Characterization, Bioactivity and Application of Chitosan-Based Nanoparticles in a Food Emulsion Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Chitosan Nanoparticles
2.3. Nanoparticle Characterization
2.4. Entrapment Efficiency
2.5. ABTS Radical Scavenging Activity
2.6. Antimicrobial Activity
2.7. ACE-Inhibitory Capacity
2.8. Morphology
2.9. Application in a Food Emulsion Model
2.9.1. Stability and Lipid Release
2.9.2. Rheological Analyses
2.10. Statistical Analysis
3. Results and discussion
3.1. Size, PDI and Zeta Potential
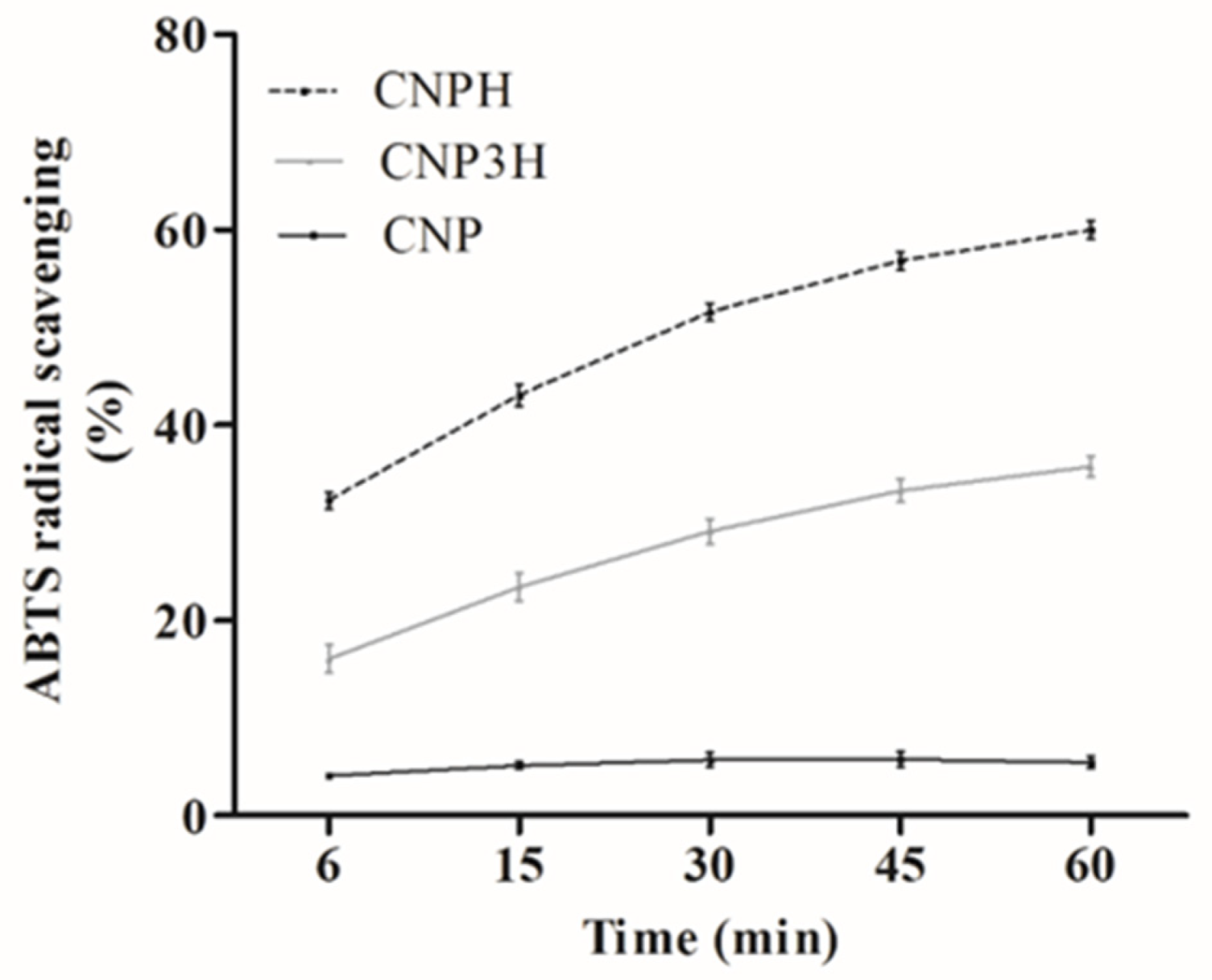
3.2. ABTS Radical Scavenging Activity
3.3. Antibacterial Activity
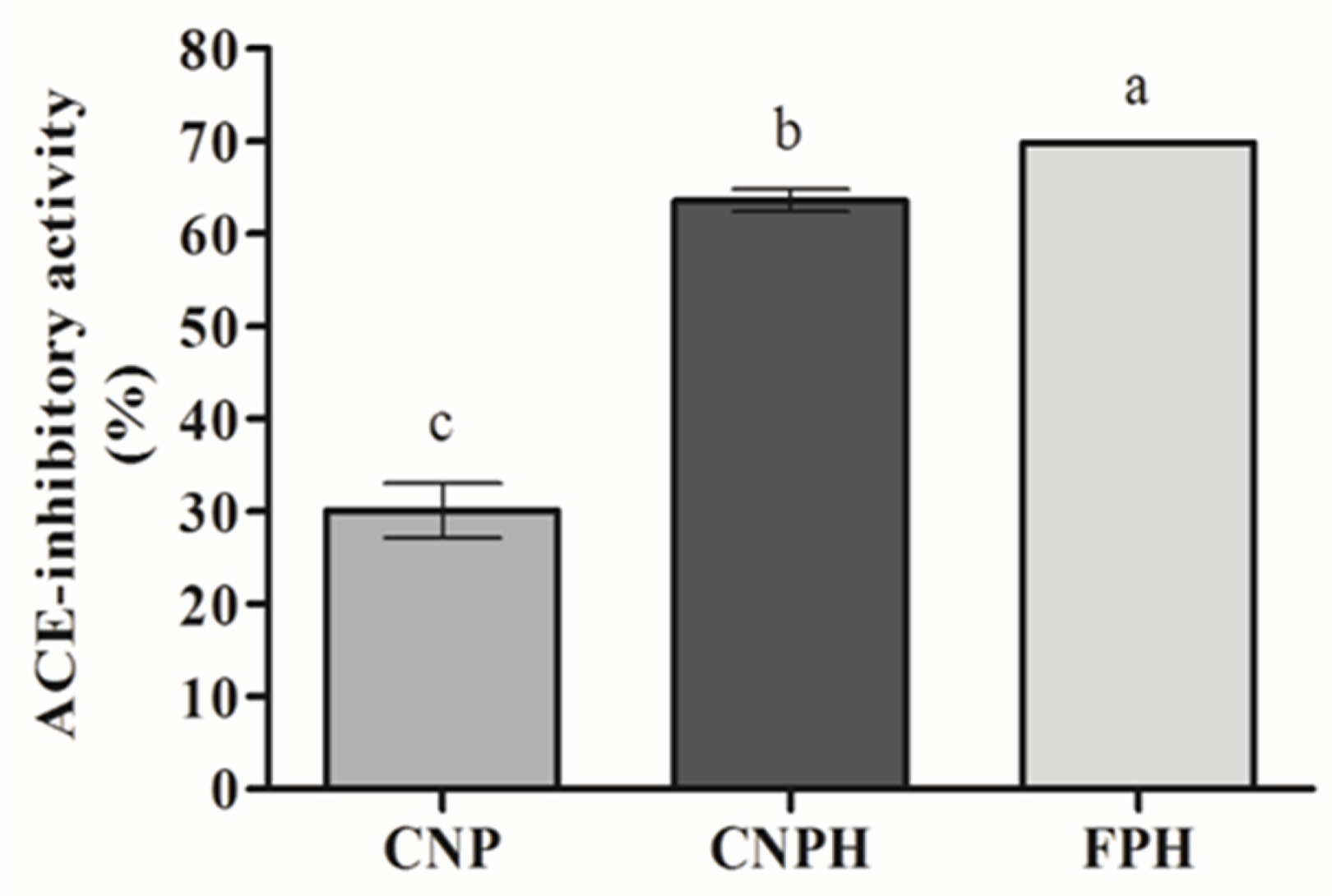
3.4. ACE-Inhibitory Activity of Nanoparticles
3.5. Morphology of Nanoparticles
3.6. Food Emulsion Model System
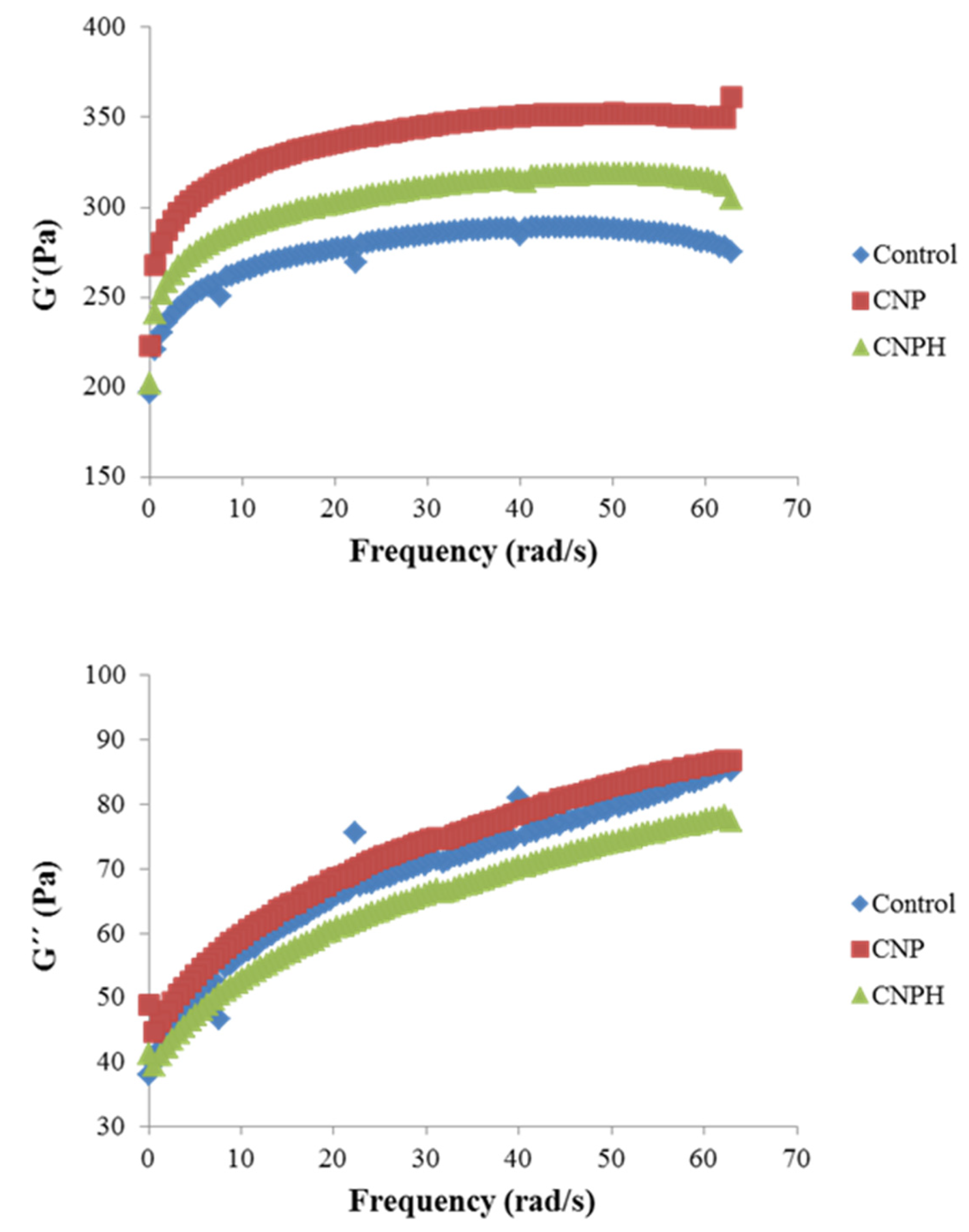
Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros Ciência Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Jancikova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active Edible Films Fortified with Natural Extracts: Case Study with Fresh-Cut Apple Pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial activity of chitosan nanoparticles: A review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Alison, L.; Rühs, P.A.; Tervoort, E.; Teleki, A.; Zanini, M.; Isa, L.; Studart, A.R. Pickering and Network Stabilization of Biocompatible Emulsions Using Chitosan-Modified Silica Nanoparticles. Langmuir ACS J. Surf. Colloids 2016, 32, 13446–13457. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Hu, Y.-Q.; Yin, S.-W.; Yang, X.-Q.; Lai, F.-R.; Wang, S.-Q. Fabrication and characterization of antioxidant pickering emulsions stabilized by zein/chitosan complex particles (ZCPs). J. Agric. Food Chem. 2015, 63, 2514–2524. [Google Scholar] [CrossRef]
- Dammak, I.; do Amaral Sobral, P.J. Investigation into the physicochemical stability and rheological properties of rutin emulsions stabilized by chitosan and lecithin. J. Food Eng. 2018, 229, 12–20. [Google Scholar] [CrossRef]
- Klinkesorn, U. The Role of Chitosan in Emulsion Formation and Stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
