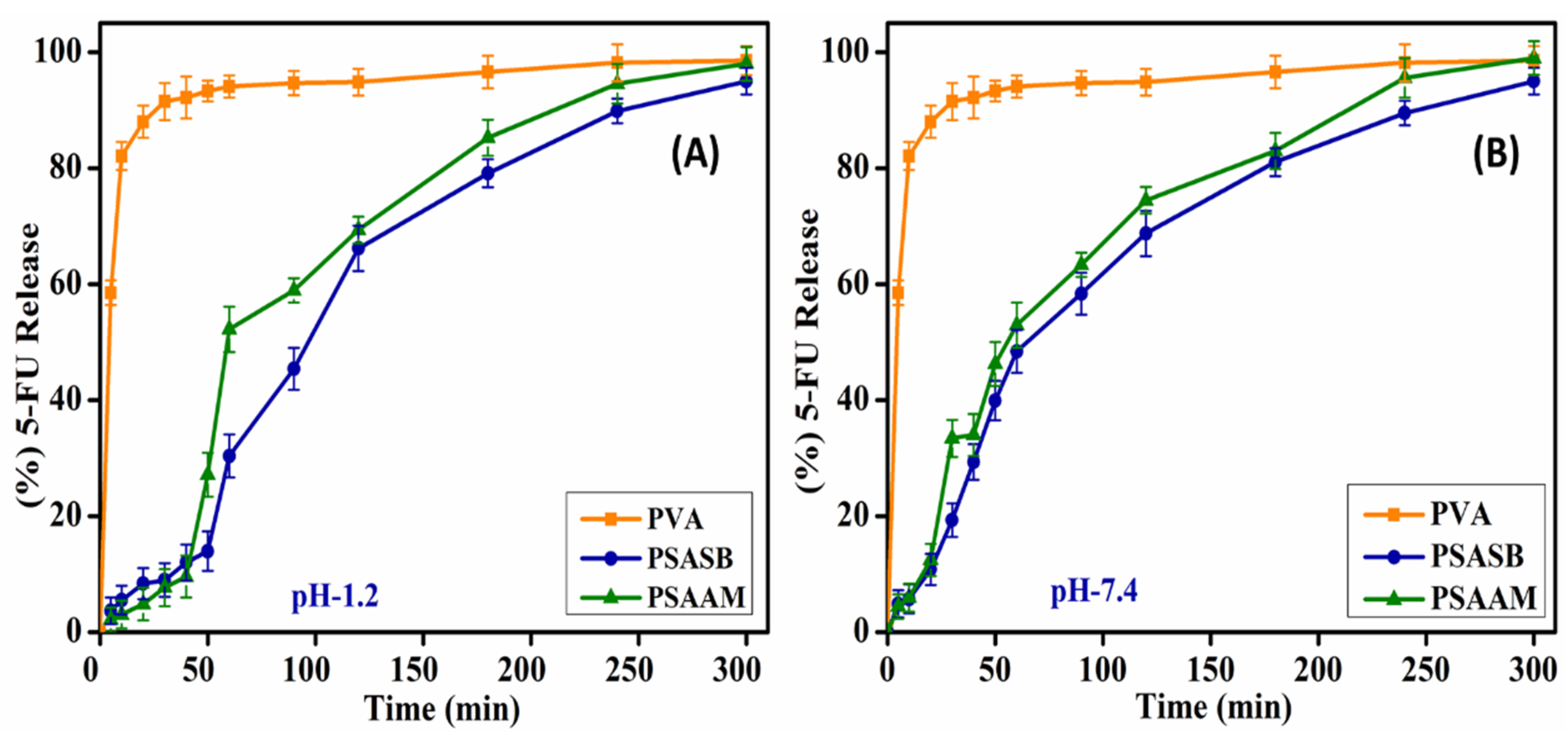
3.6. 5-Fluorouracil Drug Delivery
3.7. Copper(II) Ion Removal
3.8. Ion Exchange Capacity, Oxidative Stability Proton Conductivity and Methanol Permeability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klitzing, R.V.; Tieke, B. Polyelectrolyte membranes. Adv. Polym. Sci. 2004, 165, 177–210. [Google Scholar]
- Anirudhan, T.S.; Nair, S.S. Deposition of gold-cellulose hybrid nanofiller on a polyelectrolyte membrane constructed using guar gum and poly(vinyl alcohol) for transdermal drug delivery. J. Membr. Sci. 2017, 539, 344–357. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Vani, T.J.S.; Reddy, N.S.; Rao, K.S.V.K.; Popuri, S.R. Development of novel blend membranes based on carbohydrate polymers for the removal of toxic metal ions through sorption. Desalin. Water Treat. 2016, 57, 25729–25738. [Google Scholar] [CrossRef]
- Zhao, Q.; An, Q.F.; Ji, Y.; Qian, J.; Gao, C. Polyelectrolyte complex membranes for pervaporation, nanofiltration and fuel cell applications. J. Membr. Sci. 2011, 379, 19–45. [Google Scholar] [CrossRef]
- Makinouchi, T.; Tanaka, M.; Kawakami, H. Improvement in characteristics of a Nafion membrane by proton conductive nanofibers for fuel cell applications. J. Membr. Sci. 2017, 530, 65–72. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 2008, 22, 169–176. [Google Scholar] [CrossRef]
- Mishra, R.; Maiti, T.K.; Bhattacharyya, T.K. Feasibility studies on Nafion membrane actuated micropump integrated with hollow microneedles for insulin delivery device. J. Microelectromech. Syst. 2019, 28, 987–996. [Google Scholar] [CrossRef]
- Sharma, D.K.; Li, F.; Wu, Y.-N. Electrospinning of Nafion and polyvinyl alcohol into nanofiber membranes: A facile approach to fabricate functional adsorbent for heavy metals. Colloids Surf. A Physicochem. Eng. Aspects 2014, 457, 236–243. [Google Scholar] [CrossRef]
- Olejnik, A.; Karczewski, J.; Dołęga, A.; Siuzdak, K.; Grochowska, K. Novel approach to interference analysis of glucose sensing materials coated with Nafion. Bioelectrochemistry 2020, 135, 107575. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O. Comparison of alternative membranes to replace high cost Nafion ones in microbial fuel cells. Int. J. Hydrogen Energy 2016, 41, 23354–23362. [Google Scholar] [CrossRef]
