RETRACTED: Investigation of the Ordered Structure in Partially Melted Isotactic Polypropylene
Abstract
:1. Introduction
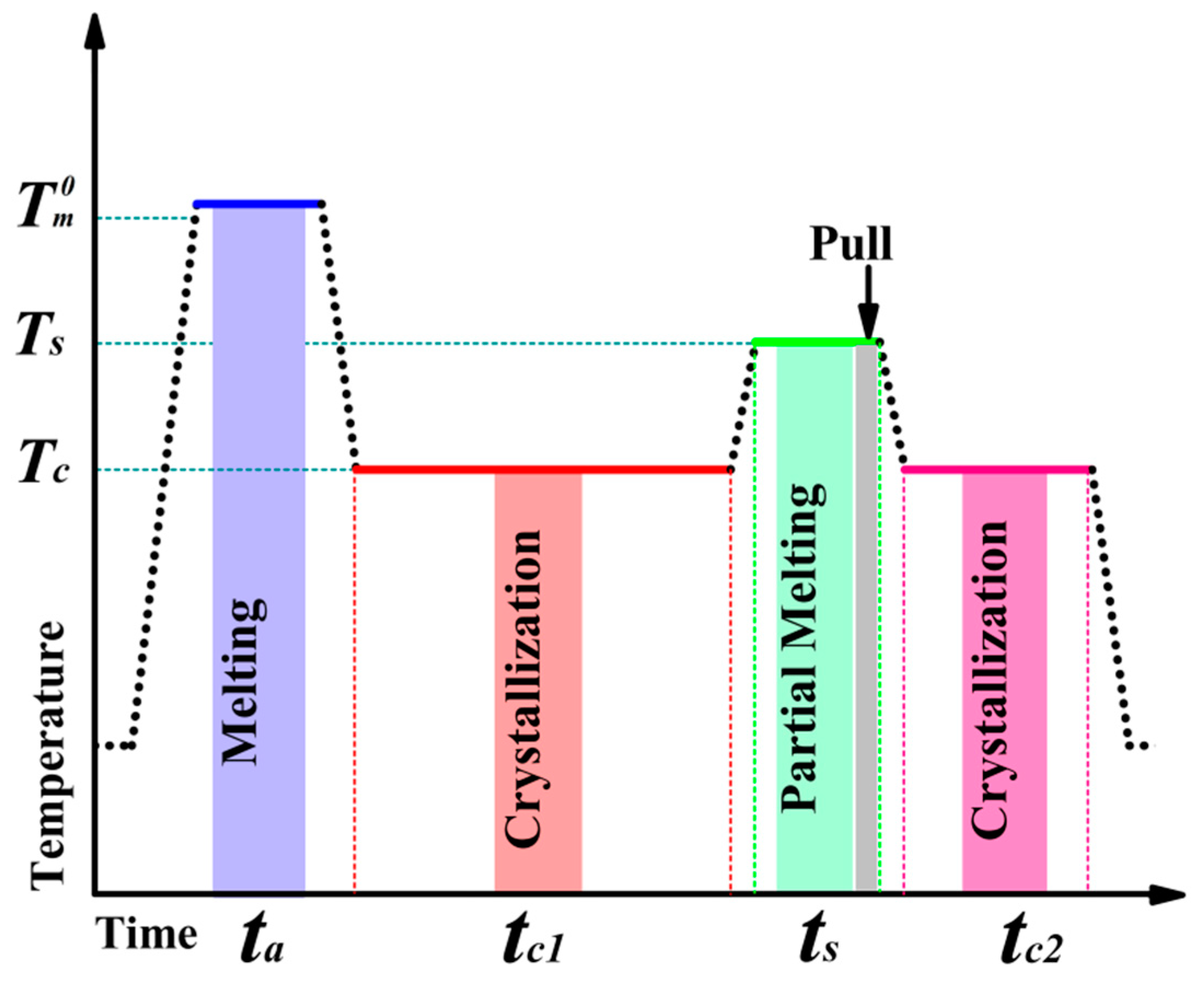
2. Materials and Methods
2.1. Material and Sample Preparation
2.2. Polarized Optical Microscopy (POM)
2.3. X-ray Measurements
2.4. SAXS and WAXS Data Analysis
2.4.1. SAXS Data Analysis
2.4.2. WAXS Data Analysis
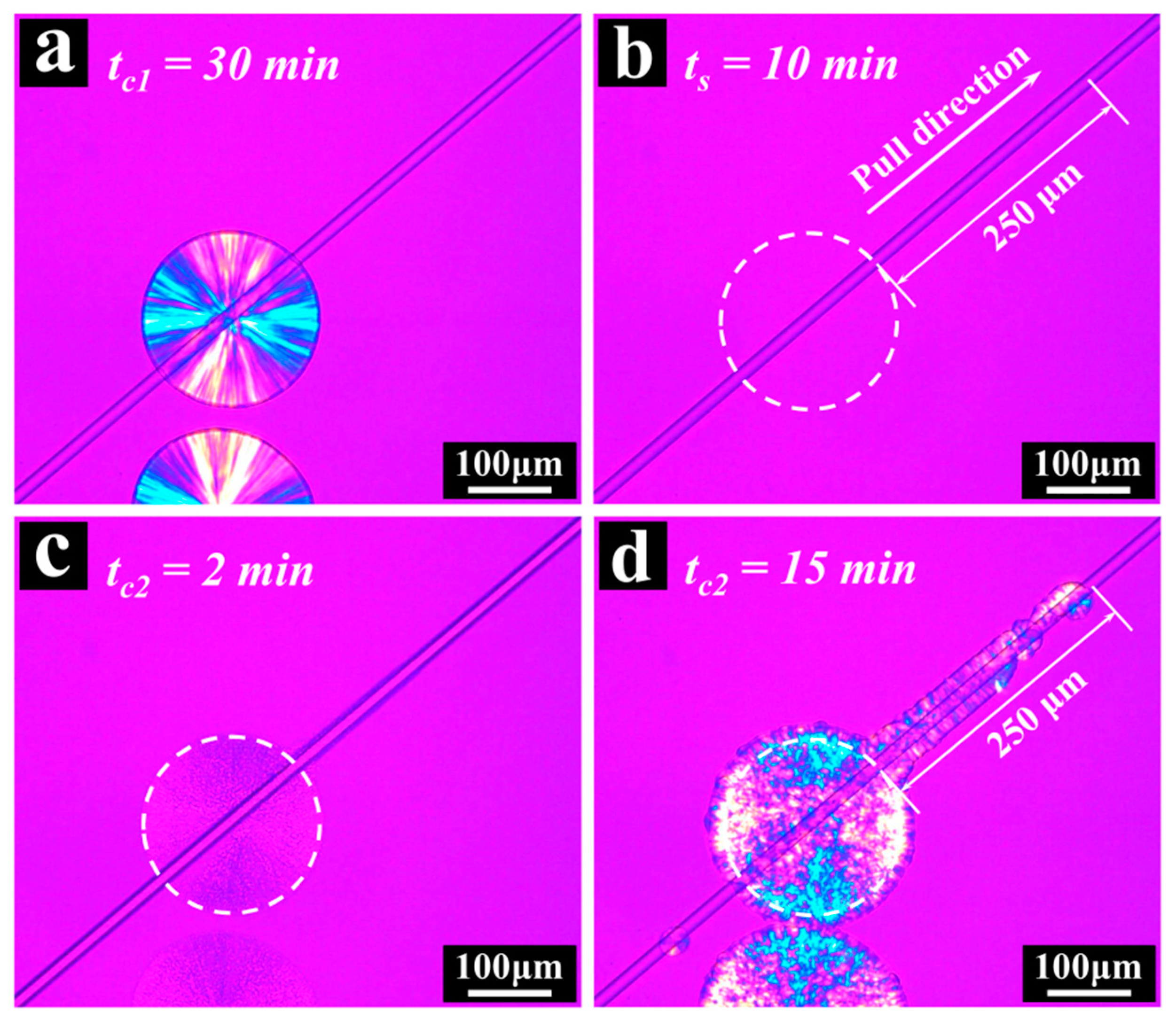
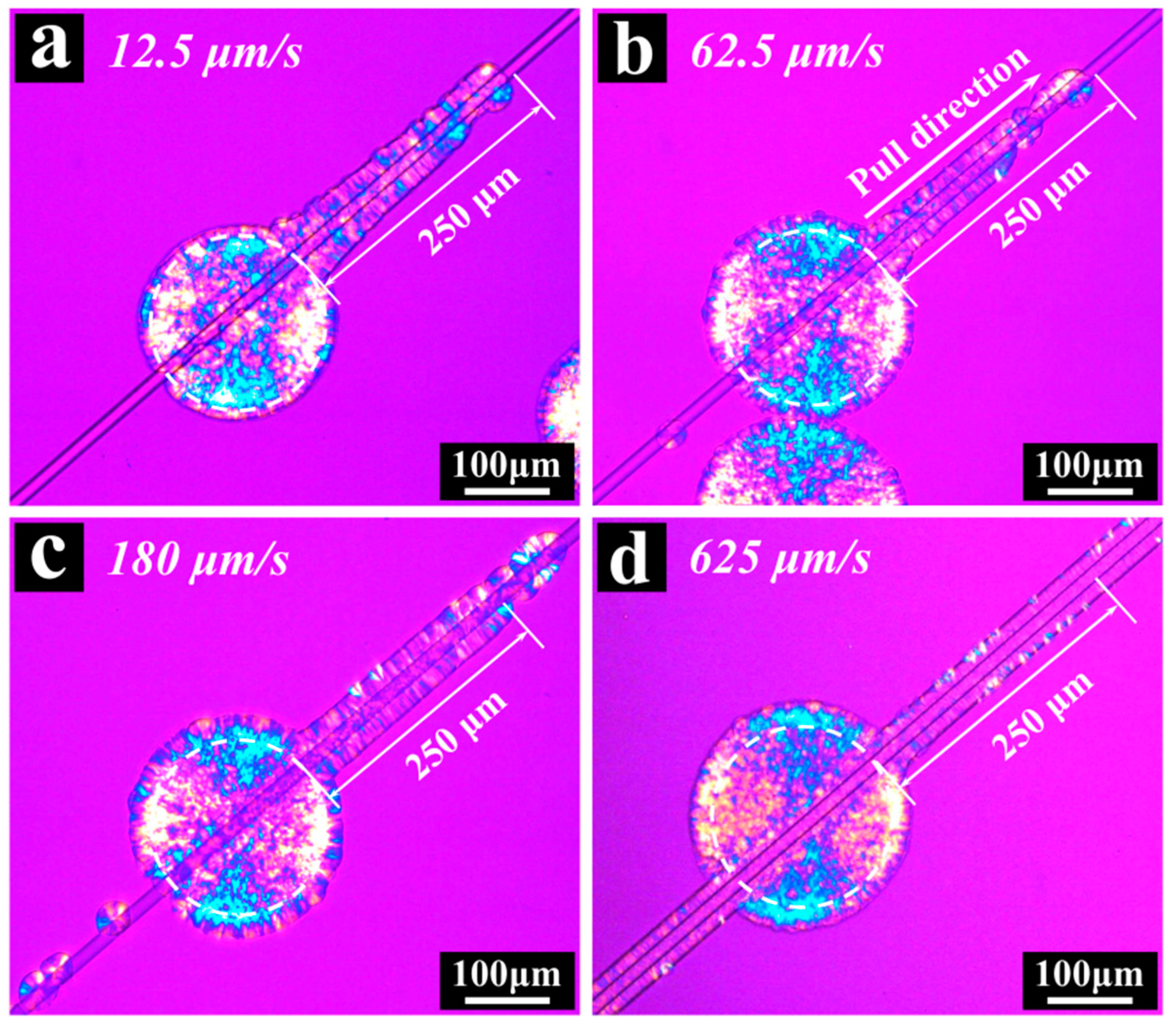
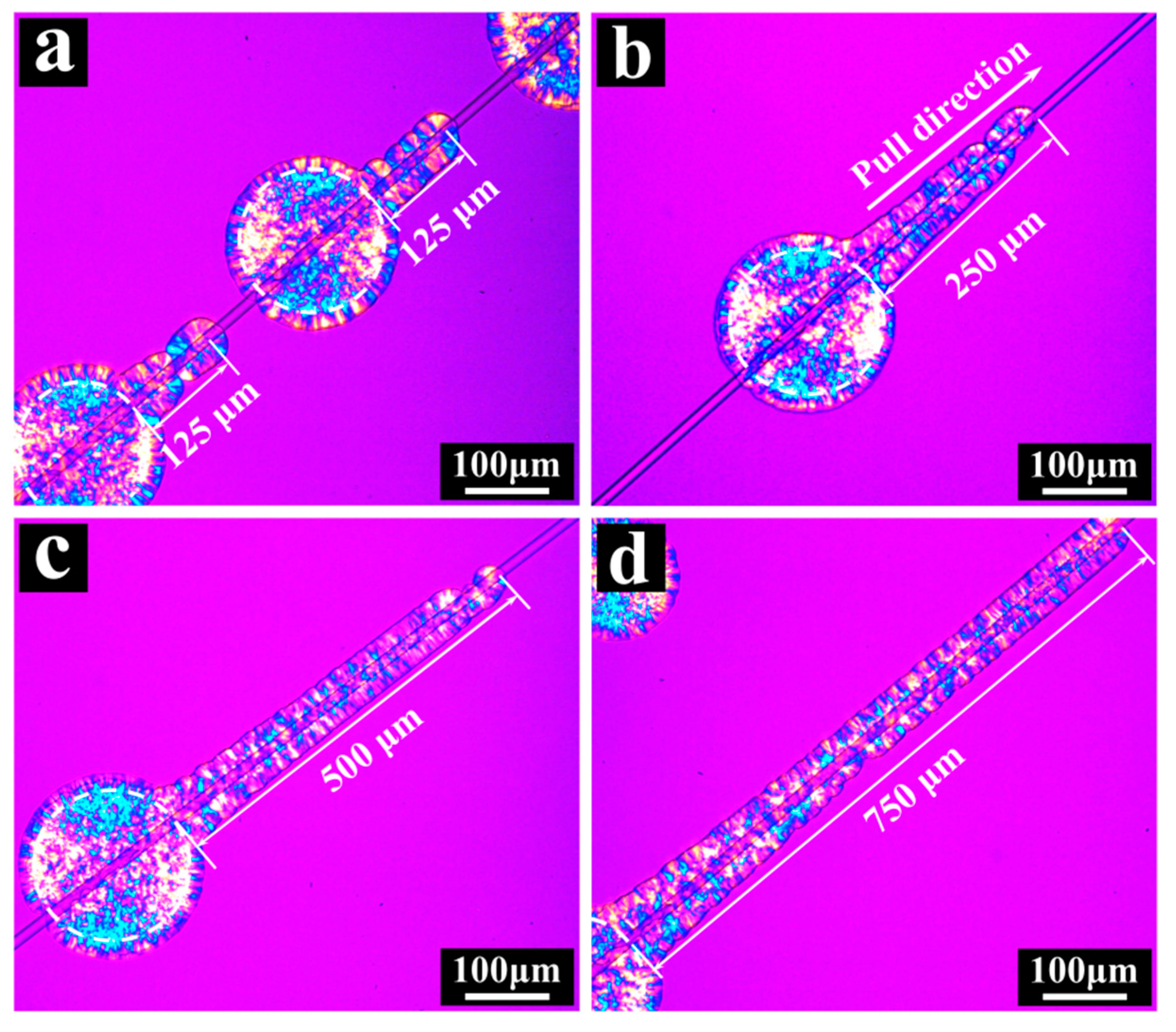
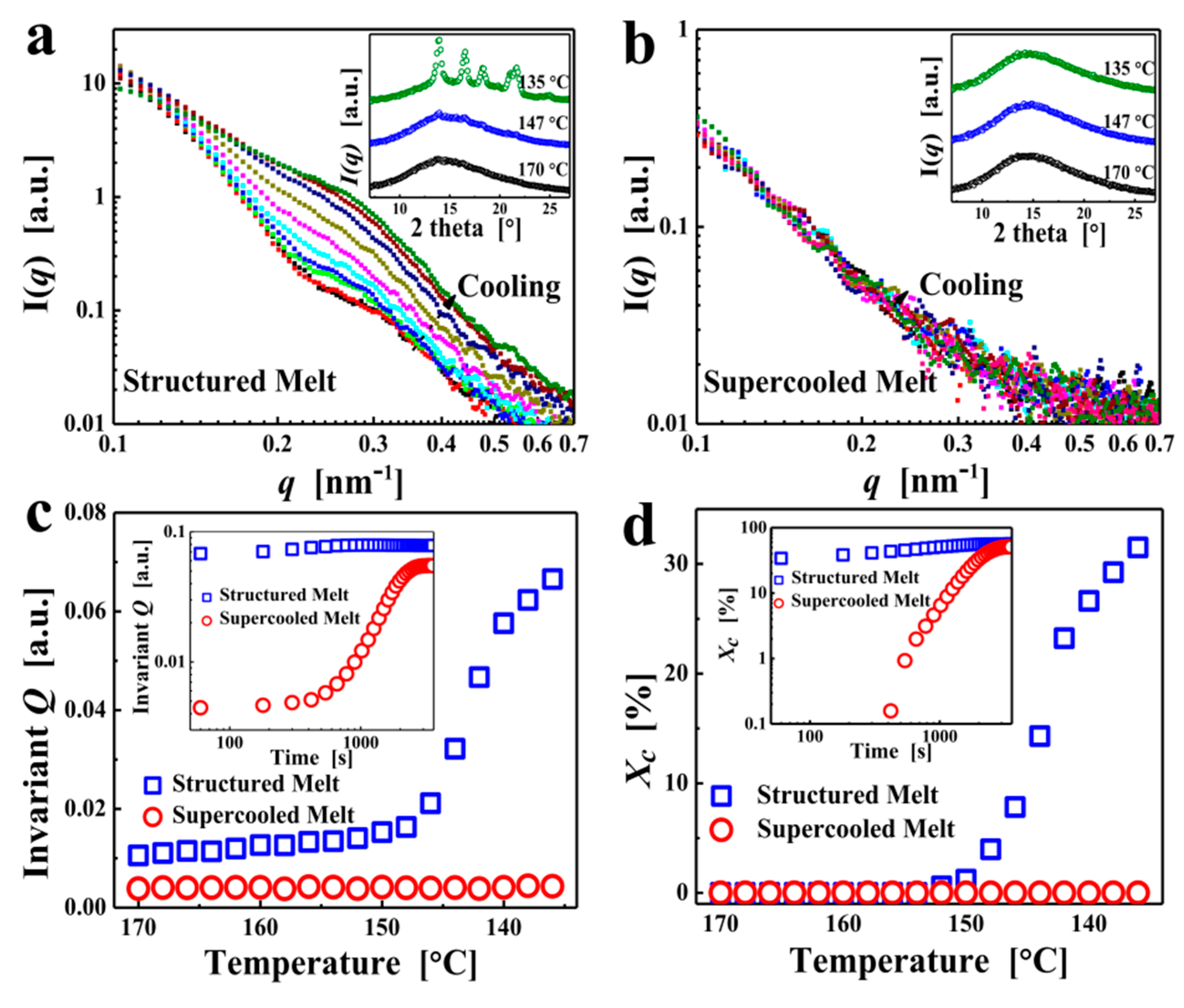
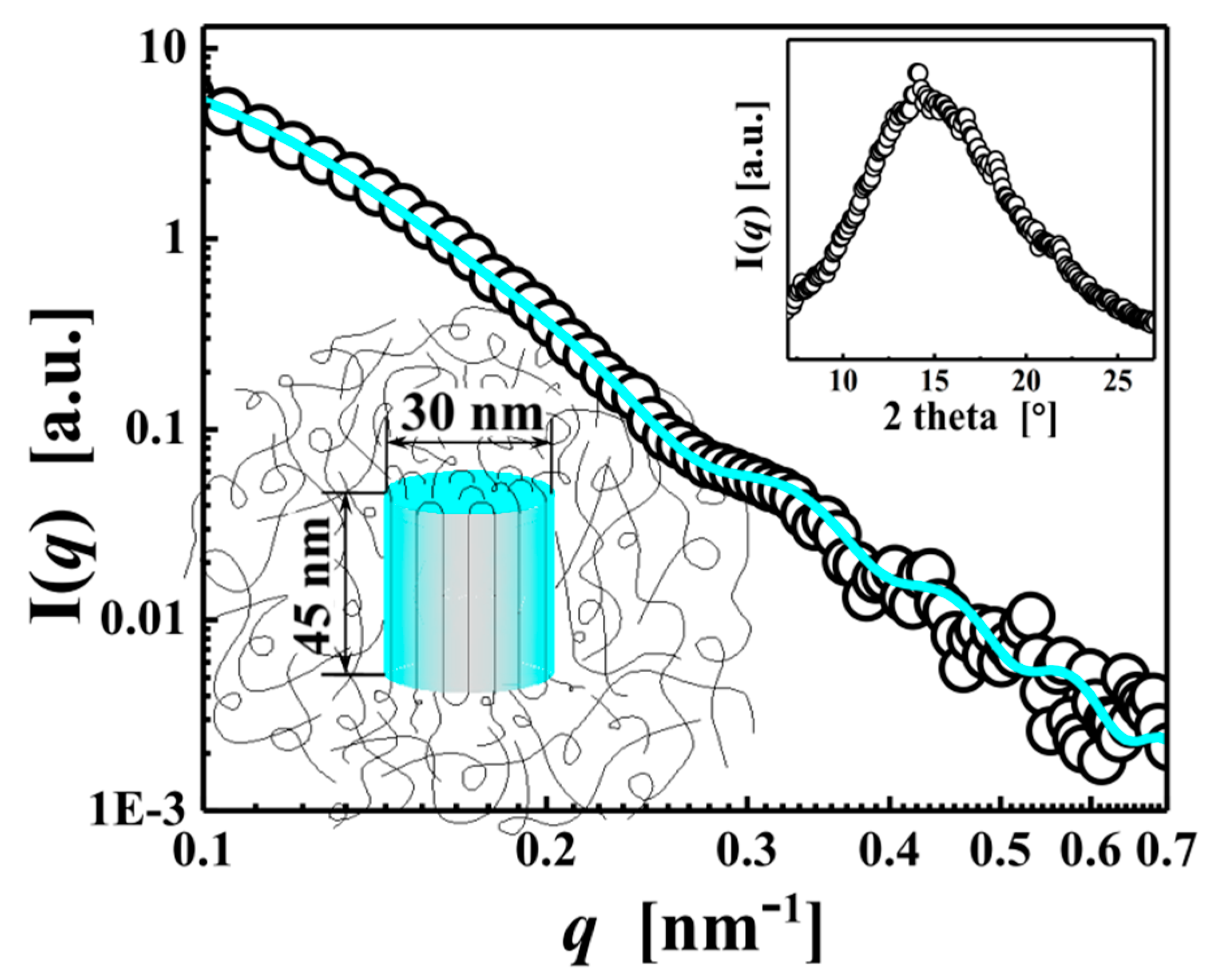
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armitstead, K.; Goldbeck-Wood, G. Polymer Crystallization Theories. Adv. Polym. Sci. 1992, 100, 219–312. [Google Scholar]
- Zhang, J.; Duan, Y.; Sato, H.; Shen, D.; Yan, S.; Noda, I.; Ozaki, Y. Initial Crystallization Mechanism of Isotactic Polystyrene from Different States. J. Phys. Chem. B 2005, 109, 5586–5591. [Google Scholar] [CrossRef]
- Schmidtke, J.; Strobl, G.; Thurn-Albrecht, T. A Four-State Scheme for Treating Polymer Crystallization and Melting Suggested by Calorimetric and Small Angle X-ray Scattering Experiments on Syndiotactic Polypropylene. Macromolecules 1997, 30, 5804–5821. [Google Scholar] [CrossRef]
- Alfonso, G.C.; Russell, T.P. Kinetics of Crystallization in Semicrystalline/Amorphous Polymer Mixtures. Macromolecules 1986, 19, 1143–1152. [Google Scholar] [CrossRef]