- Hanazawa, T.; Murray, B.S. Effect of oil droplets and their solid/liquid composition on the phase separation of protein-polysaccharide mixtures. Langmuir 2013, 29, 9841–9848. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Dammak, I.; do Amaral Sobral, P.J. Formulation optimization of lecithin-enhanced pickering emulsions stabilized by chitosan nanoparticles for hesperidin encapsulation. J. Food Eng. 2018, 229, 2–11. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- McClements, D.J. Recent advances in the production and application of nano-enabled bioactive food ingredients. Curr. Opin. Food Sci. 2020, 33, 85–90. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.A.S.; Silva, C.M.; Rosa, G.F.; Prentice, C. Peptides obtained from proteins of cobia (Rachycentron canadum): A study of potentially safe antioxidants for food. Int. Food Res. J. 2017, 24, 2500–2508. [Google Scholar]
- Latorres, J.M.; Rios, D.G.; Saggiomo, G.; Wasielesky, W.; Prentice-Hernandez, C. Functional and antioxidant properties of protein hydrolysates obtained from white shrimp (Litopenaeus vannamei). J. Food Sci. Technol. 2018, 55, 721–729. [Google Scholar] [CrossRef]
- Da Rocha, M.; Alemán, A.; Baccan, G.C.; López-Caballero, M.E.; Gómez-Guillén, C.; Montero, P.; Prentice, C. Anti-Inflammatory, Antioxidant, and Antimicrobial Effects of Underutilized Fish Protein Hydrolysate. J. Aquat. Food Prod. Technol. 2018, 27, 592–608. [Google Scholar] [CrossRef]
- Ug, Y.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- Lima, K.O.; de Quadros, C.D.C.; da Rocha, M.; de Lacerda, J.T.J.G.; Juliano, M.A.; Dias, M.; Mendes, M.A.; Prentice, C. Bioactivity and bioaccessibility of protein hydrolyzates from industrial byproducts of Stripped weakfish (Cynoscion guatucupa). LWT—Food Sci. Technol. 2019, 111, 408–413. [Google Scholar] [CrossRef]
- Lima, K.O.; Alemán, A.; López-Caballero, M.E.; del Carmen Gómez-Guillén, M.; Montero, M.P.; Prentice, C.; Huisa, A.J.T.; Monserrat, J.M. Characterization, stability, and in vivo effects in Caenorhabditis elegans of microencapsulated protein hydrolysates from stripped weakfish (Cynoscion guatucupa) industrial byproducts. Food Chem. 2021, 364, 130380. [Google Scholar] [CrossRef]