- Mukoma, P.; Jooste, B.R.; Vosloo, H.C.M. A comparison of methanol permeability in Chitosan and Nafion 117 membranes at high to medium methanol concentrations. J. Membr. Sci. 2004, 243, 293–299. [Google Scholar] [CrossRef]
- Eswaramma, S.; Rao, K.S.V.K. Synthesis of dual responsive carbohydrate polymer based IPN microbeads for controlled release of anti-HIV drug. Carbohydr. Polym. 2017, 156, 125–134. [Google Scholar] [CrossRef]
- Gao, C.; Liu, M.; Chen, J.; Zhang, X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym. Degrad. Stab. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Jabeen, S.; Chat, O.A.; Maswal, M.; Ashraf, U.; Rather, G.M.; Dar, A.A. Hydrogels of sodium alginate in cationic surfactants: Surfactant dependent modulation of encapsulation/release toward Ibuprofen. Carbohyd. Polym. 2015, 133, 144–153. [Google Scholar] [CrossRef]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohyd. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef]
- Chahibakhsh, N.; Hosseini, E.; Islam, M.S.; Rahbar, A.R. Bitter almond gum reduces body mass index, serum triglyceride, hyperinsulinemia and insulin resistance in overweight subjects with hyperlipidemia. J. Funct. Foods 2019, 55, 343–351. [Google Scholar] [CrossRef]
- Reddy, N.S.; Vijitha, R.; Rao, K.S.V.K. Polymer electrolyte membranes for fuel cell and drug delivery applications. In Advances in Chemical Science & Biotechnology for Water Purification, Energy Production and Stress Management; KROS Publications: Andhra Pradesh, India, 2021; Volume 1, pp. 95–134. [Google Scholar]
- Rao, K.S.V.K.; Chung, I.; Ha, C.-S. Synthesis and characterization of poly(acrylamidoglycolic acid) grafted onto chitosan and its polyelectrolyte complexes with hydroxyapatite. React. Funct. Polym. 2008, 68, 943–953. [Google Scholar]
- Liwei, J.I.N.; Qi, H.; Gu, X.; Zhang, X.; Zhang, Y.; Zhang, X.; Mao, S. Effect of sodium alginate type on drug release from chitosan-sodium alginate–based in situ film-forming tablets. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Ghaffar, A.; Huang, Q.; Zafar, M.S.; Usman, M.; Latif, M. Controlled-release behavior of ciprofloxacin from a biocompatible polymeric system based on sodium alginate/poly(ethylene glycol) mono methyl ether. Int. J. Biol. Macromol. 2020, 165, 1047–1054. [Google Scholar] [CrossRef]