- Keller, A.; Cheng, S.Z.D. The role of metastability in polymer phase transitions. Polymer 1998, 39, 4461–4487. [Google Scholar] [CrossRef]
- Langer, J.S. Instabilities and pattern formation in crystal growth. Rev. Mod. Phys. 1980, 52, 1–30. [Google Scholar] [CrossRef]
- Furushima, Y.; Nakada, M.; Murakami, M.; Yamane, T.; Toda, A.; Schick, C. Method for Calculation of the Lamellar Thickness Distribution of Not-Reorganized Linear Polyethylene Using Fast Scanning Calorimetry in Heating. Macromolecules 2015, 48, 8831–8837. [Google Scholar] [CrossRef]
- Lu, L.; Alamo, R.G.; Mandelkern, L. Lamellar Thickness Distributions in Linear Polyethylene and Ethylene Copolymers. Macromolecules 1994, 27, 6571–6576. [Google Scholar] [CrossRef]
- Zhou, H.; Wilkes, G.L. Comparison of lamellar thickness and its distribution determined from d.s.c., SAXS, TEM and AFM for high-density polyethylenefilms having a stacked lamellar morphology. Polymer 1997, 38, 5735–5747. [Google Scholar] [CrossRef]
- Ryan, A.J.; Bras, W.; Mant, G.R.; Derbyshire, G.E. A direct method to determine the degree of crystallinity and lamellar thickness of polymers: Application to polyethylene. Polymer 1994, 35, 4537–4544. [Google Scholar] [CrossRef]
- Marega, C.; Marigo, A.; Cingano, G.; Zannetti, R.; Paganetto, G. Small-angle X-ray scattering from high-density polyethylene: Iamellar thickness distributions. Polymer 1996, 37, 5549–5557. [Google Scholar] [CrossRef]