- Fernández-Martín, F.; Arancibia, M.; López-Caballero, E.; Gómez-Guillén, C.; Montero, P.; Fernández-García, M. Preparation and Molecular Characterization of Chitosans Obtained from Shrimp (Litopenaeus vannamei) Shells. J. Food Sci. 2014, 79, E1722–E1731. [Google Scholar] [CrossRef]
- Piras, A.M.; Sandreschi, S.; Maisetta, G.; Esin, S.; Batoni, G.; Chiellini, F. Chitosan Nanoparticles for the Linear Release of Model Cationic Peptide. Pharm. Res. 2015, 32, 2259–2265. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Soleimani, M.R.; Nikkhah, M. Chitosan/sodium tripolyphosphate nanoparticles as efficient vehicles for antioxidant peptidic fraction from common kilka. Int. J. Biol. Macromol. 2018, 111, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef]
- Kasiri, N.; Fathi, M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, M.R.; Fathi, M. Production and characterization of chitin nanocrystals from prawn shell and their application for stabilization of Pickering emulsions. Food Hydrocoll. 2018, 82, 338–345. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Sun, Y.; Gao, X.; Liang, J. Controlled release and antioxidant activity of chitosan and β-lactoglobulin complex nanoparticles loaded with epigallocatechin gallate. Colloids Surf. B Biointerfaces 2020, 188, 110802. [Google Scholar] [CrossRef]
- Hu, B.; Pan, C.; Sun, Y.; Hou, Z.; Ye, H.; Hu, B.; Zeng, X. Optimization of fabrication parameters to produce chitosan-tripolyphosphate nanoparticles for delivery of tea catechins. J. Agric. Food Chem. 2008, 56, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Qian, J.; Zhao, C.; Yang, H.; Zhao, X.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Liu, J.; Zhang, T.; Yu, Y.; Zhang, Y.; Zhai, J.; Huang, H.; Wei, S.; Ding, L.; Liu, B. A study on the preparation of chitosan-tripolyphosphate nanoparticles and its entrapment mechanism for egg white derived peptides. Food Chem. 2019, 286, 530–536. [Google Scholar] [CrossRef]