- Hanafiah, M.M.; Hashim, N.A.; Ahmed, S.T.; Muhammad, A.A. Removal of chromium from aqueous solutions using a palm kernel shell adsorbent. Desalin. Water Treat. 2018, 18, 172–180. [Google Scholar] [CrossRef]
- Al-Raad, A.A.; Hanafiah, M.M.; Naje, A.S.; Ajeel, M.A. Optimized parameters of the electrocoagulation process using a novel reactor with rotating anode for saline water treatment. Environ. Pollut. 2020, 265, 115049. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nizam, N.U.; Mohd Hanafiah, M.; Mohd Noor, I.; Abd Karim, H.I. Efficiency of five selected aquatic plants in phytoremediation of aquaculture wastewater. Appl. Sci. 2020, 10, 2712. [Google Scholar] [CrossRef] [Green Version]
- Halim, A.A.; Han, K.K.; Hanafiah, M.M. Removal of methylene blue from dye wastewater using river sand by adsorption. Nat. Environ. Pollut. Technol. 2015, 14, 89–94. [Google Scholar]
- Amira‘Ainaa’Idris, S.; Hanafiah, M.M.; Khan, M.F.; Abd Hamid, H.H. Indoor generated PM2.5 compositions and volatile organic compounds: Potential sources and health risk implications. Chemosphere 2020, 255, 126932. [Google Scholar]
- Reddy, N.S.; Eswaramma, S.; Rao, K.S.V.K.; Reddy, A.V.R.; Ramkumar, J. Development of hybrid hydrogel networks from poly(acrylamide-co-acrylamido glycolic acid)/cloisite sodium for adsorption of methylene blue. Ind. J. Adv. Chem. Sci. 2014, 2, 107–110. [Google Scholar]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Industr. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Reddy, N.S.; Rao, K.M.; Vani, T.J.S.; Rao, K.S.V.K.; Lee, Y.I. Pectin/poly(acrylamide-co-acrylamidoglycolic acid) pH sensitive semi-IPN hydrogels: Selective removal of Cu2+ and Ni2+, modeling, and kinetic studies. Desalin. Water Treat. 2016, 57, 6503–6514. [Google Scholar] [CrossRef]