- Volgt-Martin, I.G. Numerical analysis of lamellar thickness distributions. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 967–991. [Google Scholar] [CrossRef]
- Mezghani, K.; Campbell, R.A.; Phillips, P.J. Lamellar Thickening and the Equilibrium Melting Point of Polypropylene. Macromolecules 1994, 27, 997–1002. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Müller, A.J.; Lin, M.-C.; Chen, H.-L. SAXS/DSC Analysis of the Lamellar Thickness Distribution on a SSA Thermally Fractionated Model Polyethylene. Macromol. Chem. Phys. 2011, 212, 2009–2016. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Wu, S.S.; Chen, J.; Zhuo, Q.; Quirk, R.P. Isothermal Thickening and Thinning Processes in Low Molecular Weight Poly(ethy1ene oxide) Fractions Crystallized from the Melt.4. End-Group Dependence. Macromolecules 1993, 26, 5105–5117. [Google Scholar] [CrossRef]
- Maiti, P.; Hikosaka, M.; Yamada, K.; Toda, A.; Gu, F. Lamellar Thickening in Isotactic Polypropylene with High Tacticity Crystallized at High Temperature. Macromolecules 2000, 33, 9069–9075. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Chen, J.; Barley, J.S.; Zhang, A. Isothermal Thickening and Thinning Processes in Low Molecular Weight Poly(ethy1ene oxide) Fractions Crystallized from the Melt.3. Molecular Weight Dependence. Macromolecules 1992, 25, 1453–1460. [Google Scholar] [CrossRef]
- Ryan, A.J.; Stanford, J.L.; Bras, W.; Nye, T.M.W. A synchrotron X-ray study of melting and recrystallization in isotactic polypropylene. Polymer 1997, 38, 759–768. [Google Scholar] [CrossRef]