- Kiaie, N.; Aghdam, R.M.; Tafti, S.H.A.; Emami, S.H. Statistical optimization of chitosan nanoparticles as protein vehicles, using response surface methodology. J. Appl. Biomater. Funct. Mater. 2016, 14, e413–e422. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier-Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Yuan, D.; Jacquier, J.C.; O’Riordan, E.D. Entrapment of protein in chitosan-tripolyphosphate beads and its release in an in vitro digestive model. Food Chem. 2017, 229, 495–501. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Wei, L.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 2019, 226, 115256. [Google Scholar] [CrossRef]
- Morales-Olán, G.; Luna-Suárez, S.; Figueroa-Cárdenas, J.D.D.; Corea, M.; Rojas-López, M. Synthesis and Characterization of Chitosan Particles Loaded with Antioxidants Extracted from Chia (Salvia hispanica L.) Seeds. Int. J. Anal. Chem. 2021, 2021, 5540543. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef] [PubMed]
- Alamdaran, M.; Movahedi, B.; Mohabatkar, H.; Behbahani, M. In-vitro study of the novel nanocarrier of chitosan-based nanoparticles conjugated HIV-1 P24 protein-derived peptides. J. Mol. Liq. 2018, 265, 243–250. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications—A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kwak, H.S.; Kim, S.M. Physicochemical and Biofunctional Properties of Crab Chitosan Nanoparticles. J. Nanosci. Nanotechnol. 2013, 13, 5296–5304. [Google Scholar] [CrossRef]
- Pei, F.X.; Kim, S.M. Physicochemical and Biological Characteristics of Squid β-Chitosan Nanoparticle. Int. J. Food Eng. 2017, 3, 12–17. [Google Scholar] [CrossRef][Green Version]
- Tan, H.-F.; Gan, C.-Y. Polysaccharide with antioxidant, α-amylase inhibitory and ACE inhibitory activities from Momordica charantia. Int. J. Biol. Macromol. 2016, 85, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Wang, Z.; Chen, S.; Luo, Y. Production and identification of antioxidant and angiotensin-converting enzyme inhibition and dipeptidyl peptidase IV inhibitory peptides from bighead carp (Hypophthalmichthys nobilis) muscle hydrolysate. J. Funct. Foods 2017, 35, 224–235. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. The role of chitosan on oral delivery of peptide-loaded nanoparticle formulation. J. Drug Target. 2018, 26, 551–562. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zarei, M.; Tan, C.P.; Basri, M.; Saari, N. Enhanced physicochemical stability and efficacy of angiotensin I-converting enzyme (ACE)—Inhibitory biopeptides by chitosan nanoparticles optimized using Box-Behnken design. Sci. Rep. 2018, 8, 10411. [Google Scholar] [CrossRef]
- Danish, M.K.; Vozza, G.; Byrne, H.J.; Frias, J.M.; Ryan, S.M. Comparative study of the structural and physicochemical properties of two food derived antihypertensive tri-peptides, Isoleucine-Proline-Proline and Leucine-Lysine-Proline encapsulated into a chitosan based nanoparticle system. Innov. Food Sci. Emerg. Technol. 2017, 44, 139–148. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, W.; Wu, H.; Wu, M.; Liu, Z.; Dong, S. Chitosan/sodium tripolyphosphate nanoparticles as efficient vehicles for enhancing the cellular uptake of fish-derived peptide. J. Food Biochem. 2019, 43, e12730. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-based Pickering emulsions and their applications: A review. Carbohydr. Polym. 2020, 250, 116885. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; de Barros-Alexandrino, T.T.; Assis, O.B.G.; Junior, A.C.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Chitosan and crosslinked chitosan nanoparticles: Synthesis, characterization and their role as Pickering emulsifiers. Carbohydr. Polym. 2020, 250, 116878. [Google Scholar] [CrossRef]
- Rosalina, I.; Bhattacharya, M. Dynamic rheological measurements and analysis of starch gels. Carbohydr. Polym. 2002, 48, 191–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, W. Dipole—Dipole and H-Bonding Interactions Significantly Enhance the Multifaceted Mechanical Properties of Thermoresponsive Shape Memory Hydrogels. Adv. Funct. Mater. 2015, 25, 471–480. [Google Scholar] [CrossRef]
- Campo-Deaño, L.; Tovar, C. The effect of egg albumen on the viscoelasticity of crab sticks made from Alaska Pollock and Pacific Whiting surimi. Food Hydrocoll. 2009, 23, 1641–1646. [Google Scholar] [CrossRef]