- Vani, T.J.S.; Reddy, N.S.; Reddy, P.R.; Rao, K.S.V.K.; Ramkumar, J.; Reddy, A.V.R. Synthesis, characterization, and metal uptake capacity of a new polyaniline and poly(acrylic acid) grafted sodium alginate/gelatin adsorbent. Desalin. Water Treat. 2014, 52, 526–535. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, F.; Wu, Y.; Xu, R.; Li, G. Preparation of novel poly(vinyl alcohol)/SiO2 composite nanofiber membranes with mesostructure and their application for removal of Cu2+ from waste water. Chem. Commun. 2010, 46, 1694–1696. [Google Scholar] [CrossRef]
- Mahapatra, A.; Mishra, B.G.; Hota, G. Electrospun Fe2O3–Al2O3 nanocomposite fibers as efficient adsorbent for removal of heavy metal ions from aqueous solution. J. Hazard. Mater. 2013, 258, 116–123. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Shahi, V.K. Sulfonated poly(styrene-co-maleic anhydride)−poly(ethylene glycol)−silica nanocomposite polyelectrolyte membranes for fuel cell applications. J. Phys. Chem. B 2007, 111, 12454–12461. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton conductivity of composite polyelectrolyte membranes with metal–organic frameworks for fuel cell applications. Adv. Mater. Interfaces 2019, 6, 1801146. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Kabiri, K.; Mokarram, N.; Solati-Hashjin, M.; Moaddel, H. Novel high-performance nanohybrid polyelectrolyte membranes based on bio-functionalized montmorillonite for fuel cell applications. Chem. Commun. 2010, 46, 6500–6502. [Google Scholar] [CrossRef] [PubMed]
- Eldin, M.S.M.; Hashem, A.E.; Tamer, T.M.; Omer, A.M.; Yossuf, M.E.; Sabet, M.M. Development of cross linked chitosan/alginate polyelectrolyte proton exchanger membranes for fuel cell applications. Int. J. Electrochem. Sci. 2017, 12, 3840–3858. [Google Scholar] [CrossRef]
- Nasirinezhad, M.; Ghaffarian, S.R.; Tohidian, M. Eco-friendly polyelectrolyte nanocomposite membranes based on chitosan and sulfonated chitin nanowhiskers for fuel cell applications. Iran. Polym. J. 2021, 30, 355–367. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sour. 2015, 289, 71–80. [Google Scholar] [CrossRef]
- Santamaria, M.; Pecoraro, C.M.; Quarto, F.D.; Bocchetta, P. Chitosan–phosphotungstic acid complex as membranes for low temperature H2–O2 fuel cell. J. Power Sour. 2015, 276, 189–194. [Google Scholar] [CrossRef]