- Organ, S.J.; Hobbs, J.K.; Miles, M.J. Reorganization and Melting of Polyethylene Single Crystals: Complementary TEM, DSC, and Real-Time AFM Studies. Macromolecules 2004, 37, 4562–4572. [Google Scholar] [CrossRef]
- Albrecht, T.; Strobl, G. Temperature-Dependent Crystalline-Amorphous Structures in Linear Polyethylene: Surface Melting and the Thickness of the Amorphous Layers. Macromolecules 1995, 28, 5827–5833. [Google Scholar] [CrossRef]
- Strobl, G. A thermodynamic multiphase scheme treating polymer crystallization and melting. Eur. Phys. J. E Soft Matter 2005, 18, 295–309. [Google Scholar] [CrossRef]
- Lijima, M.; Strobl, G. Isothermal Crystallization and Melting of Isotactic Polypropylene Analyzed by Time- and Temperature-Dependent Small-Angle X-ray Scattering Experiments. Macromolecules 2000, 33, 5204–5214. [Google Scholar]
- Strobl, G. Crystallization and melting of bulk polymers: New observations, conclusions and a thermodynamic scheme. Prog. Polym. Sci. 2006, 31, 398–442. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Jiang, Z.; Men, Y. Molecular Weight Dependency of Crystallization Line, Recrystallization Line, and Melting Line of Polybutene-1. Macromolecules 2014, 47, 6401–6407. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Fu, L.; Lu, Y.; Men, Y. Stretching Temperature Dependency of Lamellar Thickness in Stress-Induced Localized Melting and Recrystallized Polybutene-1. Macromolecules 2013, 46, 7874–7879. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Jiang, Z.; Men, Y. Molecular Weight Dependency of Surface Free Energy of Native and Stabilized Crystallites in Isotactic Polypropylene. ACS Macro Lett. 2014, 3, 1101–1105. [Google Scholar] [CrossRef]
- Martins, J.A.; Zhang, W.; Brito, A.M. Origin of the melt memory effect in polymer crystallization. Polymer 2010, 51, 4185–4194. [Google Scholar] [CrossRef]
- Chen, E.-Q.; Cheng, S.Z.D. Primary Nucleation in Polymer Crystallization. Macromol. Rapid Commun 2001, 22, 611–615. [Google Scholar] [CrossRef]
- Alfonso, G.C.; Ziabicki, A. Memory effects in isothermal crystallization II. Isotactic olypropylene. Colloid Polym. Sci. 1995, 273, 317–323. [Google Scholar] [CrossRef]
- Alfonso, G.C.; Scardigli, P. Melt memory effects in polymer crystallization. Macrornol. Symp. 1997, 118, 323–328. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Hu, W.; Rehahn, M.; Reiter, G. Cloning polymer single crystals through self-seeding. Nat. Mater. 2009, 8, 348–353. [Google Scholar] [CrossRef]
- Kovacs, A.J.; Gonthier, A. Crystallization and fusion of self-seeded polymers. Polymer 1972, 250, 530–551. [Google Scholar] [CrossRef]
- Blundell, D.J.; Keller, A.; Kovacs, A.J. A new self-nucleation phenomenon and its application to the growing of polymer crystals from solution. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 481–486. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Hsiao, B.S.; Sirota, E.B.; Agarwal, P.; Srinivas, S. Probing the Early Stages of Melt Crystallization in Polypropylene by Simultaneous Small- and Wide-Angle X-ray Scattering and Laser Light Scattering. Macromolecules 2000, 33, 978–989. [Google Scholar] [CrossRef]