- Borderías, A.J.; Tovar, C.A.; Domínguez-timón, F.; Díaz, M.T.; Pedrosa, M.M.; Moreno, H.M. Characterization of healthier mixed surimi gels obtained through partial substitution of myofibrillar proteins by pea protein isolates. Food Hydrocoll. 2020, 107, 105976. [Google Scholar] [CrossRef]
- Chang, C.; Li, J.; Li, X.; Wang, C.; Zhou, B.; Su, Y.; Yang, Y. Effect of protein microparticle and pectin on properties of light mayonnaise. LWT—Food Sci. Technol. 2017, 82, 8–14. [Google Scholar] [CrossRef]
- Huang, X.-N.; Zhou, F.-Z.; Yang, T.; Yin, S.-W.; Tang, C.-H.; Yang, X.-Q. Fabrication and Characterization of Pickering High Internal Phase Emulsions (HIPEs) Stabilized by Chitosan-Caseinophosphopeptides Nanocomplexes as Oral Delivery Vehicles. Food Hydrocoll. 2019, 93, 34–45. [Google Scholar] [CrossRef]
- Moreno, H.M.; Bargiela, V.; Tovar, C.A.; Cando, D.; Borderias, A.J.; Herranz, B. High pressure applied to frozen flying fish (Parexocoetus brachyterus) surimi: Effect on physicochemical and rheological properties of gels. Food Hydrocoll. 2015, 48, 127–134. [Google Scholar] [CrossRef]



| Chitosan (mg/mL) | Ultrasound/Time | Size | PdI | Zeta |
|---|---|---|---|---|
| 1 | - | 342.6 ± 1.1 c | 0.279 ± 0.01 a | 20.5 ± 1.6 c |
| 1 | 50%/2 min | 164.8 ± 0.3 d | 0.166 ± 0.02 b | 16.3 ± 0.9 d |
| 3 | 50%/2 min | 314.5 ± 6.1 c | 0.254 ± 0.01 a | 40.0 ± 1.1 b |
| 7 | 50%/2 min | 486.8 ± 14.0 b | 0.316 ± 0.04 a | 43.2 ± 1.0 a |
| 10 | 50%/2 min | 601.9 ± 29.3 a | 0.264 ± 0.04 a | 42.1 ± 0.9 ab |
| Sample | Chitosan (mg/mL) | Acetic Acid (%) | TPP (mg/mL) | Size (nm) | PdI | ζ Potential (mV) |
|---|---|---|---|---|---|---|
| CNP | 3 | 1 | 1 | 205.7 ± 6.5 bB | 0.281 ± 0.05 bA | 39.1 ± 1.6 aA |
| CNP13 | 3 | 1 | 3 | 273.6 ± 1.5 a | 0.171 ± 0.03 c | 28.1 ± 0.6 b |
| CNP3 | 3 | 3 | 3 | 264.3 ± 0.8 aA | 0.146 ± 0.01 cC | 27.3 ± 0.8 bB |
| CNP31 | 3 | 3 | 1 | 199.8 ± 7.4 b | 0.371 ± 0.02 a | 37.1 ± 1.7 a |
| CNPH * | 3 | 1 | 1 | 202.2 ± 2.4 B | 0.237 ± 0.01 AB | 37.7 ± 1.2 A |
| CNP3H * | 3 | 3 | 3 | 263.1 ± 1.9 A | 0.169 ± 0.05 BC | 26.0 ± 0.9 B |