- Kourasi, M.; Wills, R.G.A.; Shah, A.A.; Walsh, F.C. Heteropolyacids for fuel cell applications. Electrochim. Acta 2014, 127, 454–466. [Google Scholar] [CrossRef]
- Ponce, M.L.; de A. Prado, L.A.S.; Silva, V.; Nunes, S.P. Membranes for direct methanol fuel cell based on modified heteropolyacids. Desalination 2004, 162, 383–391. [Google Scholar] [CrossRef]
- Rao, K.S.V.K.; Rao, K.M. A review on Radical Polymerization Used for Design and Development of Biomaterials. In Radical Polymerization: New Developments; Nova Science Publishers Inc.: New York, NY, USA, 2012; Volume 1, pp. 175–198. [Google Scholar]
- Prasad, S.S.; Rao, K.M.; Reddy, P.R.S.; Reddy, N.S.; Rao, K.S.V.K.; Subha, M.C.S. Synthesis and characterisation of guar gum-g-poly(acrylamidoglycolic acid) by redox initiator. Ind. J. Adv. Chem. Sci. 2012, 1, 28–32. [Google Scholar]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Nagar, H.; Rao, V.V.B.; Sridhar, S. Synthesis and characterization of Torlon-based polyion complex for direct methanol and polymer electrolyte membrane fuel cells. J. Mater. Sci. 2017, 52, 8052–8069. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Basri, S.; Shyuan, L.K.; Masdar, M.S.; Nordin, D. Enhanced mechanical flexibility and performance of sodium alginate polymer electrolyte bio-membrane for application in direct methanol fuel cell. J. Appl. Polym. Sci. 2018, 135, 46666. [Google Scholar] [CrossRef]
- Bary, E.M.A.; Soliman, Y.A.; Fekri, A.; Harmal, A.N. Aging of novel membranes made of PVA and cellulose nanocrystals extracted from Egyptian rice husk manufactured by compression moulding process. Int. J. Environ. Stud. 2018, 75, 750–762. [Google Scholar] [CrossRef]
- Bhat, A.H.; Bhat, I.U.H.; Khalil, H.A.S.A.; Mishra, R.K.; Datt, M.; Banthia, A.K. Development and material properties of chitosan and phosphomolybdic acid-based composites. J. Compos. Mater. 2011, 45, 39–49. [Google Scholar] [CrossRef]
- Guo, R.; Hu, C.; Pan, F.; Wu, H.; Jiang, Z. PVA–GPTMS/TEOS hybrid pervaporation membrane for dehydration of ethylene glycol aqueous solution. J. Membr. Sci. 2006, 281, 454–462. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, L.; Xue, Z.; Yusef, K.K.; Hu, H.; Wu, M. Adsorption of Cu (II) and Cd (II) from wastewater by sodium alginate modified materials. J. Chem. 2020, 2020, 5496712. [Google Scholar] [CrossRef]