- Gutiérrez, M.-C.; Alfonso, G.C.; Riekel, C.; Azzurri, F. Spatially Resolved Flow-Induced Crystallization Precursors in Isotactic Polystyrene by Simultaneous Small- and Wide-Angle X-ray Microdiffraction. Macromolecules 2004, 37, 478–485. [Google Scholar] [CrossRef]
- Lippits, D.R.; Rastogi, S.; Hohne, G.W. Melting kinetics in polymers. Phys. Rev. Lett. 2006, 96, 218303. [Google Scholar] [CrossRef]
- Pandey, A.; Toda, A.; Rastogi, S. Influence of Amorphous Component on Melting of Semicrystalline Polymers. Macromolecules 2011, 44, 8042–8055. [Google Scholar] [CrossRef]
- Rastogi, S.; Yao, Y.; Lippits, D.R.; Hohne, G.W.; Graf, R.; Spiess, H.W.; Lemstra, P.J. Segmental Mobility in the Non-crystalline Regions of Semicrystalline Polymers and its Implications on Melting. Macromol. Rapid Commun. 2009, 30, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Sommer, J.U. Coexistence of melting and growth during heating of a semicrystalline polymer. Phys. Rev. Lett. 2009, 102, 147801. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Cheng, H.; Zhang, L.; Li, Y.; Han, C.C. Early stages of nucleation and growth in melt crystallized polyethylene. Polymer 2012, 53, 2315–2319. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Cui, J.; Zhang, H.; Ji, F.; Zheng, G.; Heck, B.; Reiter, G.; Shen, C. Effect of Shear Stress on Crystallization of Isotactic Polypropylene from a Structured Melt. Macromolecules 2012, 45, 8933–8937. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Y.; Lu, Y.; Wang, B.; Shen, C.; Chen, J.; Zhang, B. Later Stage Melting of Isotactic Polypropylene. Macromolecules 2020, 53, 2136–2144. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Ji, F.; Zhang, X.; Zheng, G.; Shen, C. Effects of melt structure on shear-induced β-cylindrites of isotactic polypropylene. Polymer 2012, 53, 1791–1800. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Wang, J.; Yan, S.; Schultz, J.M. On the development of special positive isotactic polypropylene spherulites. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1114–1121. [Google Scholar] [CrossRef]
- Varga, J.; Karger-Kocsis, J. Direct evidence of row-nucleated cylindritic crystallization in glass fiber-reinforced polypropylene composites. Polym. Bull. 1993, 30, 105–110. [Google Scholar] [CrossRef]
- Varga, J.; Karger-Kocsis, J. Rules of Supermolecular Structure Formation in Sheared Isotactic Polypropylene Melts. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 657–670. [Google Scholar] [CrossRef]
- Somani, R.H.; Hsiao, B.S.; Nogales, A.; Fruitwala, H.; Srinivas, S.; Tsou, A.H. Structure Development during Shear Flow Induced Crystallization of i-PP: In Situ Wide-Angle X-ray Diffraction Study. Macromolecules 2001, 34, 5902–5909. [Google Scholar] [CrossRef]