| Sample | Inhibition Zone (mm) | ||||
|---|---|---|---|---|---|
| S. sonnei | S. aureus | A. hydrophila | E. coli | L. monocytogenes | |
| CNP | 6.37 ± 0.11 bB | 0.0 ± 0.0 | 9.69 ± 0.57 aAB | 0.0 | 0.0 |
| CNPH | 9.54 ± 1.01 bA | 18.6 ± 0.38 aA | 10.64 ± 1.14 bA | 0.0 | 0.0 |
| CNP3 | 8.40 ± 0.49 bA | 11.75 ± 1.25 aB | 0.00 ± 0.0 | 0.0 | 0.0 |
| CNP3H | 9.12 ± 0.23 aA | 7.59 ± 0.24 bC | 8.17 ± 0.53 bB | 0.0 | 0.0 |
| Control | CNP | CNPH | |
|---|---|---|---|
| G0′ (Pa) | 256.33 ± 4.94 c | 310.70 ± 12.10 a | 279.25 ± 3.69 b |
| n′ | 0.0568 ± 0.004 a | 0.0637 ± 0.001 a | 0.0639 ± 0.003 a |
| R (eq) | 0.9278 | 0.9809 | 0.9744 |
| G0″ (Pa) | 52.15 ± 1.30 ab | 55.27 ± 2.84 a | 48.91 ± 1.02 b |
| n″ | 0.1660 ± 0.007 a | 0.1441 ± 0.001 b | 0.1500 ± 0.002 b |
| R (eq) | 0.9109 | 0.8669 | 0.8804 |
| tan δ | 11.50 ± 0.06 a | 10.09 ± 0.13 b | 9.94 ± 0.08 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira Lima, K.; Barreto Pinilla, C.M.; Alemán, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Characterization, Bioactivity and Application of Chitosan-Based Nanoparticles in a Food Emulsion Model. Polymers 2021, 13, 3331. https://doi.org/10.3390/polym13193331
Oliveira Lima K, Barreto Pinilla CM, Alemán A, López-Caballero ME, Gómez-Guillén MC, Montero P, Prentice C. Characterization, Bioactivity and Application of Chitosan-Based Nanoparticles in a Food Emulsion Model. Polymers. 2021; 13(19):3331. https://doi.org/10.3390/polym13193331
Chicago/Turabian StyleOliveira Lima, Karina, Cristian Mauricio Barreto Pinilla, Ailén Alemán, M. Elvira López-Caballero, M. Carmen Gómez-Guillén, Pilar Montero, and Carlos Prentice. 2021. "Characterization, Bioactivity and Application of Chitosan-Based Nanoparticles in a Food Emulsion Model" Polymers 13, no. 19: 3331. https://doi.org/10.3390/polym13193331
APA StyleOliveira Lima, K., Barreto Pinilla, C. M., Alemán, A., López-Caballero, M. E., Gómez-Guillén, M. C., Montero, P., & Prentice, C. (2021). Characterization, Bioactivity and Application of Chitosan-Based Nanoparticles in a Food Emulsion Model. Polymers, 13(19), 3331. https://doi.org/10.3390/polym13193331