- Li, Y.; Xia, B.; Zhao, Q.; Liu, F.; Zhang, P.; Du, Q.; Wang, D.; Li, D.; Wang, Z.; Xia, Y. Removal of copper ions from aqueous solution by calcium alginate immobilized kaolin. J. Environ. Sci. 2011, 23, 404–411. [Google Scholar] [CrossRef]
- Yang, N.; Wang, R.; Rao, P.; Yan, L.; Zhang, W.; Wang, J.; Chai, F. The fabrication of calcium alginate beads as a green sorbent for selective recovery of Cu(II) from metal mixtures. Crystals 2019, 9, 255. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-L.; Thirumavalavan, M.; Lee, J.-F. Effective adsorption of heavy metal ions (Cu2+, Pb2+, Zn2+) from aqueous solution by immobilization of adsorbents on Ca-alginate beads. Toxicol. Environ. Chem. 2010, 92, 697–705. [Google Scholar] [CrossRef]
- Petrovič, A.; Simonič, M. Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana. Int. J. Environ. Sci. Technol. 2016, 13, 1761–1780. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Shin, S.H.; Chang, I.S.; Moon, S.H. Characterization of uncharged and sulfonated porous poly (vinylidene fluoride) membranes and their performance in microbial fuel cells. J. Membr. Sci. 2014, 463, 205–214. [Google Scholar] [CrossRef]
- Chen, G.; Wei, B.; Luo, Y.; Logan, B.E.; Hickner, M.A. Polymer separators for high-power, high-efficiency microbial fuel cells. ACS Appl. Mater. Interfaces 2012, 4, 6454–6457. [Google Scholar] [CrossRef]
- Misono, M. Heterogeneous catalysis by heteropoly compounds of molybdenum and tungsten. Catal. Rev. Sci. Eng. 1987, 29, 269–321. [Google Scholar] [CrossRef]
- Bielański, A.; Micek-Ilnicka, A. Kinetics and mechanism of gas phase MTBE and ETBE formation on Keggin and Wells–Dawson heteropolyacids as catalysts. Inorg. Chim. Acta 2010, 363, 4158–4162. [Google Scholar] [CrossRef]


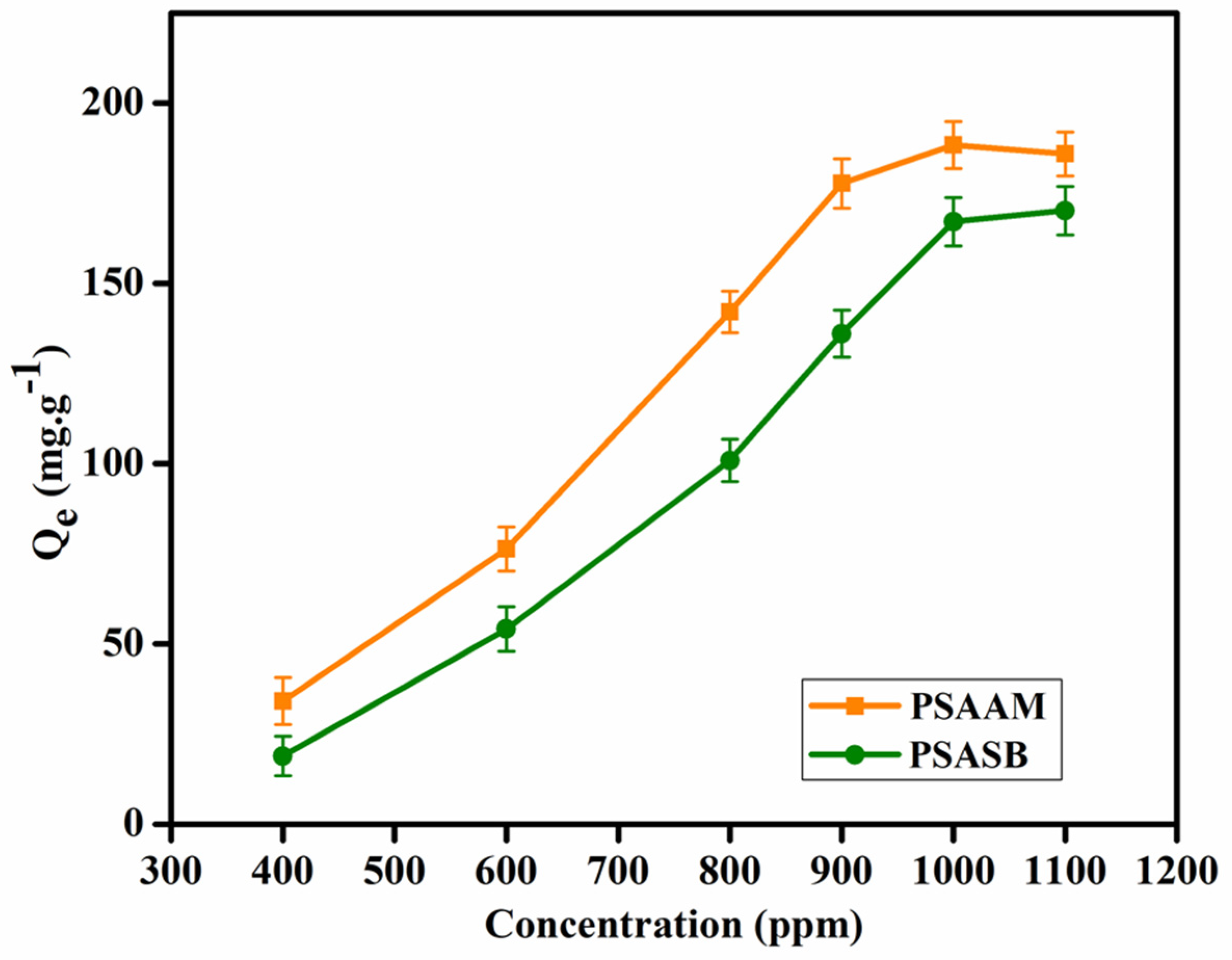
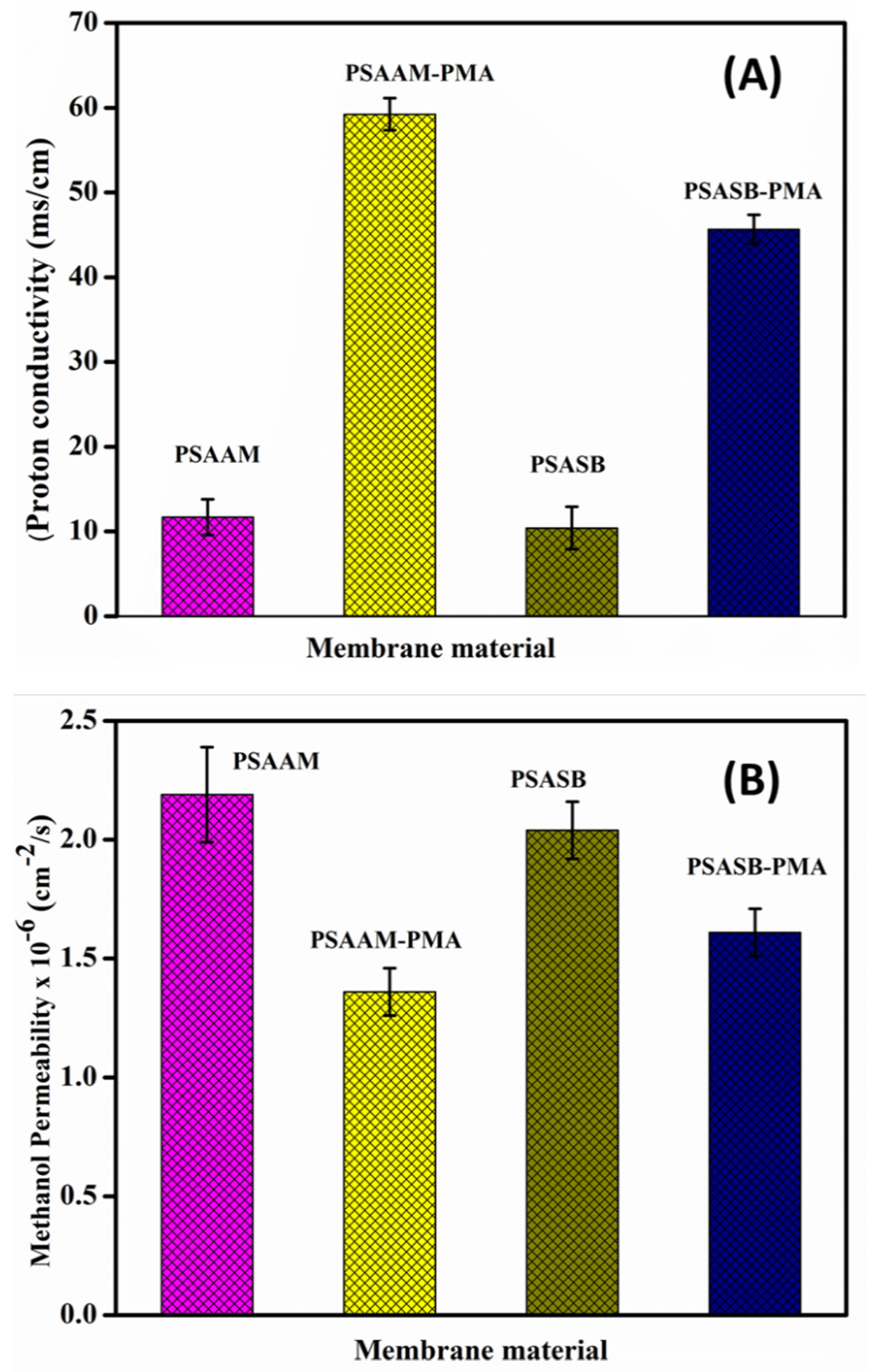
| Type of Membrane | PVA (g) | SA-g-AMPS/SA-g-SVBS (g) | PMA (g) | %EE of 5FU | Qe mg.g–1 | %Se | IEC | Oxidative Stability (RW%) | Proton Conductivity (mS/cm) | Methanol Permeability (cm2/s) (10−6) |
|---|---|---|---|---|---|---|---|---|---|---|