- Li, X.-Y.; Ma, Z.; Su, F.-M.; Tian, N.; Ji, Y.-X.; Lu, J.; Wang, Z.; Li, L.-B. New understanding on the memory effect of crystallized iPP. Chin. J. Polym. Sci. 2014, 32, 1224–1233. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, C.; Yang, J.; Xu, Y.; Wang, D. In-situ investigation on the structural evolution of mesomorphic isotactic polypropylene in a continuous heating process. Polymer 2016, 105, 133–143. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Chang, H.; Yan, S.; Yang, C.; Takahashi, I.; Ozaki, Y. Melting Behavior of Epitaxially Crystallized Polycarprolactone on a Highly Oriented Polyethylene Thin Film Investigated byin SituSynchrotron SAXS and Polarized Infrared Spectroscopy. Macromolecules 2010, 43, 5315–5322. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Zhu, D.; An, J.; Min, Z.; Chen, J. RETRACTED: Investigation of the Ordered Structure in Partially Melted Isotactic Polypropylene. Polymers 2021, 13, 3354. https://doi.org/10.3390/polym13193354
Shen J, Zhu D, An J, Min Z, Chen J. RETRACTED: Investigation of the Ordered Structure in Partially Melted Isotactic Polypropylene. Polymers. 2021; 13(19):3354. https://doi.org/10.3390/polym13193354
Chicago/Turabian StyleShen, Junfang, Derong Zhu, Junchao An, Zhiyu Min, and Jingbo Chen. 2021. "RETRACTED: Investigation of the Ordered Structure in Partially Melted Isotactic Polypropylene" Polymers 13, no. 19: 3354. https://doi.org/10.3390/polym13193354