| PSAAM | 3 | 2 | 0.5 | 78 ± 2.3 | 188.91 ± 6.1 | 369 ± 6.3 | 0.64 ± 0.09 | 79 ± 1.5 | 11.69 ± 2.1 | 2.19 ± 0.22 |
| PSAAM-PMA | 3 | 2 | 0.5 | -- | -- | 300 ± 4.9 | 0.69 ± 0.12 | 93 ± 2.1 | 59.23 ± 1.9 | 1.36 ± 0.12 |
| PSASB | 3 | 2 | 0.5 | 66 ± 4.7 | 181.22 ± 6.7 | 258 ± 7.1 | 0.57 ± 0.11 | 82 ± 3.4 | 10.41 ± 2.5 | 2.04 ± 0.12 |
| PSASB-PMA | 3 | 2 | 0.5 | -- | -- | 169 ± 4.8 | 0.84 ± 0.16 | 94 ± 2.2 | 45.66 ± 1.7 | 1.61 ± 0.14 |
| Sl. No. | Name of the Polymer | Qe (mg.g–1) | Ref. |
|---|---|---|---|
| 1. | Sodium alginate-polyethylene glycol oxide-nano materials | 6.78 | [51] |
| 2. | Sodium alginate-gelatin | 43.51 | [4] |
| 3. | Polyaniline and poly(acrylic acid)-g-sodium alginate/gelatin | 53.29 | [31] |
| 4. | Calcium alginate immobilized kaolin | 53.63 | [52] |
| 5. | Sodium alginate | 140.55 | [53] |
| 6. | Calcium alginate (CA) immobilized range peel cellulose (OPC), banana peel cellulose (BPC) | CA-OPC: 166.67 CA-BPC: 163.93 | [54] |
| 7. | Calcium alginate-immobilized Chlorella Sorokiniana | 179.90 | [55] |
| 8. | PSASB | 181.22 | Present work |
| 9. | PSAAM | 188.91 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijitha, R.; Nagaraja, K.; Hanafiah, M.M.; Rao, K.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Rao, K.S.V.K. Fabrication of Eco-Friendly Polyelectrolyte Membranes Based on Sulfonate Grafted Sodium Alginate for Drug Delivery, Toxic Metal Ion Removal and Fuel Cell Applications. Polymers 2021, 13, 3293. https://doi.org/10.3390/polym13193293
Vijitha R, Nagaraja K, Hanafiah MM, Rao KM, Venkateswarlu K, Lakkaboyana SK, Rao KSVK. Fabrication of Eco-Friendly Polyelectrolyte Membranes Based on Sulfonate Grafted Sodium Alginate for Drug Delivery, Toxic Metal Ion Removal and Fuel Cell Applications. Polymers. 2021; 13(19):3293. https://doi.org/10.3390/polym13193293
Chicago/Turabian StyleVijitha, Raagala, Kasula Nagaraja, Marlia M. Hanafiah, Kummara Madhusudana Rao, Katta Venkateswarlu, Sivarama Krishna Lakkaboyana, and Kummari S. V. Krishna Rao. 2021. "Fabrication of Eco-Friendly Polyelectrolyte Membranes Based on Sulfonate Grafted Sodium Alginate for Drug Delivery, Toxic Metal Ion Removal and Fuel Cell Applications" Polymers 13, no. 19: 3293. https://doi.org/10.3390/polym13193293









